Advances in reduction of NOxand N2O1 emission formation in an oxyfi red fluidized bed boiler☆
2019-03-20KhalidElSheikhMohammadJakirHossainKhanMaharDianaHamidSiddharthaShresthaBrahimSiAliRyabovLyaDolgushinMohdAzlanHussainTatianaBukharkinaElenaGorelova
Khalid El Sheikh*,Mohammad Jakir Hossain Khan,Mahar Diana Hamid,Siddhartha Shrestha,Brahim Si Ali,G.A.Ryabov,Lya A.Dolgushin,Mohd Azlan Hussain,Tatiana V.Bukharkina,Elena A.Gorelova
1Dmitry Mendeleev University of Chemical Technology of Russia,Moscow 125047,Russia
2Department of Chemical Engineering,Faculty of Engineering,University of Malaya,Kuala Lumpur 50603,Malaysia
3Laboratory for Simulation and Modelling of Particulate Systems,Department of Chemical Engineering,Monash University,Victoria 3800,Australia.
4All-Russian Thermal Engineering Institute,VTI,14/23 Avtozavodskaya str,Moscow,Russia
Keywords:Oxy-fuel combustion Fluidized bed technology Biomass blend Primary/secondary measures NOxemission
A B S T R A C T Fossil fuel combustion is one of the major means to meet the mounting global energy demand.However,the increasingNOxandN2Oemissionsarisingfromfossilfuelcombustionprocesshavehazardouseffects.Thus,mitigating these gases is vital to attain a sustainable environment.Interestingly,oxy-fuel combustion in fluidized bed for carboncaptureandminimizedNOxemissionsisstronglysustainablecomparetotheotherapproaches.Itwasassessed thatNOxformationandfuel-Nconversionhavesignificantlimitationunderoxy-fluidizedbedcomparedtoairmode and the mechanism of NOxformation is still deficient and requires further development.In addition,this review paper discussed the potential of primary measure as low emission process with others supplementary techniques for feasible NOxreduction.The influences of combustion mode,operating parameters,and reduction techniques suchasfluegasrecirculation,oxygenstaging,biomassco-firing,catalyst,influenceoffluidizedbeddesignandstructure,decouplingcombustionandtheirmergesarerespectively evaluated.Findingsshowthatsignificantminimization of NOxemission can be achieved through combination of primary and secondary reduction techniques.
1.Introduction
The current bulk production of energy to meet the needs of the society is primarily dependent on the combustion of fossil fuels,such as natural gas,petroleum products,and coal.Among these fuels,the use of coal for power generation is dominant because of its abundance and low cost compared with other non-conventional methods of power generation.However,thecombustionprocessofthecoalisalwaysassociated with the release of harmful air pollutants,such as CO2,SOx,and NOx.Nitrogenoxidesfromcoalcombustionconsistofnitricoxide(NO),nitrogen dioxide(NO2),and nitrous oxide(N2O).NOxis a generic term for NO and NO2collectively.These derivatives of nitrogen oxides can cause a wide range of health and environmental damages.One of the major effects is acid rain;when NOxand SOxreact with other substances in the air to form acids and fall to the earth as rain,snow,fog or dry particles,they can directly damage the buildings and historical monuments,besides,they can cause lakes and water streams to become acidic and dangerous for human purposes and other living organism[1].In addition,this increased nitrogen loading in water bodies,particularly coastal estuaries,upsets the chemical balance of nutrients used by aquatic plants and animals.Secondly,the nitrogen oxides also play a major role in Global Warming.The continuous accumulation of NOxand N2O in the atmosphere may cause gradual rise in earth's temperature,which causes the rise of the sea level as well as affects the plant and animal habitat adversely[2-6].The impact of N2O on global warming of the atmosphere isalmost300timeshigherthanthatcausedduetoeffectofCO2[7].Moreover,the toxic chemical in the air occurring by NOxand,N2O reacts with ozoneandcommonorganicchemicalswhichmayleadthebiologicalmutations and can deeply harm visibility[8-12].By considering their hazardous effects,the standards for regulations on NOxand N2O emission levels from combustion plants have been set worldwide[13,14].Table 1 shows the NOxemission standards in several EU countries[15].These emission standards are met by undertaking measures to control NOxand N2O emission,which also raised the global awareness and the need for extensive research toward the utilization of coal in an environment-friendly manner.
The major sources of the NOxand N2O emissions are the coal-fired power plants,such as pulverized,fixed,moving,and fluidized bed boilers.Three mechanisms are considered for the formation of nitrogenoxide:(i)thermal-NOx,(ii)prompt-NOx,and(iii)fuel-NOx.Thermal-NOxis formed by the reaction of atmospheric nitrogen with oxygen atoms,especially at higher temperatures(T>1000°C).The prompt-NOxalso forms at higher temperatures,but is induced by fuel radicals under fuel-rich conditions.The fuel-NOxoriginates from the oxidation of nitrogen compounds chemically bounded in the fuel.Compared with the conventional power plants(pulverized,fixed,and moving beds),fluidized beds have more advantages because they offer relatively high mixing and reaction rate and can be scaled up to medium and large sizes[16,17].Moreover,fluidized beds are suitable for wide varieties of feed-stocks(from anthracite to lignite,petroleum coke,biomass,and so on)[18,19].The operating temperature range in fluidized bed combustors is fairly low(800-900°C)[20].The formation of thermal and prompt NOxis less than 5%of the total NOxemitted and can thus be considered negligible;only NOxformed from fuel-N is important.

Table 1 NOxemission standards in some EU countries for coal and biomass[70]
Although the use of fluidized bed for power generation is relatively efficient and emits relatively less amount of NOxcompared with pulverized combustion,it is still below the requirement set by the legislated emission standards.To meet the imposed emission standards,NOxreduction technologies play an important role.The NOxreduction techniques are classified into two broad categories,namely,primary and secondary measures.Primary measures are applied during the combustion process,whereas secondary measures are operated in the downstream of the combustion process.Secondary measures,often referred as post combustion treatment or flue gas treatment,are techniques designed to remove NOxfrom flue gas.Several secondary measures are available including(i)selective catalytic reduction,(ii)selective non-catalytic reduction,(iii)non-selective catalytic reduction,(iv)pulsed corona discharge,and(v)electron beam flue gas treatments[21-23].Although NOxreduction achieved from secondary measures is competent,it is not economical because the process is energy extensive and requires periodic replacement of expensive catalysts[24].
Meanwhile,primary measures are techniques that aim to adjust the combustion parameters to reduce NOxformation during combustion.Several experts have suggested that primary measures can be an efficient way to mitigate NOxemissions[25-27].Primary measures are cost effective but are rather challenging and largely dependent on the process parameters,such as temperature,fluidizing velocity,excess air,air staging,and flue gas recirculation(FGR).Aside from modification of the combustion parameters,advancement of the technology has also led to the realization in the modification of combustion technology.
The oxy-fuel combustion technology has recently been regarded as an emerging technology and has been under extensive research.This technology also has numerous benefits over other technologies,mostly toward CO2capture[28-32].Oxy-fuel combustion is a process in which fuel is combusted in a stream of pure oxygen instead of air.The combustion byproducts mainly consist of CO2and water vapors,where CO2can be easily separated and sequestrated.In addition to CO2capture,oxy-fuel combustion is also gaining importance in NOxreduction studies because this technology is always associated with low NOxemission[27].NOxemission from the oxy-fuel system is less than about one-third that of combustion in air[33,34].Given that pure oxygen is used to combust the fuel,oxy-fuel combustion is always associated with higher temperatures than conventional air combustion.Therefore,moderationintemperatureisrequired,whichisgenerallyaccomplished by recirculation of flue gas(mainly CO2and water vapor).Similar to air combustion,oxy-fuel combustion can also be performed in any type of combustors:pulverized,fixed,moving,and fluidized bed.Oxy-fuel combustion in fluidized bed has been reported to have the following additional advantages[16,35,36]:
(1)Fuel flexibility
(2)Operable under high oxygen concentration
(3)Reductioninareaupto50%canbeachievedgiventhebettercontrol of combustion temperature by solid recirculation.
(4)Significant reduction in the amount of recirculation of flue gas
(5)Increased SO2capture.
Currently,the oxy-fuel technology has been undergoing a rapid advancement.A number of pilot plants have been established and under study to scale-up the process for commercialization[37,38].However,this method is still considered to be under development and requires more insight for advancement.Several reviews have been published discussingthe oxy-fuel technology as viable,efficient,and environment friendly emerging technology for sustainable development[39,40].However,the process of oxygen separation from air to generate pure oxygen stream is energy extensive and requires higher operational cost.The emission units are generally expressed in terms of milligram per mega joule(mg·MJ−1).
Although this technology is preferred and has been extensively studied for the implementation of CO2capture,studies on NOxemission are limited.Similar to NOxemission from fluidized bed airfi red combustors,NOxemission from oxy-fuel fluidized bed is also affected by several parameters[41-43];the major control parameters include temperature,fluidizing velocity,excess percentage of oxygen presence,oxygen staging,types of Fluidized Bed Gasification Reactor(FGR),catalyst,and so on.These parameters significantly affect the NOxreduction and remain as challenges in the application of oxy-fuelfluidized bed because they largely vary with the process conditions[20,25,35,44,45].
The objective of this review paper is to discuss the formation of nitrogen emission in fluidized bed conditions during the last two decades.The first part discusses the reaction mechanism for the formation of NOxand N2O and the effect of key parameters during fluidization.The second part summarizes the current research conducted on the reduction of NOxand N2O emission from coal.Both lab and pilot scale research works have been critically reviewed by considering the influence of operating conditions.The effect of controlling the amount of biomass and catalyst dosages was also taken into account.Lastly,the third part summarizes the recent studies on the role of combination primary and certain secondary measures for reduction NO emissions.
2.The Mechanism of Formation and Destruction of NOxand N2O
The formation and destruction of NOxis a complex mechanism where series of reactions take place and are highly influenced by combustion conditions and forms intermediate radical compositions[20].Under typical fluidized bed conditions,temperatures are usually kept low,around 800-900°C.Therefore,the amount of formation of NOxis acceptable as the outcome of nitrogen presents in the fuel(i.e.,fuel-N).The mechanism is a function of several factors including the amount,phase,and volatility of the nitrogen present in the fuel,oxy/fuel ratio(stoichiometry),bed temperature,and fixed carbon/volatile matter ratio[30].Nevertheless,the formation of NOxis reliant on O and OH radicals instead of O2.Moreover,the formation of N2O dependent on the presence of cyanides than ammonia was discussed in details by Aemand et al.[47].
2.1.Reaction mechanism of Fuel-N
The major percentage of NOxformed in oxy-fluidized bed condition is caused by the oxidation of nitrogen content that is chemically bound to the fuel.[47].The N content in coal can range from 0.5 wt%-2.5 wt%,whereas 0.1 wt%(wood)-0.5 wt%(agricultural residues)in biomass[49],as shown in Table 2.Nitrogen is chemically bounded in the form of pyridines,quinolones,iso-quinolines,amines,indoles,and carbazoles.The formation of NOxis divided into two fractions,namely,volatile-N(mainly HCN and NH3)and char-N(nitrogen remained in the char)[52],based on nitrogen conversion during devolatilization of fuel nitrogen.The fraction of volatile-N formed depends on the devolatilization temperature,heating rate,and coal type.The formation of NOxdepends on the subsequent reactions of volatile-N and char-N[53].

Table 2 Chemical composition of varieties of biomass(dry basis)and solid fuel types based on volatility fuel-N content[25,50]
2.1.1.Decomposition of N through volatilization
Volatile-N is mainly decomposed into intermediate species of nitrogen,such as HCN and tar-N[54-56].Other species,such as NH3and CNO,are also available in relevant concentrations.These species more readily form fuel-NOxthan thermal NOxbecause the N-H and N-C bonds in fuel-N are weaker than the triple bond in molecular nitrogen that needs to be dissociated to produce thermal NO.Moreover,the formation of fuel-NOxdepends on the type of volatile nitrogen compounds formed and proceeds with the subsequent reactions.The process is relatively complex and results in a large number of homogenous and heterogeneous reactions.In general,the formation of NOxand N2O from volatile-N is attributed to HCN and NH3shown in Table 3[46,57-59].

Table 3 Formation and decomposition NOx,N2O in fluidized bed conditions
2.1.2.Formation of Char-N
Besides volatile-N,a part of the total fuel-N persists in char after devolatilization,which is known as char-N.During char combustion,char-N results in the formation of NOxand N2O.The formation of NOxand N2O from char-N under fluidized bed conditions depends mostly on the quality and quantity of char[60-62].Dong et al.[63]studied the influence of operating conditions on char texture by pyrolysis and concluded that char reactivity increases with heating rate and coal category.This increase is attributed to enhanced NO heterogeneous reduction on the char surface[64].Meanwhile,nitrogen distribution between char and volatiles during coal pyrolysis is weakly dependent on coal rank[62].According to Wójtowicz et al.[65],approximately 60%of the initial fuel-N remains in the char during coal combustion.
The study of Czakiert et al.[66]identified the noticeable drawbacks of application of the oxy-fuel combustion in the case of fuel-N conversion to NOx.Nevertheless,it has been identified that compared to airfiring technique,NOxformation is lower during oxy-coal combustion because of the additional reduction in NO by reaction with carbon dioxide[52,67,68].
2.2.Influence of operation parameters
2.2.1.Bed temperature
Coal devolatilization as well as the formation of volatile-N/char-N are strongly dependent on bed temperature.Glarborg et al.[69]reported that higher bed temperature resulted in higher NO emissions.
Tourunenetal.[61]investigatedthedependencyofCO,NO,andN2O emissions on bed temperature and oxygen concentration in circulatingfluidizedbedcombustion(CFB).Theirstudymainlyfocusedonnitrogen emission formation in the lower furnace area.Their experiments showed that NO emissions above the dense bed decreases,whereas N2Oemissionincreaseswithdecreasingtemperatureoroxygenconcentration.These trends can be explained by the heterogeneous reactions between NO and char that decreased with temperature or oxygen concentration and increased the bed char inventory.Oxygen concentration and temperature also affected NO emissions directly.Correlations for CO,NO,N2O,NH3,and HCN concentrations at the exit of dense bed werealsodeveloped[61].Recently,Royetal.[70]confirmedN2Oformation is high under oxy-fuel combustion than an air.However,N2O decreased with an increase in bed temperature.
Duanetal.[25]studiedtheeffectofbedtemperatureonNOemission for bituminous and anthracite coals under different combustion atmospheres in CFB.As a result,NO emission increased with bed temperature,which can be explained in three points:
(1)The coal char burnt more completely,and more char-N was released from the N site in the coal matrix into the gas phase to form NO precursors.
(2)Active O and OH radicals were produced from molecular O2,and the precursors were easier to oxidize to NO.
(3)Thechar and COconcentrationsin the combustor decreased,and the NO reduction reaction was suppressed.
De diego et al.[71]showed the increment of NO emission with development in bed temperature.In their experiments,NO emission increased until the bed temperature was 900°C and remained constant up to 950°C.The increase in NO emission with bed temperature was attributed to reduced char and CO concentrations,which led to the heterogeneous reduction of NO on the char surface.Moreover,higher temperature tended to promote NCO oxidation to NO.
Lupianez et al.[43]studied NOxemissions from bituminous and lignite coal under oxy-firing in bubbling fluidized bed(BFB).They found that the effect of bed temperature on NOxemissions is less intense and without a clear tendency.They suggested that fuel-NOxformation is mainly controlled by the oxygen partial pressure in the surroundings of the burning particle.Moreover,the temperature influence is not significant,and the differences on emissions relied on the coal type.However,the effect of temperature increased.
Lasek et al.[72]studied the influence of temperature and pressure on NOx,N2O emissions in pressurized fluidized bed combustion.They reported significant decrease in nitrogen emissions with the increase of pressure.These results are shown in Fig.1,
2.2.2.Excess oxygen
Excess oxygen(λ)is stoichiometric oxygen ratio,which can be controlled by varying fluidizing gas flow rate or fuel flow rate in oxy-fired condition.Several researchers have studied the effect of excess oxygen ratio on NOxformation in oxy-fired fluidized bed condition[25,35,73].Lupianez et al.[20]investigated the effect of excess oxygen in 90 kWth BFB.The range of oxygen concentrations in the O2/CO2(25:75,55:45)atmospheres was varied to change the excess oxygen percentage;whereas,other parameters,such as bed temperature and catalyst,were maintained constant.Their results showed significant rise of NO emission at higher range of excess oxygen(ƛ =1.5-1.7)compare to the lower range(ƛ =1.1-1.3).The NOxemission increased from 590 mg·Nm−3to 685 mg·Nm−3when ƛ was increased from 1.1 to 1.7.Tan et al.[20,74]also observed similar trend from their experiments in a 0.1 MWthoxy-fuel CFB.These results suggest that at higher‘excess oxygen'ratio the formation of NO increased,which may depend on the coal type used during fluidization.Duan et al.[25]studied the effect of excess oxygen ratio(ƛ=1.1-1.3)by using two different coal types in recator.Their results are shown in Fig.2.

Fig.2.Effect of excess oxygen on NO emission[2].

Fig.1.Influence of the pressure on NO during the air/oxy condition[1].
The figure shows that the NO emission from bituminous coal increased from 116 mg·MJ−1to 130 mg·MJ−1,whereas emmission rate was 72 mg·MJ−1to 97 mg·MJ−1from anthracite,when the value of ƛ was 1.1 to 1.3.The NOxemission from bituminous coal was higher at lowervalueofƛ.Thereasonbehindthisistheremarkableamountofnitrogenretainedintheunburntcharofanthracitecoalwhichwasgreater amount than the bituminous coal.
Reduction in conversion of nitrogen species from char or solid fuel using reasonable reduction techniques can minimize the formation of NOxand N2O.The next sub-section reviews the different reduction techniques together with optimized operating parameters in oxy-fired in CFB conditions.
3.The NOxReduction Emission Systems
Numerous studies have been conducted on NOxand N2O emission reduction technologies in oxy-fuel fluidized bed in lab and pilot scalesince 2005.Carbon Capture and Storage(CCS)technology has been intensively studied in large scale to improve with the passage of time by some research centers such as:VTT in Finland,CIRCE in Spain,Alstom in USA and others.In lab scale,Czakiert et al.[66]studied the fuel behavior under oxy-fired CFB conditions and their effect on NOxemissions;they found an increase nitrogen conversion with increase in excess oxygen.Jia et al.[75]investigated the effect of recycling flue gas on reduction NOx,and also extended their studies on SOxemission.Their results exhibited low NOxemissions for the oxy-fired condition,even at higher temperature.Moreover,they showed that high oxygen concentration can increase the rate of NOxreduction.Thereafter,Li et al.[76]studied NOxand SOxemissions under CFBC under high oxygen,and observed higher NOx(ppm)in oxy-condition compared to the air combustion condition.On the other hand,Duan et al.[77]studied NOxemission during cofi ring biomass with coal under oxy-fuel condition and noticed that the addition of biomass can have a positive effect on NOxreduction.Another study by Stewart et al.[42]and Tan et al.[78,79]using lab and pilot scale power plant CFBC under air and oxy-fuel mode examined the effect of H2O(g)on SOx,formation and NOxemission with adding limestone to optimize the reduction process with temperature.In addition,Hofbauer et al.[80],Duan et al.[77]and Li et al.[81]investigated the effect of recycling flue gas on emissions.They confirmed higher reduction in wet and dry recycling flue gases.Li[76]and Li et al.[82]investigated the effect of steam with various oxygen concentration on NOx,and achieved significant reduction in high oxygen concentration.However,N2O decreased by height of the boiler.

Table 4 Review techniques of NOxemission issues in air and oxy firing fluidized bed
Accordingtotheseresearches,theformation of NOxgreatlydepends on combustion parameters,such as the characteristics of solid fuel,operating temperature,oxygen availability,combustor design,andfiring configuration.All the combustion parameters need to be monitored to develop an effective reduction technique.This process may be developed by implementing the primary measures that aim to adjust the combustion operating parameters(temperature,oxygen availability,and so on),either to reduce the formation of NOxor deformed the NOxformed inside the furnace.
Internal modifications of combustion system and conducting reactions at minimum reaction temperature and/or the reducing contact period of nitrogen to the fuel along with oxygen in the reaction phase,while creating a fuel-rich zone.These techniques reduce NOxemissions around 50%-80%,and are simple and cost effective.The available literature discussed on NOxreductiontechniquesin air/oxy-firingtechniques has been shown in Table 4.
3.1.NOxand CO2recirculation profile
Flue Gas Recycle(FGR)is known as an effective method in emissions reduction.In(FGR)the flue gas is recycled back to the combustion zone to reduce the formation of NOx,either by reducing the flame temperature and overall excess oxygen or by increasing the concentration of CO2[34,87].The effect of recycling flue gas(O2/CO2)and(NOx/CO2)on NOx,N2O has been studied in fluidized bed condition by several authors[34,42,50,71,77,88].Qian et al.[88]investigated the effect of RFG on NOxreduction in a vortex fluidized bed under air condition.They reported that recycling NOxnot only minimized but also inhibited the formation NOxby reducing the combustion temperature.
The effects of FGR on nitrogen emissions have been investigated at different compositions under air and oxy-fuel combustion conditions as shown in Fig.3(a)and(b)[50].The results indicated that the implementation of FGR in air firing mode tends to increase the formation of NOx,whereas it seems to increase the formation of N2O in oxycombustion.The formation of N2O increased at the inlet of the reactor because of recirculation of N2O;however,its contribution was lessened along the height,as shown in Fig.3(b).Moreover,Hosoda and Hirama[73]observed by recycling flue gas the fractional conversions of fuel-N into NOxand N2O in oxy-mode to be significantly lower than in the air combustion.They explained the effect of RFG on NOx,N2O,can extend the reduction from 80%to 95%including the fractional contributions of CO2and H2O.

Fig.3.Effects of combustion atmosphere on nitrogen emissions[3].
The effect of FGR in Bubbling Fluidized Bed(BFB)combustor was studied by De Diego et al.De Diego et al.[71]used various gases(CO2,SO2,and NO)on dry base at two different temperatures(850°C,950°C)to simulate different compositions of dry recycle flue gas.Their experimental results showed that the recycled flue gas with 650 vppm reduced NO concentration at 850°C from 60%-70%.However,Hu et al.[26]confirmed that further reduction results could be achieved when recycling NO or NO2in recycle flue gas.Compared to De Diego et al.[71]showed that the result of dry recirculation was 65%NOxto N2.Moreover,they mentioned 850°C as an optimum temperature.In addition,the recycled flue gas can be dry or wet.De Diego et al.[71]compared the result between dry and wet FGR in oxy-fuel condition at 950°C and suggested that Wet FGR could be more effective in reducing NOx.Stewart et al.[42]and Zhu et al.[89]have studied the effect of wet FGR on NOxconcentration in(ppm)and fuel-N conversion to NOxin percentage using(30%O2-70%CO2)oxygen concentrations at varied temperatures.Results of Zhu et al.[89,90]are shown in Fig.4.
Reduction in both NOxand N2O was observed using steam.The use of steam may be significant for nitrogen emission;however,its utilization requires more insight toward corrosion,combustion efficiency,and formation of N2O[42].

Fig.4.Conversion ratios of fuel-N to(a)NO and(b)N2O with different temperatures(BC and inlet gas composition:30%O2þ70%CO2)[4].
Yoshiie et al.[42,50]investigated the formation of NOxand N2O in a drop tube furnace by varying the combustion mode and fuel-N content in their experiments.Their results are summarized in Table 5.

Table 5 Fuel-N content and NOx,N2O formation for different types of coal[19]
The authors elaborated that the relationship of char-N to coal-N ratio was dominated by the nitrogen conversion ratio from NO and N2O.However,the N2O emission at the exit point was a function of nitrogen content in the original coal.Therefore,the N2O emission concentration seems to be high in oxy-CFB,and RFG cannot minimize such concentration.Thus,the use of different types of catalyst may be applicable.
3.2.Oxygen staging
Stagingintypicalfluidizedbedsreferstoamethodinwhichthetotal air supplied in the reactor is divided into ratios at different locations,usually as primary(under-fire)and secondary(over-fire).This investigation has focused on oxy-fuel combustion because the O2concentration is limited to 21%and can be varied independently,which is referred as oxygen staging.This process is in contrast to combustion with air,in which the formation of NOxis a function of oxygen concentration although the air is staged.Various lab and pilot scale experiments with different solid fuels have been conducted to investigate the effect of oxygen concentration in the formation of NOxand N2O[25,43,44,51,74,91].
3.2.1.Oxygen staging in primary flow
NOxemission increases with oxygen concentration in primary stream.At low temperature and oxygen concentration in the dense bed,the concentration of CO2and CO increases,consequently reducing NOxto N2[74]:

In addition,NO is reduced by CO as follows:

Char-N conversion was low with O2concentration in the primary stream because the volatile consumed a lot of oxygen.Incomplete oxidization of carbon,together with CO2gasification,produced large amount of char and CO,which enhanced the NO/Char reduction reaction.However,the NOxemission increased slightly with increasing O2concentration in the primary flow[74].The similar trend was observed by Duan et al.[25]for two different types of coal as shown in Fig.5.

Fig.5.Effect of oxygen staging on NOxusing different type of coal[2].
At 14%O2concentration in the primary stream,the NOxemission was similar for both bituminous and anthracite coals.Meanwhile,NOxemission for bituminous coal was higher by 50 and 40 mg·MJ−1at 30%and 40%O2concentration,respectively.Recently,Li et al.[82]studied the effect primary and secondary staging on formation N2O.They showed increased in excess oxygen concentration in primary stream increases N2O.However,with increasing combustion temperature concentration of N2O decreased.
3.2.2.Oxygen staging in secondary flow
Secondaryoxygenstagingis appliedat non-richfuelareato regulate formationofNOwithheightofgasifierorcombustor[25].Lupianezetal.[51]investigated the effect of staging on NOxformation in lab scale BFB under two different secondary/total ratios(S/T)of 0%-10%and 10%-20%.The staging results showed higher reduction in NOxemission at S/T ratio of 0%-10%.Experiments were carried using coal in bubblingfluidized bed reactor at 90 kWth station to compare anthracite and lignite at same atmosphere 45/55 O2/CO2.The result showed significant NOxreduction only on Lignite coal due to higher amount of volatiles released in Fig.6[51,67].
However,in Fig.7 shows NOxemission from anthracite coal at different atmospheres,the first concentration under 30/70 O2/CO2,NOxemission reduced around 37%.However,under 50/50 O2/CO2the variation is minor,the no advantages obtained by the secondary staging in this case.That explains the limitation of oxygen secondary staging on NOxemission in different types of fuel and various atmospheres.
Li et al.[76]studied the effect of oxygen concentration in secondaryflow in 1 MWth pilot scale oxy-CFB.They considered different oxygen concentrations(30%,40%,and 55%,)in secondary flow,their result showed that NOxconcentration decreased gradually to 55,50,and 42 mg·MJ−1,respectively.Another experiment was conducted by Mingxin et al.[92]in circulating fluidized bed to study the relation between primary and secondary staging ratios on NOxemission.Their results are shown in Fig.8 which delineate the change in axial profile of NO concentration in combustor.It can be seen clearly that the 50/50 staging gave the lowest emissions at FG1.Reduction of NOxby secondary air(S.A)is more obvious at higher temperature due to the intensified NOxemission[93].
4.Influence of Fluidized Bed Design and Structure
Fluidized Bed Technology(FBT)has been considered as one of the best options to reduce the NOxemission because of its user-friendly andflexible operating and design facilities such as,excellent heat and mass transfer uniformity,lower temperature requirement for operations and opportunities of conducting multiphasic reactions for energy production[94-101].

Fig.6.Normalized NOxemission from Anthracite(Left)and Lignite(right):effect of secondary gas ratio[5].
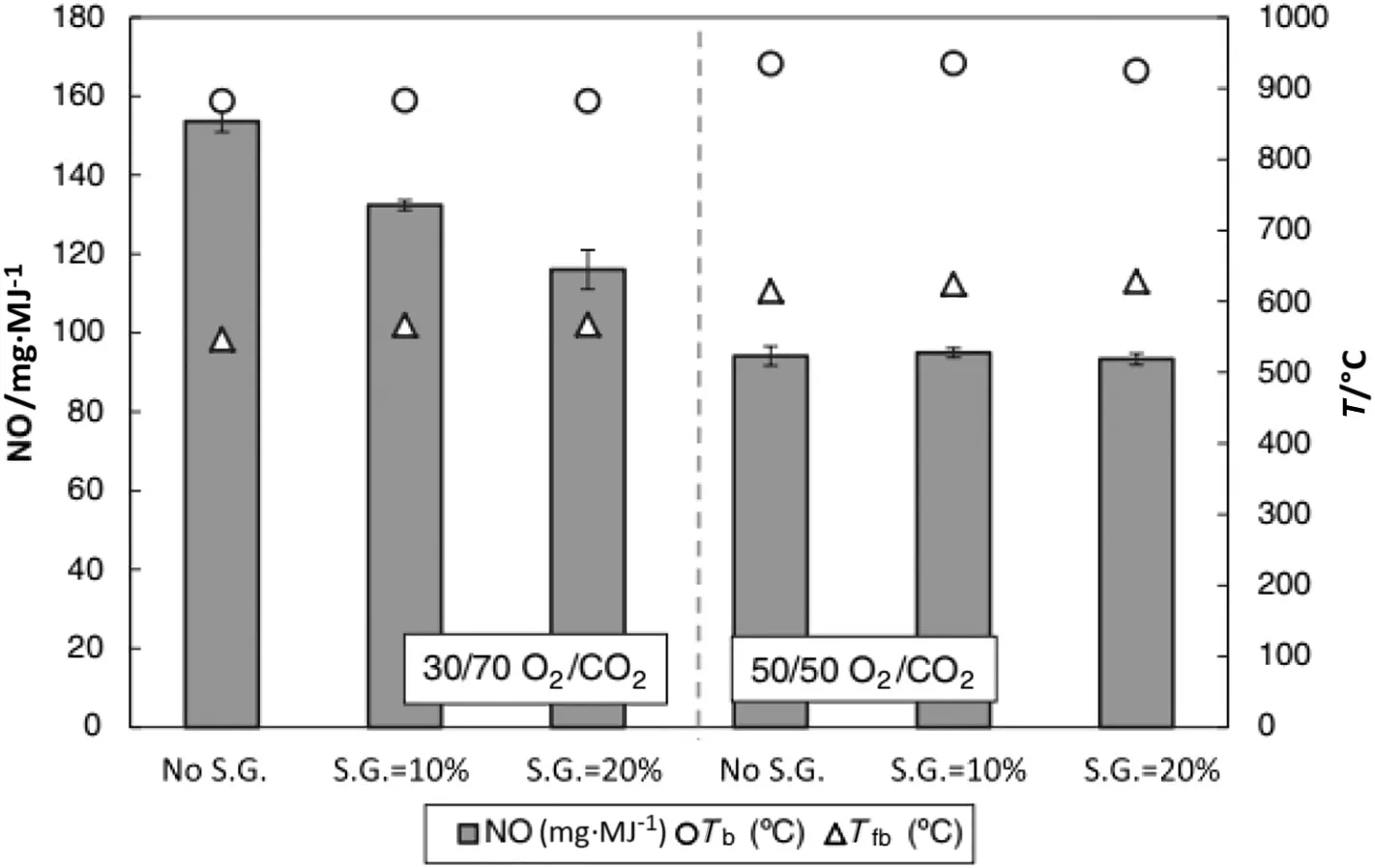
Fig.7.Normalized NOxemission from Anthracite:effect of secondary gas ratio[6].
4.1.Effects of design and structure variations of reaction systems
Theprocessdesignandstructuresprovidecontroltothefluidizedbed boiler which is beneficial for dynamic performance,efficiency,implementation cost and emission control[102].Sirisomboon et al.[103]compared biomass firing in pilot combustors with advanced conical fluidized bedatthesameoperatingcondition.Theyconcludedthatthegreaterresidence time and more uniform distribution of the combustion air across the bed allowed operation to be lower in NOxemissions.The details offluidization state specification(FSS)have been reviewed by Runxia Cai,et al.[101].They have discussed to achieve ultra-low NOxemission,some design principles have been followed and modified such as:
(1)the separators and ash coolers were improved,
(2)the position of discharging ports was modified
(3)the particle size distribution of feeding coal was reduced

Fig.8.Effect of SG and location on NO emission profile along the combustor[8].
In addition,the reduced particle size of fly ash has proven to givehigherefficiencyandfinerbedmaterial.Hence,comparedwithtraditional CFB boilers,the pressure drop of the dilute zone increased to over 1.0 kPa.Higher-pressure drop indicated the higher solid concentration in the dilute phase and the higher solid inventory.As a result,the NOxemission was significantly reduced.The influence of nozzle structure in NOxemission was investigated by Zhu et al.[104].They studied pulverized coal combustion in circulating fluidized bed boiler by using 3 different types of secondary air nozzle structure(center,annular,and circular ports),with various ratios and tertiary air position of the boiler.Fig.9 shows the results obtained from their study.The variation in NOxconcentration is identical under both annular and circular secondary air nozzles.The released-N remained stable in the reducing zone.When tertiary air position changed from 600 mm to 1200 mm belowthetop,i.e.,thelengthofthereducingzonewasgreater,NOxconcentrations decreased during combustion.Concentrations of reduced NOxincreased because the reducing ratio of NOxincreased in tandem with the length of the reducing zone,resulting in a marked drop in NOxemissions.

Fig.9.NOxemissionwithdifferentsecondaryairNozzlestructureonly/uniformlyinjected,at 600/1200 mm blew the top down-fired combustor[9].

Fig.10.Schematic of a decoupled fluidized bed reactor with NOxand NO2testing facility.
4.2.Decoupling combustion
Decoupling of fluidized bed reactors for combustion has been considered as a developed new clean technology for the purpose of NOxand N2O emission reduction[105,106].Devolatilization and fuel combustion takes place in during traditional fluidized bed combustion,whereas in the decoupled fluidization the combustion process go through three steps:(1)pyrolyzation of fuel particle takes place at the bottom section of the reactor(2)in the second stage the pyrolyzed products are burned in the reactor column at fully fluidized conditions and(3)the unburned particles are separated form gas by passing the gas-solid fluid through a cyclone and send back to the system again.This extra step plays a vital role for the reduction of NOxand NO2[107]The schematic of a standard decoupled fluidized bed reactor has been shown in Fig.10.
Asconsequence,theideaofdecouplingreactionsactuallyprovidesoptimization on the reaction behaviors and performances.Number of researcher's had been studied decoupled combustion in last decade.Li dong et al.[107]used coal and biomass and achieved around 19%NOxemissionlessthantraditionalwayofcombustionintenselyNOxreduction till 33%by merging decoupling combustion with air-staging and reburying.The comparison is shown in Fig.11[108].Moreover Zhennan Hanetal.[83,109]comparedtothedirectcombustionofDSLinCFBcombustion,theoperating modeoffirstpyrolysis orpartialgasificationoffuel andthencombustionof fuelgascanbringa stablecombustioneasily,and effectively inhibit the produced NOx,reaching the level of 50 μl·L−1compared to 160 μl·L−1in old-style combustion.

Fig.11.Comparison of NOxemissions during direct combustion and decoupling combustion[10].

Fig.12.NOxemission pathways.
5.Influence of Biomass and Catalyst Additives on NOxEmissions
5.1.Co-firing coal and biomass
Biomass is a promising renewable source of CO2neutral fuel.Co-firing or blending biomass with coal can be a cost-effective and environment-friendly method for minimizing the existing pollution problems[110-117].Given that the biomass is composed of higher volatile and lower nitrogen content,co-firing may lead to the reduction of NOxemission[118-120].Moreover,biomass is highly reactive because of the presence of cellulose,hemicelluloses,and lignin contents with weaker bonds and higher alkali/alkaline earth metallic species,which plays a key role in the thermal chemical process[121-123].The main objectives of blending biomass and coal are to reduce NOxemissions in fuel content,improve boiler efficiency,and reduce fuel cost.During co-firing,knowledge on fuel properties and qualities is required because of their significant effect on NOxemission.However,combustion conditions,for example,bed temperature,freeboard extension,and excess oxygen,are key to successful operational control.The present paper aims to establish the effect of biomass addition on fuel-N reduction and oxygen availability under various operating conditions.

Fig.13.NOxemission for different biomass bituminous coal blends.

Table 6 Proximate and ultimate analysis of bituminous possible blend with different biomass on%wet basis[20]

Fig.14.NOxemission during staging in co-combustion[11].
During co-combustion,synergistic effects of coal and biomass blending can lead to reduction in emitted pollutant,especially NOx.The co-combustion mechanism of fuel can be divided into two stages:the initial stage of devolatilization and then the combustion of the remaining char.During volatilization,some of the fuel-N will be released as volatile-N,while others will remain as char-N.Based on the first assumption for co-firing made by Glarborg et al.[48],HCN and NH3are the dominant species formed as nitrogen-bearing intermediates.The formation ratio of both species is dependent on specific combustion condition and nitrogen content of fuel.For biomass combustion,only NH3as nitrogen intermediate species can be considered based on the second assumption made by Álvarez et al.[68].NOxemission pathways from co-combustion of coal and biomass are shown in Fig.12.
NOxemissions were dependent on biomass volatility and the operating conditions,shown in Fig.13,Tables 2 and 6[124].
Fig.13 shows drop in NOxemission by their highest ratio in blends with coal.Some studies have been conducted to analyze the effect of biomass and coal co-combustion on NOxand N2O emissions in oxyfuelfluidizedbeds[38,125-129],aswellexperimentshavebeencarried out in small and large scale[125,130-133].The conducted test on thermo-gravimetric analysis[134,135]suggested that co-firing can be an effective NOxreduction technique.In addition co-combustion of coal and biomass can be adopted to reduce N2O emission in high nitrogen-content coal[136].However Duan et al.[84]recently reported that burning biomass alone has higher NOxemission than coal.Lower nitrogen concentration was evidently observed by Duan et al.in cocombustion oxy-fuel by shifting to 30%oxygen staging in 10 kWth CFBC shown in Fig.14.
Similar results were also observed by blending wood pellets at different ratios with different ranks of coal,as shown in Fig.15.
Furthermore,oxygen staging reduces NOxemission in co-firing mode during oxy-fuel combustion.Table 7 summarizes several experiments and their results under air/oxy firing/CO2-firing in fluidized bed combustor.

Fig.15.Effect of biomass ration on NOxemission in oxy-fuel combustion[12].

Table 7 Review of NOxstudies on air/oxy co-firing fluidized beds
An experimental study on the conversion of Fuel-N to NOxwas carried out by G.Pu,et al.[137]in a bubble fluidized bed under high oxygen concentration at different temperatures—first for coal alone(Antharcite)where the NOxconversion rate increased by 1.98%.Secondly by coal mixed with 20%pine powder where the nitrogen emission was decreased by 3.39%.
Lowering the fuel-N content,biomass produces significant amount of ash compared with coal,which has been shown to induce catalytic effect[138-140].Kalembkiewicz[141]reviewed ashes from cocombustion of coal and biomass and termed it as an industrial waste.However,other researchers[142,143]reported that the ash is composed of constituents,such as AL2O3,CaO,and TiO2,which are considered catalytically active compounds that reduce SOxand NOxemissions during the combustion processes.In addition,the ash of biomass also exhibits strong catalytic activity toward the oxidation of emission gas precursors,such as HCN,CO,and CH4.The selectivity of HCN to produce NO is higher than that of N2O formation[144].
5.2.Influence of catalytic reduction
Reduction in SOx,NOxand N2O by absorption-desorption using sorbent/catalyst such as bauxite,quartz sand,dolomite,limestone and other bed material has been in practice for the last two decades,been proven to reduce emission gases.But the challenges were in the selection of catalyst for NOxreduction in fluidized bed reactor.There are other factors like temperature variation,chemical stability and reaction retention time that are also proven to be considered as effective operation conditions[2,6,145].The opportunities for in-situ catalyst/s as bed material to reduce of NOxin FBC have been studied by number of researchers and classified catalyst in groups,shown in Table 8.
5.2.1.Thermal stability
The catalyst groups mentioned in Table 8 have been classified by Khanh-Quang Tran et al.[146]such as zeolites,noble metals and metals oxides;all were effective toward HC-SCR of NOxat different temperature ranges(800-900)°C in fluidized bed[147,148].Suggested Cu-Fe/ZSM-5ascatalyst,whichshowedhigherNOxconversioninlower temperature and better performance will be achieved by varying the amount of cooper and iron[147,148].
5.2.2.Mechanical stability
The second major concerns in fluidized bed include excessive sorbent attrition and short residence time of catalyst.Therefore,catalysts/sorbents that are mechanically stable and have higher capability of absorption are anticipated.Nevertheless,an internal circulationfluidized bed(i-CFB)was suggested by Cheng et al.[149]to increase the residence time and minimize iteration of the catalysts.The hydrodynamic behavior coupled withthereactionkineticstosimulate the performance of i-CFB for NOxreduction was also studied[149-151].
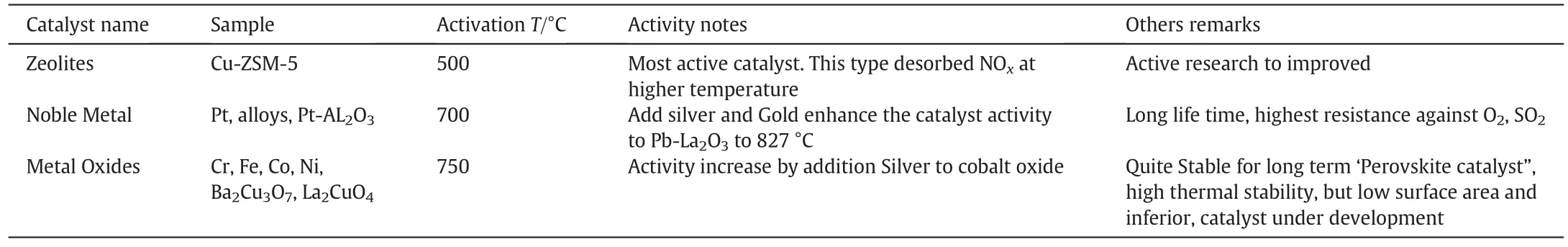
Table 8 Classification Catalysts used to reduction NOxfrom Fluidized bed combustion[30]
5.2.3.Limestone effect
Limestone in the form of Ca and CaO is widely employed as catalyst bed materials mainly for sulfur retention.The interaction between desulfurization and NOxformation in fluidized bed combustor by limestone addition has been broadly studied[126,152-154].Studies have suggested that the use of Ca and CaO as catalyst aids in sulfur retention,but results in increase of NOxemission[153].The solid phase of CaSO4and CaOhas a negligible effecton NOx,especiallyCaO restrained release fromchar-N[155].However,thecatalyzedlimestone[156,157]cansignificantly affect the formation and destruction of NO.The formation of NO from NH3occurs because of the catalytic effect of CaS:

Similarly,CaS can also catalyze NO to N2.

In addition,N2O reduction can also be examined with the use of Ca and Cao as catalysts[158].The mechanism of CaS catalytic decompositionandreductionofN2OwasstudiedbyLingnanetal.[159]andthedestruction of N2O occurred with Ca and CaO according to Reaction(19).

Zhao et al.[85]studied the effect of mineral matters(Na,Ca)on NOxreduction.Fig.16(a)and(b)showstheirresultsatdifferentoxygenconcentrations and increasing temperature for LK coal,with and without mineral matter(LK)and(LK-de).The NOxemission from LK started at lower temperatures and reached its maximum quickly.However,NOxemission from LK-de started at relatively high temperature and then changed gradually.They concluded that the mineral matter present in the coal promotes oxidation of fuel-N.
Besidesmineralmattercatalyst,metalcatalystshavealsobeenstudied to analyze their performance.Yang et al.[160]prepared Fe/ZSM-5 catalyst for adsorption/reduction of NOx.The catalyst showed a good performance in CFB in the presence of different hydrocarbons,H2O,and temperatures.Nickel-based catalysts are active for tar reduction and NH3decomposition[161].Almost all NOxprecursors were converted to N2by Ni/AL2O3catalytic reforming at 650°C in the presence and absence of steam during the pyrolysis process in gasifier,with an enrichmentinhydrogeninfixed bed reactor[162].Similarly,significant increase in NOxreduction rate was achieved in the presence of CO and solids,such as SiO2,Al2O3,Fe2O3,CaO,TiO2,and K2O in fluidized bed in the absence and presence of water vapors[142].
Finally,this paper reviewed possibility of primary reduction techniques such as combustion modification,renewable fuel,catalysts andflying ash reduction,implantation of various fluidized bed designs and structures including process decoupling combustion through oxy-firedfluidized bed boiler to meet the requirement of ultra-low NOx,N2O emission.These techniques as discussed earlier lead to virtuous contradictory NOxreduction.However,the combination of reduction techniques in oxy-fuel technology fluidized bed based power generation and CCS systems may result in high efficiency,low(SOx,NOx)emission,low energy penalty and capital costs.Nevertheless,with the important advantages of this technology some secondary measures for NOxmitigation canbeincorporated easily.Thechallengeof multi-objective optimizationparameter,design,controlstructuresandfurtherfluidizedbed modificationsis necessary to encounternew strictairpollution requirements expected in the near future.
6.Advanced Reduction

Fig.16.NOxevolution profiles during temperature programmed combustion of coals with various O2concentration;(a)LK,(b)LK-de[13].
The advanced flue-gas purification technologies were implemented by merging between primary and secondary measures to meet environmental requirements of ultra-low NOxemission.The European conditions target to estimate nitrogen emission to be less than 200 mg·Nm−3for pilot scale,therefore this combination can be considered as oneof most perspective methods.A number of studies related to the deployment of hybrid of various existing technologies such as RFG,oxy-fuel combustion,co-combustion,SNCR,etc.can potentially meet the emission reduction proficiently and economically.Recent research activity in fluidized bed condition has been targeted to merge different combinations such as less nitrogen content biomass combustion with reduction in NO emission by injecting NH3[163].They achieved around 84%NOxreduction using NH3and urea in 58 MWth CFBC.In addition,they highlight the economic impact of implementing SNCR.The demonstration unit known as CIUDEN CFB pilot plant[164]has provided an experimental platform for CCS by injecting NH3into the cyclone to sustain concentration NOxemission at 120 mg·m−3.Similarly,research work have been conducted in pulverizedcoalfacilitiesonthedevelopmentrelatedtothecombination ofco-firing,oxygenenrichmentandSNCR[86].Testswerecarriedoutin a 20 kW down-fired furnace by fixing the optimum position for ammoniainjection;oxygenenrichedover-firedair(OFA)andoptimumblending portion for two different biomass with coal.Fig.17 shows the significant reduction with high initial NO biomass for blending ratios of SM with RC2.
Ontheotherhandthenewreductionemissiontechnologyappliedin Chinaknown asdry multi emission control(D-MEC)[165]for ultra-low emissions includes the technologies of desulfurization,denitrification,dust removal and removal of multi-component pollutants.This was achieved through the installation of SCR(catalyst)binder in pulverized coal boiler or CFB boiler and was targeted to NOxreduction.This technology has been applied in a number of power projects,for example:Shanxi province power station with the capacity of 350 MW.They have achieved NOxemissions of less than 50 mg·m-3.
7.Conclusions
This review paper demonstrated several methods and experimental techniques applied in reducing NOxand N2O under oxy-fuel condition.The rules and regulations on gaseous emissions are becoming stricter each year.NOxformation and fuel-N conversion has significant limitation under oxy-fluidized bed compared to air mode.However,the mechanism formation is still not fully understood and need further studies to investigate the mechanism.

Fig.17.NOxreduction when applying NH3with co-combustion and air staging[14].
Temperature,pressure and excess oxygen are the important operating parameters influencing the NO emissions.The NO emissions strongly dependent on bed temperature,higher bed temperature leads to higher NO emissions.Moreover,NO emissions decreased withtheincreaseofpressurewhileitincreasedathigherrangeofexcess oxygen.However,the influence of the operating parameters on NO emissions was found largely relying on the type of coal and the amount of nitrogen present in it.Moreover,the formation of NOxgreatly depends on combustion parameters,such as the characteristics of solid fuel,operating temperature,oxygen availability,combustor design,and firing configuration.Depending upon the operating condition and fluidized bed design various minimization techniques,which in turn enhance the development of more complex technology toward new eco-technological system are needed.The different minimization techniques to reduce NOxand N2O emissions in oxy-FB combination technology can be summarized as follows:
(1)Recycling NOxnot only inhibits the formation NOxby reducing the combustion temperature.In addition,reduction in both NOxand N2O was observed using steam.
(2)Excess oxygen concentration in primary stream increases N2O.However,with increasing combustion temperature concentration of N2O decreases.Reduction of NOxby secondary air is observed at higher temperature due to the intensified NOxemission[93]
(3)Developing stages of decoupling fluidized bed combustion technology in oxy-fuel need to be implemented for biomass as a source of renewable energy for low emission.
(4)The role of catalyst in reducing nitrogen emissions especially on N2O in oxy-fluidized bed condition can play a dominant role in the future.
(5)NOxemissions can be further inhibited by nozzle structure by rationally arranging tertiary air positions.The challenge is to improve fluidized bed design control in oxy-combustion condition toward energy efficiency and low NOxemission.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Review on application of nanoparticles for EOR purposes:A critical review of the opportunities and challenges
- Drawdown mechanism of light particles in baffled stirred tank for the KR desulphurization process☆
- Optical inline analysis and monitoring of particle size and shape distributionsformultipleapplications:Scientificandindustrialrelevance
- Experimentalstudyofpowerconsumption,localcharacteristicsdistributions and homogenization energy in gas-liquid stirred tank reactors☆
- Molecular simulation of penetration separation for ethanol/water mixtures using two-dimensional nanoweb graphynes☆
- Chaotic characteristics of pseudoplastic fluid inducedby 6PBTimpellerin a stirred vessel☆
