Immobilization of organophosphorus hydrolase enzyme by covalent attachment on modified cellulose micro fibers using different chemical activation strategies:Characterization and stability studies☆
2019-02-15MeisamSharifiSeyedMortazaRobatjaziMinooSadriJafarMohammadianMosaabadi
Meisam Shari fi,Seyed-Mortaza Robatjazi*,Minoo Sadri,Jafar Mohammadian Mosaabadi
Department of Bioscience and Biotechnology,Malek Ashtar University of Technology,Tehran,Iran
Keywords:Organophosphorus hydrolase Cellulose powder Activation Immobilization Stability
ABSTRACT The plantcellulose powder was activated by two different methods using 1,4-butanediol diglycidylether(BTDE)and 1,1′-Carbonyldiimidazole(CDI)as the chemical coupling agents.Organophosphorus hydrolase(OPH)from Flavobacterium ATCC 27551 was immobilized on any ofactivated support through covalent bonding.The optimal conditions of affecting parameters on enzyme immobilization in both methods were found,and it was demonstrated that the highest activity yields of immobilized OPH onto epoxy and CDI treated cellulose were 68.32%and 73.51%,respectively.The surface treatment of cellulose via covalent coupling with BTDE and CDI agents was proved by FTIR analysis.The kinetic constants of the free and immobilized enzymes were determined,and it was showed that both immobilization techniques moderately increased the Km value of the free OPH.The improvements in storage and thermalstability were investigated and depicted thatthe half-life ofimmobilized OPH over the surface ofepoxy modified cellulose had a bettergrowth compared to the free and immobilized enzymes onto CDItreated support.Also,the pHstability of the immobilized preparations was enhanced relative to the free counterpart and revealed that all enzyme samples would have the same optimum pH value for stability at 9.0.Additionally,the immobilized OPH onto epoxy and CDI activated cellulose retained about 59%and 68%of their initial activity after ten turns of batch operation,respectively.The results demonstrated the high performance of OPH enzyme in immobilized state onto an inexpensive support with the potential of industrial applications.
1.Introduction
Organophosphoruscompoundsare a group ofhighly toxic substances which are extremely applied as pesticides,insecticides and chemicalwarfare agents[1].Extensive uses of these compounds enhanced general concerns because of their adverse effects on human health and environment by contamination of soil,sediments and groundwater.Considering the recent progression in bio science,the use of microorganisms for degradation of these toxic substances as an economic and less disruptive way in comparison to other conventional methods would appear to be very interesting[2].Organophosphorus hydrolase(OPH)(EC 3.1.8.1)is a biocatalyst which is capable to hydrolyze various organophosphorus compounds.However,low thermostability and specification of this enzyme restricted its industrial applications substantially[3].Therefore,different techniques such as enzyme immobilization are used currently to make the enzyme more stable even under harsh conditions.
Immobilization willimprove the enzyme characteristics by keeping its natural catalytic surrounding for repeated operations.The immobilized enzymes would have higher endurance in organic solvents and are capable to reuse for several batches of hydrolysis reactions.As well as,in the reactions which are catalyzed by immobilized enzymes in a heterogeneous environment,termination ofthe reaction is conveniently accessible justby physicalisolation ofthe enzyme from the solution[4,5].Numerous methods have been used for enzyme immobilization such as covalent coupling or physicaladsorption to a carrier,entrapmentin polymeric networks and encapsulation[6,7].The immobilization via multipoint tight covalent binding on insoluble matrices will lead to rigidify the tertiary structure of enzymes and has attracted much attention because of its diverse advantages like low enzyme leakage even in severe reaction conditions and efficient usage of bioconjugates in various types of bioreactors.The activity of biocatalyst will decrease during the covalent immobilization,but the consequent remarkable improvement in stability of the enzyme which is immobilized in optimal conditions would overcome this slight enzyme deactivation[8,9].
For immobilization of enzymes,natural polymers depict several outstanding features as support.These materials are inert,non-toxic,biodegradable and biocompatible.As well as,they can be treated with different functional groups easily by chemical reactions that occur under gentle conditions in absence of impurities.Also,the industrial applications of these carriers are justified due to their low prices and greatavailability[10,11].The recentgrowth in the use ofcarriers based on cellulose,as the cheapest natural polymer on the earth,for enzyme immobilization was noticeable because of their ideal properties like hydrophilicity,renewability,and low contamination risk to the environment.Cellulose could be plant-based or synthesized by algae,tunicates,and some bacteria.Additionally,three hydroxyl groups exist in every monomeric unit(glucose)of cellulose chemical structure with the potential of making covalent bonds with amino acids of biocatalysts.So,this polymer has a good chemical reactivity and could be applied reasonably for enzyme immobilization[12–14].But,the direct interaction between hydroxyl groups of cellulose and enzyme is not strong enough.So,in order to improve the binding efficiency,the surface of this matrix should be modified by employing chemical coupling agents to create more appropriate functional groups on its surface.Hence,enzyme immobilization will take place by stable and firm multipoint covalent linkages between activated supportand amino acid residuesofbiocatalyst(--NH2,--COOH,--SH)which are frequently involved in covalent coupling.The cellulosic supportwhich is treated by amino group would be capable to interactcovalently with carboxyl group of amino acids like aspartic acid or glutamic acid.Also,if the chemical treatment introduces aldehyde,carboxyl or epoxy group onto the matrix,the enzyme molecule willattach to cellulose through N-terminus(amine group of lysine)[12].
Several activating agents such as cyanogen bromide,cyanuric chloride,epichlorhydrine,and organic sulfonyl chlorides were used for modification of the cellulose hydroxyl groups by diversified methods in previous studies[14,15].The bisoxiranes like 1,4-butanediol diglycidyl ether(BTDE),as the chemicals which contain two epoxy functional groups,could be applied for activation of cellulose-based carriers under a mild condition.The surface treated matrices by the free reactive epoxy groups are capable to form strong covalent bond with the enzyme's amino groups[8].Also,1,1′-carbonyldiimidazole(CDI)is an active carbonylating reagent which contains two acyl imidazole leaving groups that are capable to convert the free hydroxyl groups on the cellulose surface into cyclic imidazolyl-carbamate groups.In the following,with the reaction of these groups with N-nucleophiles on enzymes,the relatively stable N-alkyl carbamates can be formed.As the CDI agent is sensitive to hydrolysis,the support modification should be conducted under nonaqueous conditions like in dry acetone[16].The enzymes which are linked to the supports via the spacer arms would have more degree of mobility and as a result,they will represent higher activity relative to the biocatalysts which are attached directly[17].As well as,the amount of loading capacity and the stability of the immobilized biocatalyst would improve noticeably by this technique[18].
In this study,the plant cellulose powder was modified using two various techniques.In the first method,BTDE and in another CDI was used as the chemical coupling agent.Afterwards,the OPH enzyme from Flavobacterium ATCC 27551 was immobilized on any of activated support by covalent attachment.The identified effective parameters on the preparation of immobilized enzyme in both methods were analyzed,and the optimal conditions were found.The processes for OPH immobilization are shown in the Fig.1.
The immobilized OPH was employed for biodegradation of ethylparathion,as the substrate and the results were investigated for detection ofthe improvementin reusability and other enzyme characteristics like thermal,pHand storage stability relative to those offree biocatalyst.All the examinations were carried out in triplicate to determine the standard deviations and the studies were performed at the obtained optimum conditions.Eventually,the outcomes demonstrated that the enzyme's performance in its immobilized state will enhance rationally.
2.Material and Methods
2.1.Chemicals
Plant cellulose powder(length of fibers:0.02–0.15 mm)was purchased from Fluka Chemie GmbH(Steinheim,Germany),ethylparathion(analytical grade)as substrate for evaluating enzyme activity from Sigma-Aldrich(St.Louis,MO,USA)and p-nitrophenol(PNP)from Aldrich.For the activated reagents,1,4-butanediol diglycidyl ether(C10H18O4)and 1,1′-Carbonyldiimidazole(C7H6N4O)were obtained from Sigma-Aldrich Chemical Co.;sodium hydroxide and acetone were purchased from Merck Co.Ltd.Germany.All the other chemicals and materials were used as analytical grade in this research.
2.2.Bacterial culture and OPH purification
Flavobacterium ATCC 27551 was attained from Microbial Type Culture Collection(MTCC,Chandigarh,India),and it was maintained in Wakimoto medium on slant[7].The bacterial cells were cultured in a modified LB broth at 30°C for 48 h.The liquid culture was centrifuged at 5000g to separate the cells.Then,the cells were washed twice with Tris–HCl buffer(10 mol·m-3,pH 8.0).The purified OPH was obtained by the procedure described in the previous literature[19].For this purpose,bacterial cells were lysed employing an ultrasonic disintegrator(Decon FS400B,UK),and the sample was centrifuged at 10000g to remove insoluble cellular materials.The OPH enzyme in the supernatant was precipitated by addition of saturated ammonium sulfate solution.Then,the precipitated was isolated by centrifugation at 5000g and resuspended in 10 mol·m-3Tris–HClbuffer(pH 8.0).The extracts were desalted by dialysis against Tris–HCl buffer(10 mol·m-3,pH 8.0)for 24 h using a membrane bag with a 12000cut-off molecular weight and applied to a column(70 cm×1.6 cm dia)of Superdex 75.The selected fractions from this column were pooled and injected to a DEAESepHarose anion exchange chromatography column,for further puri fication.In both chromatography stages,all the output samples were analyzed in terms of enzymatic activity and the protein concentration by SDS-PAGE gel electrophoresis.Finally,the OPH enzyme with the least purity of 90%was achieved.
2.3.Activation of cellulose powder by epoxy method
Modification of cellulose powder using the epoxy method was done by the following steps[20].First,different amounts of BTDE(10–40 μl)as chemical coupling agent were added to a suspension of 0.1-g plant cellulose powder in 0.45 ml of 1.0 mol·L-1NaOH in a 1.5 ml microfuge tube.Then,the activation was conducted by agitating the mixture at various temperatures(30–60 °C)for different time periods(1–2.5 h)in a shaker incubator.Afterwards,the modified cellulose was separated from the suspension by centrifugation and washed with distilled water frequently.
2.4.Activation of cellulose powder using CDI agent
Cellulose powder treatment using the CDI agent was performed based on the previous study[16].Accordingly,plant cellulose powder(0.1 g)was transferred to a 1.5-ml microfuge tube and washed sequentially with water,acetone/water 3:7,acetone/water 5:5,acetone/water 7:3,and acetone.This step will lead to remove any impurities to enhance the immobilization efficiency[21].Then,the cellulose powder was separated by centrifugation at 5000g,and surface treatment was conducted under stirring at different temperatures(0–28 °C)in dry acetone by the addition of 0.03–0.2 g CDI for 1–5 h.The modified powder was thoroughly rinsed with dry acetone and instantly employed for enzyme immobilization.Before the immobilization,surface treated cellulose was washed frequently with phosphate buffer(50 mol·m-3,pH 8.0)to remove the acetone.It should be emphasized that the selection of investigated ranges for variable parameters was done based on our preliminary results achieved from laboratory experiments in this area to find the optimal conditions of activation process.

Fig.1.Covalent immobilization of enzyme onto the surface of modified cellulose by chemical coupling agents:(A)Enzyme immobilization on epoxy activated cellulose;(B)Enzyme immobilization on CDI activated cellulose.
2.5.Preparation of immobilized OPH
For immobilization of enzyme onto modified support,0.1 g of wellrinsed activated cellulose was added to 0.5 ml of OPH solution with a specified enzyme activity(0.2 U·ml-1)provided in 50-mol·m-3phosphate buffer at different pHs(5.5–9.0),containing 1-mol·m-3CoCl2,and the suspension was agitated at 4°C for 1 h in epoxy method and for 24 h in activated cellulose using CDI agent.Next,the bioconjugates were isolated from the mixture and washed frequently with phosphate buffer containing 0.05(v/v)%Tween 20 to separate the free OPH and the enzymes which are immobilized only by hydrogen bonding,hydrophobic interactions or physical adsorption.Then,the excess reactive groups were blocked with ethanolamine as a quenching agent.Finally,the resulted samples were thoroughly washed again with phosphate buffer containing 0.05(v/v)%Tween 20 and prepared for determining the immobilized OPH activity[16,20].
2.6.FTIR analysis
Fourier-transforminfrared(FTIR)spectra was recorded on a Thermo Scientific Nicolet 800 infrared spectrometer(Thermo Nicolet Corp.,Madison,WI,USA)at room temperature in the spectral range from 4000 to 400 cm-1in a transmittance mode(16 scans,resolution 4.0 cm-1).The dried state of prepared formulations were dispersed in KBr and then pressed into transparent tablets before measurements.
2.7.Activity assay of the free and immobilized OPH
Aspectrophotometric method was applied to determine the enzyme activity[2,7].The reaction rate of ethyl-parathion hydrolysis was assessed by evaluating the amount of liberated PNP as a product of substrate decomposition.The activity of free OPH was measured by addition of 50 μl of the biocatalyst sample to 445 μl of 50-mol·m-3phosphate buffer pH 8.0,containing 1-mol·m-3CoCl2.Then,to reach the temperature equilibrium,the mixture was incubated for 15 min in a 1-ml microfuge tube at 30°C.After addition of substrate to the solution,it was incubated for 3 min,and the absorbance was specified at 410 nm relative to a blank solution by employing a spectrophotometer(Jenway 6310,UK).Here,a solution comprise of all ingredients except the ethyl-parathion was used as the blank solution.
The activity assay of immobilized OPH was carried out by preparing a suspension containing 485 μl of phosphate buffer(50 mol·m-3,pH 8.0),10 μl of 50-mol·m-3CoCl2and 0.1 g of bioconjugates in a 1.5-ml microfuge tube and agitating it for 15 min at 200 r·min-1in a shaker incubator at 30 °C.To start the hydrolysis reaction,5 μl of 40-mol·m-3ethyl-parathion was added to the mixture,and it was agitated gently for 5 min.Then,to put an end to the reaction,the bioconjugates were removed from the suspension,and the product formation rate was evaluated by determining the absorbance of supernatant solution spectrophotometrically at 410 nm relative to a blank solution.One unit of phosphotriesterase activity was defined as the amount of enzyme required to hydrolyze 1 μmol of ethyl-parathion per minute at 30 °C[2,7].The enzyme activity of native and immobilized samples could be determined by specifying the amount of change in absorbance value during incubation,as described before by Robatjazi et al.[2].As well as,the results were converted to relative activity using the following relationship:

2.8.Steady-state kinetics
The kinetic constants were determined to compare the features of immobilized enzymes onto epoxy and CDI treated cellulose with the free enzyme.The OPH activity as a function of substrate concentration was calculated for native enzyme and bioconjugates.The ethylparathion concentration was changed in phosphate buffer(50 mol·m-3,pH 8.0)at 30 °C,and the initial reaction rates were measured by the explained procedure for enzyme assay.Finally,the maximum reaction rate(Vmax)and Michaelis–Menten constant(Km)were evaluated employing the Lineweaver–Burk plot from the intercepts at x and y axes,respectively.
2.9.Thermal and pH stability of the free and immobilized OPH
Forthermalstability,the native and immobilized OPHwere preincubated in 50-mol·m-3phosphate buffer pH 8.0 at 30 °C,45 °C,and 55 °C forvarious time intervals.Later,the samples were keptin an ice bath for 5 min in order to coolthem quickly.Afterincubation for 15 min at30°C,the residual enzyme activities were determined as reported in earlier sections.The free and immobilized states of enzyme were compared to each other by computing the thermal deactivation rate constant(ki)at different temperatures in a first order unimolecular irreversible reaction.This constant was evaluated according to the exponential“one step-two states”model by the below equation:

where A0is the initial enzyme activity,and Atis the activity after time t[22].
The pH stability of free and immobilized enzymes was investigated by incubation of the preparations in the pH range of 4.0 to 10.6 using 50-mol·m-3acetate buffer(pH 4.0–5.0),50-mol·m-3phosphate buffer(pH 6.0–8.0)and 50-mol·m-3glycine-NaOH buffer(pH 9.0–10.6)for 8 h at 25°C.Lastly,the residual enzymatic activities were calculated by evaluating the enzyme activity as explained before and were considered to specify the pH stability.
2.10.Reusability and storage stability
The immobilized enzymes can retain high amount of their original activity even after using for multiple batches of hydrolysis reaction.The reusability of the bioconjugates was determined by measuring their activity in ten replications.After each reaction cycle,to prepare the immobilized OPH for next repeated use,it was isolated by centrifugalforce and extensively rinsed with phosphate bufferatpH 8.0 in order to remove any residual substrate.The ratio of the immobilized enzyme activity in each reaction cycle to its initialactivity is defined as efficiency of reusability.This value was decreased gradually,and after the tenth run,it was 59%and 68%for immobilized OPH onto modified cellulose by BTDE and CDI activating agents,respectively.
The storage stability of free and immobilized enzymes was investigated by maintaining them in phosphate buffer(50 mol·m-3,pH 8.0)at 4°C and room temperature.The activity of the biocatalyst was followed within a month at certain period of times by the procedure mentioned previously for OPH activity assay.The storage stability would exhibit the viability of the enzyme after storage for a long time.This parameter was checked out by determining the residual activity calculated from the ratio of the native or immobilized enzyme activity after storage for specific time intervals to their original activity.
3.Results and Discussion
3.1.Optimum conditions for enzyme immobilization
Two strategies for cellulose activation were considered and the OPH was immobilized on both types of modified carriers.The affecting parameters on the processes were changed in specified ranges with the objective ofobtaining the optimalconditions ofenzyme immobilization.The responses revealed that the highest immobilized enzyme activity would be achieved by choosing the variable parameters with the values illustrated in Table 1.
At the identified optimum conditions of immobilization processes,the specific enzymatic activity of bioconjugates prepared by epoxy method was 0.68 U·g-1ofcellulose,whereas the immobilized enzymes onto CDIactivated cellulose exhibited a specific activity of0.74 U·g-1of cellulose.So the derivative immobilization yields in optimum states for immobilized OPHonto epoxy and CDItreated cellulose were 68.32%and 73.51%,respectively.Therefore,higher immobilization efficiency can be observed by modification of the carrier with CDI agent relative to the other method.However,the yields obtained for both immobilization techniques were close to each other.The reduction in enzyme catalytic activity which is a common phenomenon during covalent immobilization methods could be as a consequence of conformational changes in the three-dimensional structure and loss of dynamic properties of enzyme upon immobilization.The strong multipoint and multisubunit covalent interactions between the enzymes and the functional groups on the modified support surface would destruct the enzyme conformation and reduce its flexibility by alteration of structural integrity[23–26].Additionally,unfavorable orientation of the bound enzyme molecules and mass transfer constraints for substrate caused by the carrier material would limit the accessibility of the substrate to the catalytic active centers of the bioconjugates and decrease the activity recovery of the process[23,27,28].

Table 1 Optimum values of different affecting parameters on the overall enzyme loading
Furthermore,covalent immobilization might involve the amino acid residues essential for catalytic activity of enzyme in binding to the support and inactivate the enzyme active sites to some extent.Whatever the coupling agent prevents undesirable side attachment and incompatible interactions between the enzyme molecules and carrier,greater enzyme loading and more activity recovery can be obtained[29,30].Also,as the intermolecular steric hindrance of enzyme could be more decreased using a spacer arm and the vigorous chemical reaction conditions which are often accompanied by covalentimmobilization processes cause less deformation of enzyme active conformation,the higher immobilization percentage would be achieved.Besidesthe lossofenzyme structure,also the limitations ofthe localand globalmolecular mobility of the enzyme by anchoring to a support and fine movements of the backbone and side chains of the biocatalyst after covalent immobilization can play a crucial role in the loss of enzymatic activity[23,25,31].
3.2.FTIR characterization of the activated samples

Fig.2.FTIR spectra of the native cellulose powder(A),cellulose powder activated using epoxy method(B)and cellulose powder activated using CDI agent(C).
Fig.2 shows the FTIR spectrums of the unmodified cellulose powder and activated cellulose with investigated chemical coupling agents.The most indicative infrared bands in the spectra of cellulose could be as follows:The obvious peak recorded around 2902 cm-1due to the symmetrical and asymmetrical CH2stretching and the signal at 1636 cm-1corresponds to the O--H bending of the water associated with fibers.The presence of the bands at the wavenumber of 1429 cm-1and 1317 cm-1in the spectra were referred to the symmetric CH2bending vibration and wagging,respectively.The absorption bands found at 1163 cm-1and 893 cm-1were assigned to C--O--C stretching at the β-(1,4)-glycosidic linkages between the glucose units in the glucan chains.Also,the two absorption peaks at 1370 cm-1and 1281 cm-1arose from the C--H bending vibration of cellulose.In addition,the peak at 1163 cm-1was belonged to the ring breathing of C--C.Strong bands at 1058 cm-1and 1029 cm-1are characteristic signal of the C--O group of secondary alcohols and ethers functions which are present in the chain backbone of the cellulose.Also,the typical small peak of outof-plane bending of C--O--H appeared at 666 cm-1[32–35].
The broad and obvious peak which was emerged in all spectrums at the region of 3500–3200 cm-1is characteristic signal of the free O--H stretching vibrations,intramolecular and intermolecular hydrogen bonds of the O--H groups in cellulose molecule.In addition,the water associated with fibers will affect the intensity of the observed peak.So it is not possible to detect the change in intensity of this peak for evaluating the amountof activated hydroxyl groups of cellulose after support modification steps[36].
As represented in Fig.2,the enhancement in peak intensity at around 890 cm-1for BTDE treated cellulose confirmed the existence of new epoxy groups onto its surface[37,38].Furthermore,two bands were appeared in the spectrum of CDI treated cellulose around the wavenumber of 1767 cm-1and 1660 cm-1which are referred to the C=O stretching mode of the imidazole ester and to C=C and C=N stretching modes of the imidazole heterocycle.Also,the absorption bands at 1430 cm-1and 1340 cm-1which were assigned to the imidazole cycle and C--O,enhanced the intensity ofnative cellulose peaksin this wavenumber area[39,40].
3.3.Kinetic constants of free and immobilized enzymes
Kinetic parameters of enzyme preparations were evaluated by determination of the free and immobilized enzymes activity at different substrate concentrations at 30°C,pH 8.0.Then,the initial reaction rates of ethyl-parathion hydrolysis were calculated to estimate the Michaelis constant(Km)and maximum rate of reaction(Vmax)from the intercepts on x and y axes of the Lineweaver-Burk plot(1/V vs.1/[S]),respectively as shown in Fig.3.The kinetic properties of free and immobilized enzymes onto epoxy and CDI treated supports are represented in Table 2.

Fig.3.Evaluation of K m and V max values forfree and immobilized enzymes by Lineweaver-Burk plot method.Symbols:(▲)Free OPH;(■)Immobilized OPH onto activated cellulose by CDI agent;(♦)Immobilized OPH onto activated cellulose by epoxy method.

Table 2 Kinetic parameters of free and immobilized OPH onto epoxy and CDI activated cellulose
As a result,the Kmvalues of the immobilized OPH onto epoxy and CDI treated cellulose increased about 1.81 and 2.06 times compared to the soluble biocatalyst,respectively.Also,the apparent maximum reaction rates of the immobilized enzymes using epoxy method and CDI agent were about 2.68 and 3.32 times lower than that of free OPH,respectively.The structural changes of enzyme after immobilization and the diffusional limitations created for reacting species are among the reasons for enhancing the Kmvalues of the immobilized enzymes[41,42].In addition,the decrease in activity by autolysis or denaturation,the steric blockage of the active sites by the support,and the reduction in af finity of biocatalyst toward its substrates after immobilization process are the other possible explanations for the differences between the kinetic constants of free and immobilized enzymes[43,44].
However,the moderate changes in kinetic parameters ofimmobilized enzymes onto epoxy and CDI activated cellulose relative to these constants for free enzyme revealed that the OPH active sites were not affected remarkably by the used immobilization techniques.Therefore,the obtained bioconjugates would be capable to hydrolyze even low concentrations of organophosphate compounds in the environment.Richins et al.[45]stated similar changes in Michaelis constants for bifunctional fusion proteins consisting of OPH moieties linked to a Clostridium-derived cellulose-binding domain(CBD)immobilized onto the cellulose matrix.
3.4.Thermal stability of free and immobilized OPH
Thermal stability is a significant characteristic for commercialization ofan enzyme which could be changed by immobilization[30].The evaluation of this parameter for soluble and immobilized OPH was performed by incubation of the samples in 50-mol·m-3phosphate buffer pH 8.0 at 30,45,and 55°C in the absence of substrate.Then,the activity loss of free and immobilized enzymes was determined at certain time intervals and used to measure the relative activity for specifying the thermal stability.As represented in Fig.4,the relative activity of free OPH which was incubated at 55°C for 24 h decreased about 92%,whereas at the same conditions the immobilized enzymes onto epoxy and CDI modified cellulose were more stable and retained 35%and 26.4%of their initial activity,respectively.
El-Boubbou et al.[46]showed that the thermal stability of immobilized OPH in functionalized mesoporous silica particles was enhanced efficiently relative to the free enzyme.Dennis et al.[47]reported that entrapment of OPH in silk fibroin leads to increase in thermal stability of OPH under a variety of conditions.Furthermore,Raynes et al.[48]determined the thermal stability of immobilized OPH by a covalent method on the insulin amyloid fibrils as a nanoscaffold,using glutaraldehyde as the crosslinking reagent.It was shown that the relative activities of immobilized enzymes held at 40,45,and 50°C after the final incubation time were about 3 times more than that of free enzymes which are lower than the improvement in thermal stability results observed for immobilized OPH onto epoxy and CDI treated cellulose.In the other report,thermal stability results similar to this study were achieved for immobilized OPH on polyester textiles.The produced bioconjugates were stable over 3 h at 55°C which was in contrast with the high decrease in the relative activity of soluble enzyme during the same treatment[4].However,the enhancement in thermal stability of immobilized phosphotriesterase within a polyurethane foammatrix at50°C was higher than the results obtained in this study for immobilized OPH onto epoxy and CDI activated cellulose[49].The immobilization will restrict the conformational changes in the tertiary structure of enzyme and improve its thermal stability and denaturation resistance at high temperatures relative to its free counterpart[8,50].

Fig.4.Thermal stability of the free and immobilized OPH at 30 °C(A),45 °C(B)and 55 °C(C)in phosphate buffer(50 mol·m-3,pH 8.0).Symbols:(▲)Free enzyme;(■)Immobilized enzyme onto activated cellulose by CDI agent;(♦)Immobilized enzyme onto activated cellulose by epoxy method.
The half-life values and thermal inactivation rate constants of free and immobilized enzymes at various temperatures were studied[51].According to Table 3,it was concluded that the half-life of immobilized OPH onto epoxy treated cellulose had a better growth compared to immobilized enzyme onto CDI activated cellulose.
The improvement in half-life values of immobilized OPH relative to the free enzyme atvarious temperatures is consistentwith previous observations.Caldwell et al.[52]evaluated the thermal stability of immobilized phosphotriesterase from Pseudomonas diminuta onto trityl agarose at pH 9.0 using paraxon as the substrate.It was found that the half-life value of immobilized enzyme onto trityl agarose at 55°C is lower than these values for the immobilized OPH onto epoxy and CDI activated cellulose.Also,Robatjazi et al.[53]determined that the immobilized OPH on functionalized ferric magnetic nanoparticles showed about 50%decrease in relative activity after 62 h of incubation at 45°C which is almost in agreement with the results achieved in this study.

Table 3 Half-life(t1/2)values of the soluble and immobilized OPH at various temperatures
3.5.pH stability of free and immobilized enzymes
The stability of soluble and immobilized OPH on activated cellulose by two described methods at various pH values is shown in Fig.5.

Fig.5.Effect of different pH values on the stability of free and immobilized OPH for 8-h storage at 25 °C.Symbols:(▲)Free enzyme;(■)Immobilized enzyme onto activated cellulose by CDI agent;(♦)Immobilized enzyme onto activated cellulose by epoxy method.
As can be observed,the pH stability of immobilized enzyme in both cases was enhanced relative to the free OPHand the immobilized enzyme onto epoxy activated cellulose exhibited better results,so that its relative activity was remained more than 90%when incubated atpHvalues ranging from 7.0 to 10.0.The enzymatic activity of the free enzyme after 8-h incubation at pH 6.0 was decreased to less than 12%of its initial activity,while the relative activity of the immobilized enzymes onto epoxy and CDI modified cellulose was about 45%and 36.6%under the same conditions,respectively.The results proved that all enzyme samples would have the same optimum pH value for stability at 9.0.Milani et al.[54]evaluated the increase in pH stability of immobilized OPH on chitosan beads containing the glutaraldehyde cross-linker in the pH range of 2.0 to 12.0 for 5 h.It was exhibited that the stability of enzyme preparations in alkaline pH is higher than that of acidic buffers which is also resulted in this research.Furthermore,Robatjazi et al.[53]reported better improvement relative to the present study for pH stability of immobilized OPH on functionalized ferric magnetic nanoparticles in acidic pHranges.In anotherinvestigation,the pHstability ofimmobilized recombinant OPHon spores of Bacillussubtilis was determined at4°C and revealed thatafterincubation for1 h atpH 3.0 and pH 5.0,the residualenzymatic activity of the bioconjugates was 53%and 94%,respectively[55].The immobilization would alter the naturalmolecular microenvironment of the enzyme and protect it against extreme pH values[51].So,as a result the enzyme will be more stable in a wider pH range and become less sensitive to pH changes after immobilization.
3.6.Reusability of immobilized OPH
In this work,the reusability of the immobilized OPH onto epoxy and CDI treated cellulose was compared to each other.As the biocatalysts commonly produce through expensive processes,their large-scale applications in batch or continuous operations require high stability after successive operational cycles[56].The reusability was evaluated by measuring the activity ofthe bioconjugates aftermultiple hydrolysis reactions.After each cycle the same OPH-cellulose system was isolated from the reaction medium by centrifugation and washed several times with phosphate buffer(50 mol·m-3,pH 8.0)to prepare for further cycle.As represented in Fig.6,the activity of OPH samples reduced gradually after consecutive usages due to active site distortion of the enzyme.
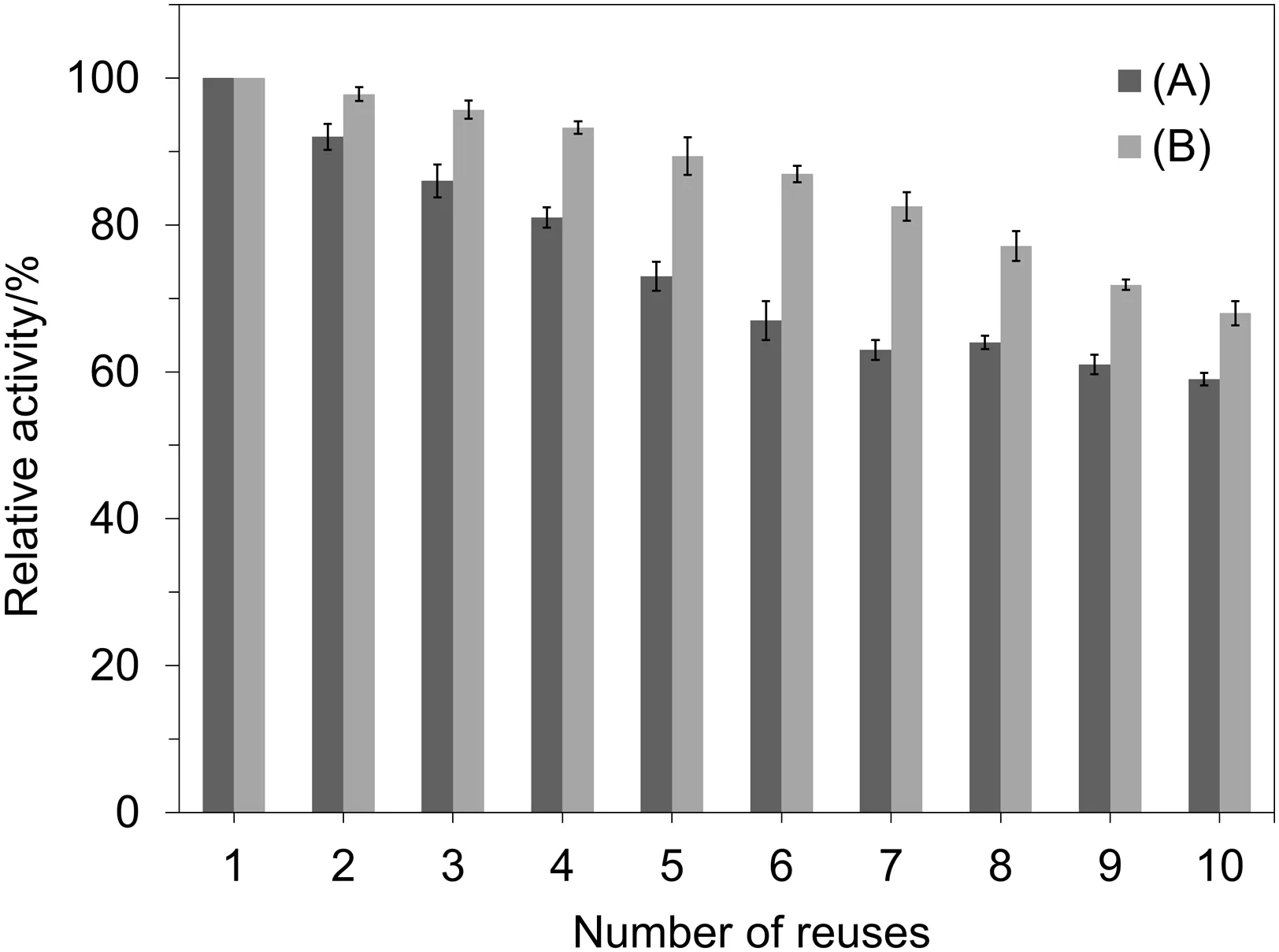
Fig.6.Reusability ofthe immobilized OPHonto:(A)Activated cellulose by epoxy method;(B)Activated cellulose by CDI agent.
According to Fig.6,the immobilized enzymes onto epoxy and CDI modified carriers kept about 59%and 68%of their original activity after ten turns of batch operation,respectively.The lower activity loss of immobilized OPH onto CDI modified cellulose represented the more resistance of enzyme molecular structure against denaturation because of stronger covalent linkages between the enzyme and carrier in this method[29,57].In the previous study,Milani et al.[54]determined that the immobilized OPH on chitosan beads containing glutaraldehyde lost more than 60%of its original activity after five repeated cycles of use.As well as,Kapoor et al.[58]immobilized the recombinant OPH by entrapment using Ca-alginate and agar-agar matrices and evaluated the reusability of produced bioconjugates.It has been shown that after three repeated cycles of use,the relative activity of immobilized recombinant OPHon Ca-alginate and agar-agarmatrices decreased to 48%and 50%,respectively.Also,the reusability of immobilized OPH on polyamide nano fibrous membrane has been reported previously and depicted that after ten repeated uses,the nano fibrous enzyme retained about 37%of its initialactivity[59].So,the immobilized OPHonto BTDE and CDItreated cellulose exhibited betterresultsin comparison with the abovementioned studies.In addition,results almost similar to the present research were obtained for reusability of immobilized OPH on the functionalized ferric magnetic nanoparticles which revealed 23%reduction in the relative enzyme activity after seven cycles ofuse[53].The binding strength between the biocatalyst and support surface would be weakened after successive reuses,and it will cause the leakage of enzyme and reduction in catalytic efficiency of immobilized preparations.Furthermore,imperfect washing steps during evaluation of the reusability is the other reason for decreasing the activity of bioconjugates[29,57].
3.7.Storage stability of free and immobilized enzymes
Storage stability is a significant characteristic that shows enzyme capability to preserve its activity after prolonged times and should be considered to use an enzyme in industrial scale[60].To determine this parameter,the enzyme preparations were stored in 50-mol·m-3phosphate buffer pH 8.0 at two different temperatures(4 °C and 25 °C),and the relative activity of the samples was evaluated over one month.It was concluded that the enzyme in the free state which was kept at 25°C lost all of its initial activity after 6.5 d,while the immobilized enzymes onto epoxy and CDI activated cellulose were more stable and showed around 25%and 6%of their initial activity within 30 d,respectively as illustrated in Fig.7.

Fig.7.Storage stability of the free and immobilized OPH:(A)T=4 °C;(B)T=25 °C.Symbols:(▲)Free enzyme;(■)Immobilized enzyme onto activated cellulose by CDI agent;(♦)Immobilized enzyme onto activated cellulose by epoxy method.
The obtained results for storage stability of immobilized OPH onto BTDE and CDI activated cellulose are supported by the results of Gao et al.[4],who reported that the immobilized organophosphate degrading enzyme on nonwoven polyester textiles could retain about 75%–80%of its initial activity after four weeks storage in phosphate buffer at 4°C.Moreover,the results of storage stability for chitosanembedded OPH immobilized on gold nanoparticles after three weeks storage at 4°C are in accordance with those achieved in the current study[61].Also,Yan et al.[59]reported comparable results for storage stability of immobilized OPH on polyamide nano fibrous membrane through glutaraldehyde as the crosslinker.The residual activity of the nano fibrous OPH remained about 40%after being stored for 30 d in a buffer solution at 25°C.However,in other studies,better results for storage stability of immobilized OPH have been achieved.In a research carried out by Dennis et al.[47],the storage stability of entrapped OPH enzyme in silk fibroin has been evaluated and indicated that during the first two weeks of storage in assay buffer at room temperature,the entrapped OPHmaintained about80%ofits initialactivity.Furthermore,Richins et al.[45]reported that CBD-OPH fusion proteins could be immobilized onto different cellulose carriers,retaining up to 85%of their initial activity after 30-d incubation at 25°C.Substantial improvement of storage stability was observed for covalently immobilized OPH on single-walled carbon nanotubes(SWNTs).The produced bioconjugates exploited for direct amperometric detection of paraoxon and proved thatthey could display a long term stability with only 25%signal loss over 7 months storage at 4°C[62].The enhancement in storage stability of immobilized OPH can be mainly attributed to the stabilization of the active conformation of enzyme upon covalent attachment to the matrix[50].
4.Conclusions
Two chemical coupling agents,BTDE and CDI,were used to activate the free hydroxyl groups of plant cellulose powder and OPH from Flavobacterium ATCC 27551 immobilized on modified carriers by covalent linkages.The highest immobilization yields obtained in optimum conditions of effective parameters on enzyme immobilization onto epoxy and CDI activated cellulose were found to be 68.32%and 73.51%,respectively.The kinetic parameters were determined,and it was showed that the apparent Kmvalues of the immobilized enzymes onto epoxy and CDI activated cellulose increased about 1.81 t and 2.06 t in comparison with the free OPH,respectively.Also,the maximum reaction rates of the immobilized enzymes using epoxy method and CDI agent were about 2.68 t and 3.32 t lower than that of free OPH,respectively.According to the results,the immobilized OPH revealed more thermal and storage stability compared with the soluble enzyme.The experiments showed that the native enzyme which was kept at 25°C lost all of its original activity after 6.5 d,whereas the immobilized OPH onto epoxy and CDI modified cellulose preserved around 25%and 6%of their initial activity within one month at the same conditions,respectively.Additionally,it was indicated that the enzyme acquired more denaturation resistance against pH variations after immobilization,and the OPH preparations have more pH stability in alkaline buffers relative to the acidic conditions.Generally,it was concluded that the enzyme immobilization onto epoxy modified cellulose showed more improvement in stability parameters relative to CDI modified cellulose.Furthermore,the reusability of immobilized preparations were studied and confirmed that after ten consecutive batch reactions,the relative activity of immobilized OPH using the CDI mediated covalent coupling was about 9%more than epoxy method.So,regarding the achieved outcomes,it is possible to choose the appropriate spacerarms forproduction ofimmobilized OPHover the cellulose surface depending on whether stability or reusability of resulted bioconjugates is desired,and it open the feasibility of various large-scale applications for biodegradation of organophosphate compounds.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Experimental investigation on flow characteristics in circular tube inserted with rotor-assembled strand using PIV☆
- PIVexperimentand large eddy simulation ofturbulence characteristics in a confined impinging jet reactor☆
- CFD predictions for hazardous area classification
- Hydrate agglomeration modeling and pipeline hydrate slurry flow behavior simulation☆
- Improving the performance of a thermoelectric power system using a flat-plate heat pipe☆
- Extending the EMMS/bubbling model to fluidization of binary particle mixture:Formulation and steady-state validation☆
