Preparation of a transparent superhydrophobic coating based on silanemodified zeolitic imidazolate frameworks and study on its properties
2018-10-23ManxinZhangTengfeiXiangChundongDongLingYangWenmingChanYingxuanZhaoChengLi
Man-xin Zhang,Teng-fei Xiang,Chun-dong Dong,Ling Yang,Wen-ming Chan,Ying-xuan Zhao,Cheng Li*
(College of Materials Science&Technology,Nanjing University of Aeronautics&Astronautics,Nanjing210016,China)
Abstract: SiO2-modified zeolitic imidazolate framework-8(ZIF-8)nanoparticles with methyl groups on their surfaces(CH3-SiO2@ZIF-8)were prepared by sol–gel method with methyltriethoxysilane(MTES)as precursor.A superhydrophobic coating was obtained by sprayingγ-aminopropyltriethoxysilane(KH-550)modified core–shell CH3-SiO2@ZIF-8particles on glass substrate.Morphological analysis revealed that the coating was of porous structure.The water contact angle(WCA)of the coating could be as large as(152±0.5)°and its sliding angle was(8±1.2)°.The visible-light transmittance of this coating reached not less than90%as compared with the glass substrate.The results of self-cleaning,water jet,and antifogging tests showed that the superhydrophobic coating possesses excellent non-wettability,stability,and antifogging properties.
Keywords: glass;zeolitic imidazolate framework;silane;sol–gel method;modification;superhydrophobic coating;water contact angle;self-cleaning
1 Introduction
Glass is commonly used for making architectural windows,optical lenses,windscreen,and so on[1-3].However,with hydrophilic nature of ordinary glass,water and dust would be adsorbed on its surface easily.The contaminants significantly affect the transmittance of glass,thus the glass surface needs to be cleaned regularly.With the demands of easy cleaning,it is urgent to develop a transparent,durable,and self-cleaning superhydrophobic glass.The superhydrophobic surfaces are generally characterized as those with a water contact angle(WCA)exceeding150°and a sliding angle(SA)less than10°.Surface roughness and surface energy are two main factors affecting the wettability of solid surface[4-6].Commonly,lowering the surface energy on a smooth surface through proper modification with effective chemicals could maximize increasing the water contact angle to119°[7].Therefore,it is worth to build a proper rough structure besides modification,which is inspired by lotus leaves in the nature.Koch et al.[8]fabricated artificial lotus leaves with hierarchical structure by a molding of the lotus leaf microstructure.They utilized lotus waxes by thermal evaporation to create the wax tubule nanostructures,which showed a self-cleaning property superior to the natural Lotus leaves.Jiang et al.[9]constructed a superhydrophobic dandelion-like3D microstructure by selfassembly from one-dimensional nanofibers of polyaniline,which behaved excellent superhydrophobicity.Zhang et al.[10]developed a superoleophobic nanofilament layer on glass surface,which exhibited a high contact angle and ultralow sliding angle.These surfaces possess enough micro-roughness with low surface energy to achieve a substantial increase in contact angle.
Generally,superhydrophobic structure on the glass surface can be fabricated by two approaches.One is direct etching of glass surface to fabricate rough structure in micro/nano-scale[11-12].For example,Jiet al.[13]prepared a superhydrophobic glass surface by one-step hydrothermal method with ammonium hydroxide followed by chemical modification.Nonetheless,this approach is unsuitable for industrial application due to its strict reaction conditions.Another is coating by organic or/and inorganic components on glass to achieve a surface with low surface energy[14].Fujishimaet al.[15]developed superhydrophobic coatings on glass using a silica–PMMA composite via sol–gel method.To date,several types of material have been reported to prepare superhydrophobic surface,such as polymers,porous materials,and fabrics.
Recently,metal–organic frameworks(MOFs)have attracted much attention because of their unique porous characteristics[16].They are formed via linking inorganic and organic units by strong bonds,which behave the flexibility with geometry,size,and functionality[17].These features have been widely applied to separation[18-19],catalysis[20-21],sensing[22-23],etc[24-27].Zeolitic imidazolate frameworks(ZIFs),a sub-family of MOFs,have a zeolite-like porous structure which are composed of transition metals and imidazolate linkers[28].With the advantages of easy preparation and unique porous structure,ZIF-8(i.e.Zn(mIm)2,mIm=2-methylimidazole)has been widely used.Jayaramuluet al.[29]synthesized a superhydrophobic/superoleophilic HFGO@ZIF-8composite in which ZIF-8in situ formed between highly fluorinated graphene oxide(HFGO)layers acted as pillars to provide the composite with additional mesoporosity.Wanget al.[30]fabricated ZIF-8@SiO2micro/nano hierarchical superhydrophobic surface,which improved corrosion resistance and abrasion resistance of the AZ31magnesium alloy.Nevertheless,few studies reported the synthesis and application of superhydrophobic ZIF-8on glass.
Due to the existence of─CH3groups,ZIF-8displays hydrophobic characteristics[31].Meanwhile,the coating consisting of rhombic dodecahedral ZIF-8particles can form a rough surface.Thus,a water droplet on ZIF-8powder exhibits spherical shape[32].But the hydrophobic ZIF-8particles are hard to adhere on hydrophilic glass.The porous structure of ZIF-8can capture CO2when it is exposed to air.However,the irreversible chemical reaction among ZIF-8,water and CO2might collapse the porous structure of ZIF-8[33].So ZIF-8cannot be used directly as a hydrophobic material for long-term exposure in air.Therefore,methyltriethoxysilane(MTES)was selected as precursor to form CH3-SiO2in situ on ZIF-8to achieve a core–shell structure,which can maintain its rhombic dodecahedral shape and hydrophobicity.In this work,a facile method was used to fabricate a superhydrophobic porous coating on glass.The coating was first fabricated from core–shell CH3-SiO2@ZIF-8particles and then modified by KH-550,which exhibited satisfied durability,self-cleaning and antifogging properties,and could be used in a wide range of substrate.
2 Experimental
2.1 Materials
Slide glasses(Sail Brand,25.4mm×76.2mm×1.2mm,composition:72.5%SiO2,13.7%Na2O,9.8%CaO,3.5%MgO,0.4%Al2O3,0.1%K2O)were used as substrate.Zinc nitrate tetrahydrate(Zn(NO3)2·6H2O,98%)and2-methyl-imidazole(2-mIm,99%)were purchased from Aladdin Corporation.Cetyltrimethylammonium bromide(CTAB),methanol,ammonia water(25%-28%),acetone,hydrogen peroxide(30%),Congo red and ethanol were used with analytical grade and provided by Nanjing Chemical Reagent Co.,Ltd.Methyltriethoxysilane(MTES,98%)was purchased from Shanghai Macklin Biochemical Co.,Ltd.γ-Aminopropyltriethoxysilane(KH-550)was purchased from Nanjing LangKe Chemical Co.,Ltd.Deionized(DI)water was homemade.
2.2 Synthesis of 100 nm ZIF-8
810mg of Zn(NO3)2⋅6H2O was added to40mL methanol,which was denoted as solution A.526mg of2-mIm was added to40mL methanol,which was denoted as solution B.After solution A and B were immediately mixed under stirring(800r/min),kept continuous stirring for30min and then stayed for12h at room temperature.The obtained white precipitates were collected by centrifugation and washed with methanol for three times,and then dried at60°C overnight.
2.3 Synthesis of core–shell CH3-SiO2@ZIF-8 particles
80mg of the above prepared ZIF-8and100mg of CTAB were well dispersed in80mL methanol under stirring(800r/min)for30min.The pH of the resulting solution was adjusted by ammonium hydroxide(25%)to11.Then100μL of MTES was added to the above solution drop by drop with stirring.Afterward,the mixture was kept being stirred for4h at room temperature.Subsequently,the turbid liquid was centrifuged and the precipitates were washed with ethanol for three times to obtain the core–shell CH3-SiO2@ZIF-8particles.
2.4 Modification of CH3-SiO2@ZIF-8 particles
All of the as-prepared CH3-SiO2@ZIF-8particles were dispersed in80mL ethanol.The resulting solution was adjusted to pH=11by ammonium hydroxide(25%),added with0.1,0.2,0.3and0.4vol.%of KH-550respectively under continues stirring(800r/min)for4h,and aged at room temperature for subsequent spray coating process.
2.5 Fabrication of superhydrophobic coating on glass
Firstly,the glass was ultrasonicated for30min in a100mL mixed solution of ethanol,acetone,and DI water at equal volume.Secondly,the glass was immersed in hydrogen peroxide(30%)for30min and then dried at60°C.Finally,the as-prepared solution of CH3-SiO2@ZIF-8particles was sprayed on the cleaned glass surface and dried at room temperature overnight.
2.6 Characterization
The microstructure and morphology of coatings were observed by scanning electron microscopy(SEM,LEO1550,ZEISS)and transmission electron microscope(TEM,JEOL).The phase composition was analyzed via X-ray diffraction(XRD,BRUKER D8,Cu Kα,40kV,40mA)at a scanning rate of10°/min.Fourier transfer infrared spectrometer(FTIR,Nicolet6700,Thermo Scientific,USA)was used to detect and identify the components of the samples’surfaces.The wettability was characterized by static water contact angle measuring system(DSA100,Kruss Corporation)at three different positions for each sample,and the average value was adopted as WCA.Transmittance measurements were performed using a UV–VIS–NIR spectrophotometer(SHIMADZU,UV-3600).All transmittance data were based on the transmittance of bare transparent glass substrate as reference in the visible wavelength(380-780nm).The antifogging test of the samples was conducted using a low constant temperature water bath(DC-6506,SOPTOP).
3 Results and discussion
A brief procedure for fabricating superhydrophobic glass by spray coating technique was shown in Figure1.During the synthesis process,ZIF-8is firstly formed by mixing Zn2+ions and2-mIm in methanol solution.Then,the MTES precursor is hydrolyzed and condensed under the catalysis of ammonia in methanol,and spontaneously cover the ZIF-8to form core–shell CH3-SiO2@ZIF-8particles via sol–gel process.Finally,the core–shell particles are modified by KH-550.After hydrolysis of MTES containing hydrolyzable─C2H5groups and non-hydrolyzable─CH3groups,the CH3-SiO2shell with hydrophobic─CH3groups endows the CH3-SiO2@ZIF-8particle with hydrophobicity.The hydrolyzable─C2H5groups are substituted by hydrogen atoms which exhibit high reactivity in the modification process.During the modification process,KH-550reacts with the active hydrogen of CH3-SiO2,forming a molecular bridge which enhances the hydrophobicity and adhesion of coating.
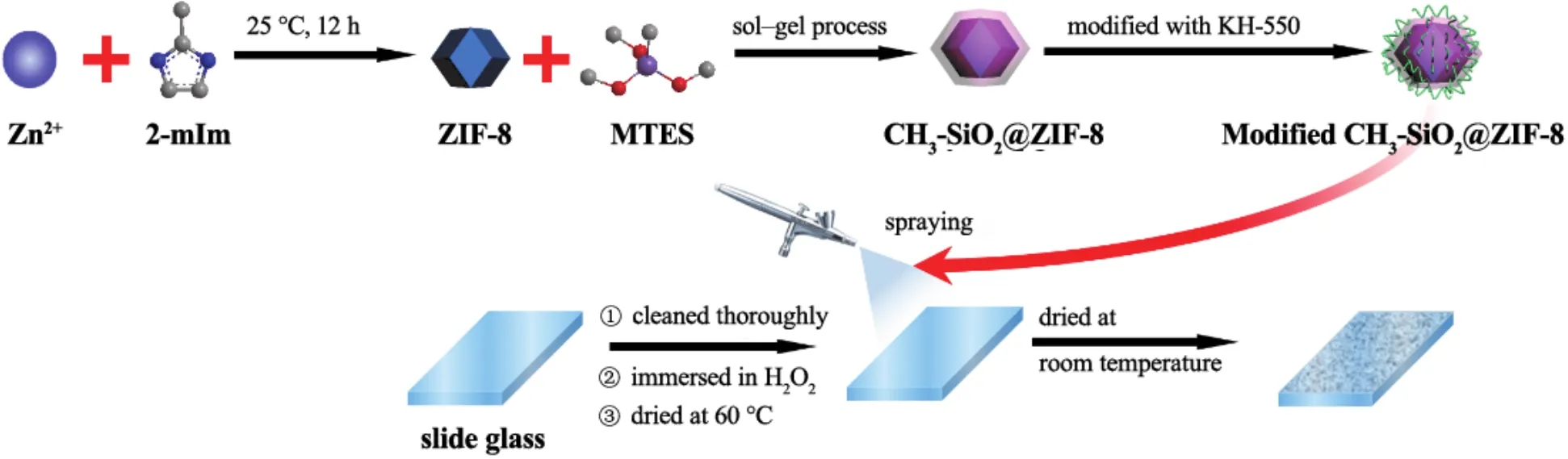
Figure 1 Schematic illustration showing the fabrication process of superhydrophobic coating on glass surface图1 玻璃表面超疏水涂层的制备过程示意图
3.1 Analysis of core–shell CH3-SiO2@ZIF-8 particles
As shown in Figure2a and2c,the ZIF-8particles presented uniformly rhombic dodecahedral shape with a diameter about100nm.After coated with CH3-SiO2,the core–shell particles can be observed from Figure2b and the inserted figure was the corresponding WCA(ca.136°).It could be seen that the rhombic dodecahedral shape of CH3-SiO2@ZIF-8particles was almost unchanged and the diameter of particles was slightly enlarged.Compared with the shape of ZIF-8,the morphology was smoothed after the formation of core–shell structure.Meanwhile,there was a few crosslinking among CH3-SiO2@ZIF-8particles from TEM image(see Figure2d).

Figure 2 SEM and TEM images of ZIF-8 and CH3-SiO2@ZIF-8图2 ZIF-8和CH3-SiO2@ZIF-8的SEM和TEM照片
The XRD patterns of ZIF-8,CH3-SiO2,and core–shell CH3-SiO2@ZIF-8particles were shown in Figure3a.The diffraction peaks found at7.2°,10.3°,12.7°,14.6°,16.4°,and18.0°indicated the(011),(002),(112),(022),(013),and(222)planes of the prepared ZIF-8particles[34].The CH3-SiO2@ZIF-8evidently showed the new diffraction peaks of CH3-SiO2at11.0°and23.0°.All the above results demonstrate that the core–shell CH3-SiO2@ZIF-8was successfully prepared with CH3-SiO2coating on the surface of ZIF-8particles.
FT-IR analysis was used to characterize the chemical bonds of the products and the related substances.Figure3b showed the infrared spectra of ZIF-8,CH3-SiO2,and CH3-SiO2@ZIF-8particles.On the spectrum of ZIF-8,the peaks at2926cm−1and1600cm−1correspond to the stretch vibrations of C─H and C=N,respectively.The bands in the spectral region from900cm−1to1350cm−1are for the in-plane bending of the imidazole ring[35].As for the CH3-SiO2particles,the stretching and bending of C─H bonds were at around2950cm−1and1400cm−1,respectively,and the swinging of Si─C bonds was at about847cm−1[36].The asymmetric stretching vibration peak of─OH groups was at around3456cm−1.The intensity of the peak at1074cm−1indicates the asymmetric stretching vibration of Si─O─Si.From the spectrum of CH3-SiO2@ZIF-8,there was a peak appeared at1090cm−1near the1145cm−1peak.It could be inferred that ZIF-8reacts with CH3-SiO2,leading to the offset of stretching vibrations of Si─O─Si.The existence of the characteristic bonds of ZIF-8and CH3-SiO2proved the growth of CH3-SiO2on the surface of ZIF-8nanoparticles.
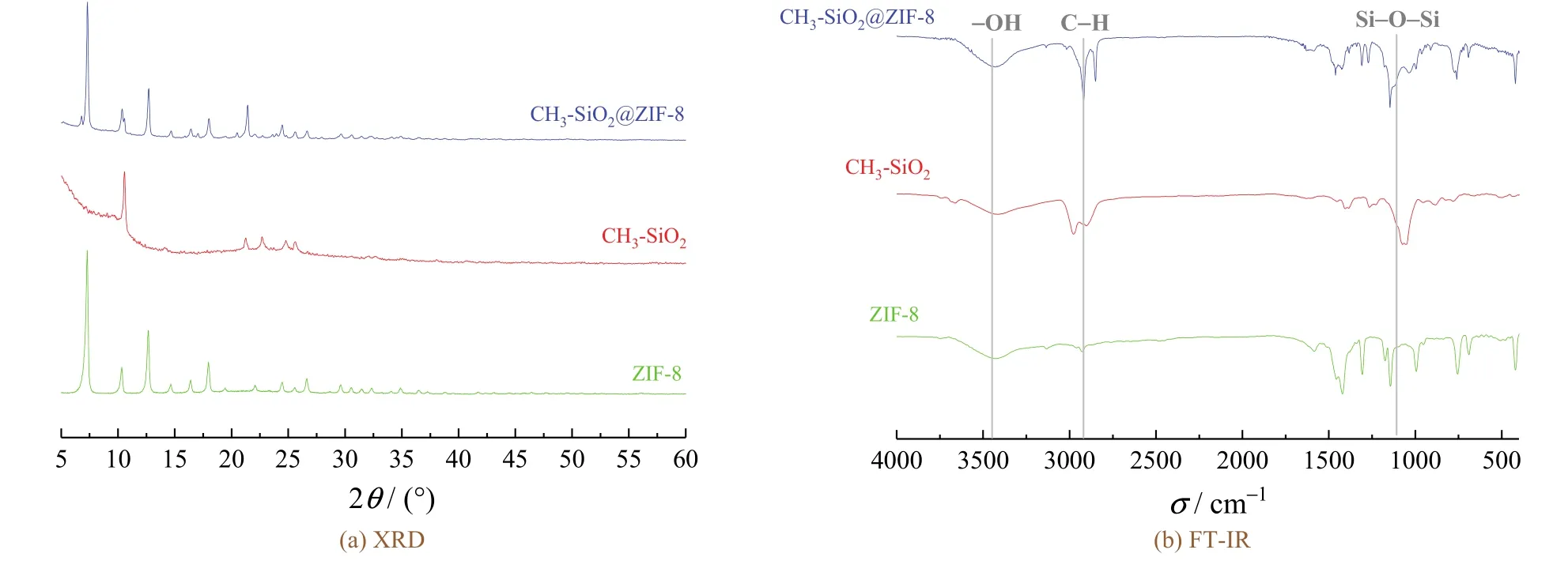
Figure 3 XRD patterns and FT-IR spectra of ZIF-8, CH3-SiO2, and CH3-SiO2@ZIF-8 particles图3 ZIF-8、CH3-SiO2和CH3-SiO2@ZIF-8三种粒子的X射线衍射和傅里叶变换红外谱图
3.2 Analysis of the superhydrophobic CH3-SiO2@ZIF-8 particles
The WCA is commonly used to evaluate the wettability of superhydrophobic coating.The wettability of surface is considered as a function of its roughness[37].It depends on the surface chemistry and surface topography.For a hydrophilic surface,the rougher it is,the more hydrophilic it becomes,i.e.the smaller the WCA is.On the contrary,a hydrophobic surface would show a larger WCA when it becomes rougher.As is known,glass is hydrophilic with a high surface energy and its WCA is about25°.As shown in the previous characterization,CH3-SiO2@ZIF-8retained the special dodecahedron structure,and the sprayed coating steeply improved the roughness of the substrate surface.In view of the existence of a large amount of hydroxyl groups on the surface of CH3-SiO2@ZIF-8particles,it is necessary to modify these particles by some materials with low surface energy.On account of this advantaged microstructure,it is believed that the modification with an organic low-surface-energy substance could provide a superhydrophobicity for this coating.
Figure4showed the SEM images of CH3-SiO2@ZIF-8coating surface modified with the increasing content of KH-550,and the inserted images showed the corresponding WCAs.Among all of samples,the coating surface exhibited the highest water contact angle about(152±0.5)°when CH3-SiO2@ZIF-8particles were modified by0.2vol.%KH-550.The porous structure(see Figure4b)makes it trap the air easily.It was likely that the amounts of CH3-SiO2@ZIF-8and KH-550reached an appropriate proportion at this situation.These low-surface-energy particles piled up on the film to form a superhydrophobic surface.From the corresponding WCA images,it is inferred that the water drop on the film surface is in the Cassie-Baxter state.The following equation[38]describes the contact angle at a heterogeneous surface composed of two different materials:
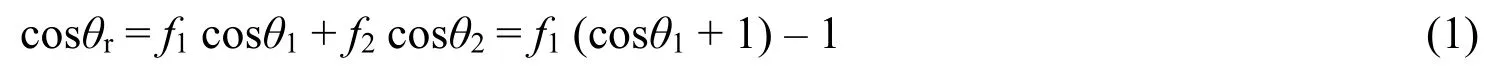
whereθris apparent contact angle,f1is the fraction of solid surface being in contact with water,f2represents the fraction of trapped air contacting with water(f1+f2=1),θ1is intrinsic contact angle on solid,θ2is intrinsic contact angle on trapped air(θ2=180°).Based on the WCA of74°for a smooth glass modified with KH-550reported in literature[39],thef2was calculated to be0.91.Such high value means that the water droplets on coating mainly contact with the trapped air,demonstrating the important role of dodecahedron structure in superhydrophobicity surface.

Figure 4 SEM and WCA images of the coatings of CH3-SiO2@ZIF-8 modified by KH-550 with different volume fractions图4 以不同体积分数的KH-550改性的CH3-SiO2@ZIF-8涂层的表面SEM照片和水接触角测试照片
For the coating obtained with CH3-SiO2@ZIF-8particles modified by0.1vol.%KH-550,by contrast,Figure4a depicted an irregular porous structure that was similar to Figure4b,but its WCA was only148°.It was speculated that KH-550totally reacts with the excess CH3-SiO2@ZIF-8particles exhibiting hydrophobicity.When adding0.3vol.%KH-550,the excess of coupling agent was completely coated on the particles,leading to a decreased interspace(see Figure4c)and even a difficulty to trap the air due to its high WCA(146°).Further increase of KH-550to0.4vol.%resulted in the self-hydrolysis of excessive KH-550firstly and its condensation,generating lots of hydrophilic silanol groups.The silanol groups enhanced the crosslinking of particles,and made several CH3-SiO2@ZIF-8particles agglomerate to form a big sphere,leading to a decrease of the porosity of coating.As a result,the hydrophobic property of coating was reduced(see Figure4d).It can be concluded that with the increasing of KH-550dosage,the WCA of coating is increased firstly and then decreased.In short,the modification by0.2vol.%KH-550greatly affects the antiwettability behavior of coating surface.
FT-IR was used to confirm the chemical modification of CH3-SiO2@ZIF-8particles by KH-550.Figure5showed that,for the modified particles,besides the characteristic transmittance peaks of CH3-SiO2@ZIF-8particles,there were two additional characteristic transmission peaks at3329cm−1and1650cm−1,being assigned to the stretching vibration and deformation vibration of N─H in KH-550.The stretching vibration of Si─O─Si located at1118cm−1and1023cm−1[40].These results indicated that CH3-SiO2@ZIF-8particles were successfully modified with KH-550.

Figure 5 FT-IR spectra of KH-550, CH3-SiO2@ZIF-8, and 0.2vol.% KH-550 modified CH3-SiO2@ZIF-8图5 KH-550、CH3-SiO2@ZIF-8和0.2%(体积分数)KH-500改性的CH3-SiO2@ZIF-8的傅里叶变换红外光谱图
3.3 Transmittance
The optical transmission spectra of the coatings sprayed on glasses were shown in Figure6.It was clearly observed that the transmittance reached as high as93%for a coating sprayed with0.4vol.%KH-550modified CH3-SiO2@ZIF-8particles,but only90%for a coating from those modified with0.2vol.%KH-550.When increasing the amount of KH-550for the modification of CH3-SiO2@ZIF-8particles,the optical transmission of the coating was also enhanced in the visible region.The gradual decrease of roughness might lead to the reduction of light scattering,thus increasing the transmittance.Therefore,the roughness had a great effect on the transmittance of coating.Figure7showed the optical image of10μL water droplets on the transparent superhydrophobic glass(modified by0.2vol.%KH-550)and the cleaned glass without coating.The water droplets were colored with Congo red for better optical clarity.As can be seen that,the water droplets were self-spreading on the bare glass,but spherical in shape on the glass with a superhydrophobic coating,under which the letters were observed clearly.

Figure 6 Optical transmission spectra of the coatings prepared from CH3-SiO2@ZIF-8 modified with different volume fractions of KH-550 in visible region图6 用不同体积分数的KH-550改性CH3-SiO2@ZIF-8后制得的涂层的可见光透过率

Figure 7 Photo showing the water droplets on the surfaces of the glass with (left) and without (right) a superhydrophobic coating prepared from CH3-SiO2@ZIF-8 modified with 0.2vol.% KH-550, respectively图7 水滴在用以0.2%(体积分数)KH-550改性的CH3-SiO2@ZIF-8制成的超疏水玻璃(左)和普通玻璃(右)表面的状态
3.4 Sliding angle analysis and self-cleaning behavior
Generally,the sliding angle is a parameter directly reflecting how easy would it be for a droplet to roll off from a surface.It also represents the contact angle hysteresis of a solid surface.Figure8showed the roll-off process of water droplet on the surface of CH3-SiO2@ZIF-8(modified by0.2vol.%KH-550)coating.The advancing and receding water contact angles were observed to be approximately150.57°and142.45°,respectively.The sliding angle was(8±1.2)°.This phenomenon reflects an ultralow adhesion between the surface and water droplet.Interestingly,this coating can be used as a self-cleaning surface.

Figure 8 Sliding behavior of a water droplet on the superhydrophobic glass obtained by coating with 0.2 vol.% KH-550 modified CH3-SiO2@ZIF-8图8 水滴在以0.2 vol.% KH-550改性的CH3-SiO2@ZIF-8制成的超疏水玻璃表面的滚动行为
The most common self-cleaning behavior is that the dirt particles on a lotus leaf are easily removed by water droplets[41].A sparse layer of carbon powder was separately sprinkled on the treated and untreated glasses(see Figure9a and9d).Then,water was dropped to wash the contaminated glass surfaces.During the cleaning process,the carbonpowder was immediately adsorbed and carried away by the water droplets on the superhydrophobic glass(see Figure9b and9c),leaving behind a clean surface.It was observed that some water droplets still maintained a spherical shape after the adsorption of contaminants.When the water was dropped on the bare glass(see Figure9e and9f),the spreading droplets were attached with the carbon powders and slowly slipped to the bottom of glass,retaining a plenty of powders on the glass surface.These results confirmed that the superhydrophobic coating plays an important role in self-cleaning.
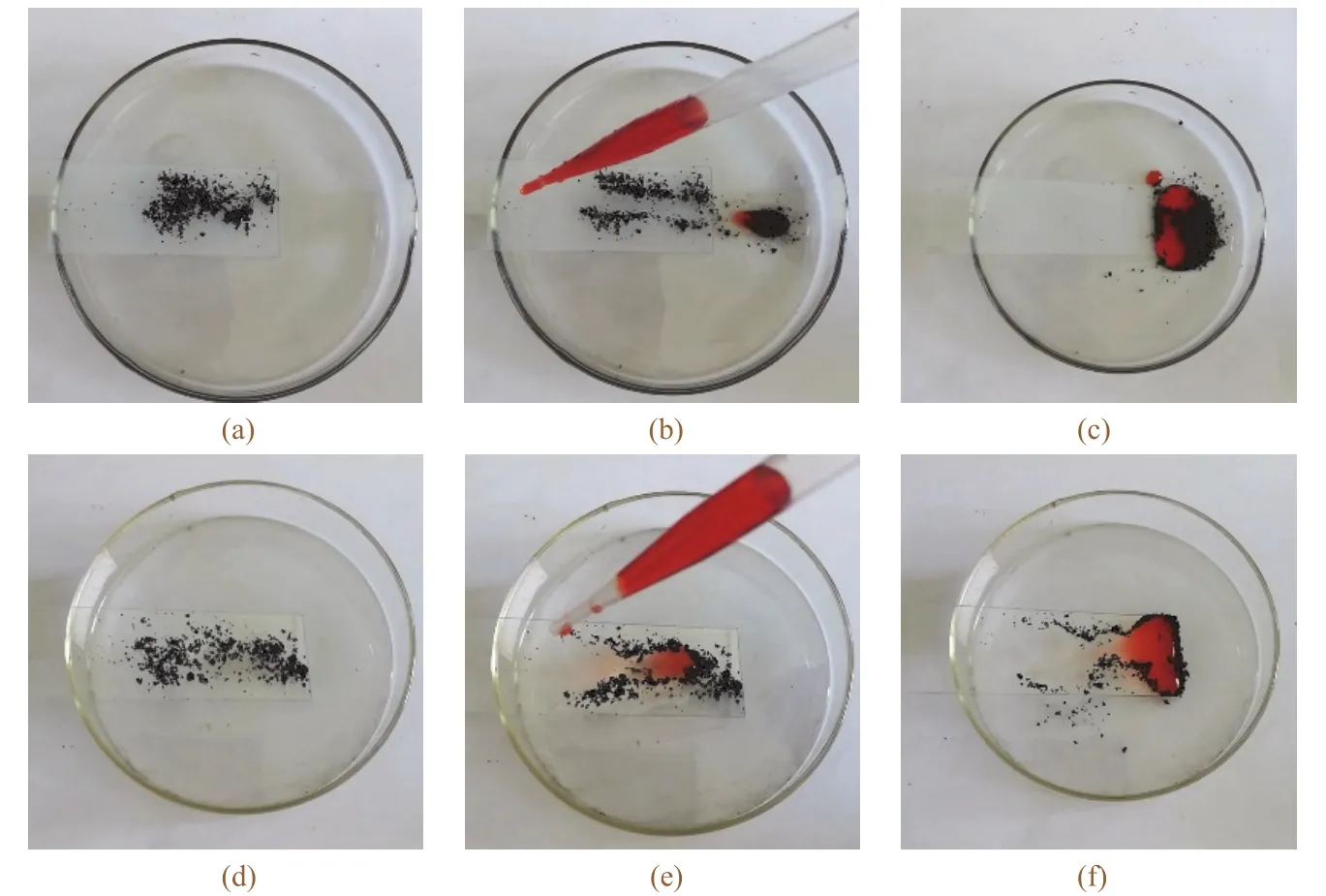
Figure 9 Self-cleaning properties of superhydrophobic glass (a, b, c) and original glass (d, e, f)图9 超疏水玻璃(a−c)和普通玻璃(d−f)的自清洁效果
3.5 Stability of the coatings
An immersion test was conducted to evaluate the stability of the superhydrophobic coating.When the superhydrophobic coating was immersed in DI water,the water was repelled and could not penetrate into the microstructure.After being immersed into DI water for72h and dried at60°C,the contact angle of coating surface changed from(152±0.5)°to(150.3±1.3)° (see Figure10).The contact angle decreased slightly but was still larger than150°.These results demonstrated that the superhydrophobic coating had excellent stability.
In order to further verify the stability of the coating,the superhydrophobic glass was exposed to the ambient condition for a month.Figure11showed that the WCA and SA displayed a quite slight decrease and increase after a month of natural exposure,respectively.The CH3-SiO2@ZIF-8coating was expected to be excellent for long-term superhydrophobicity in ambient condition.
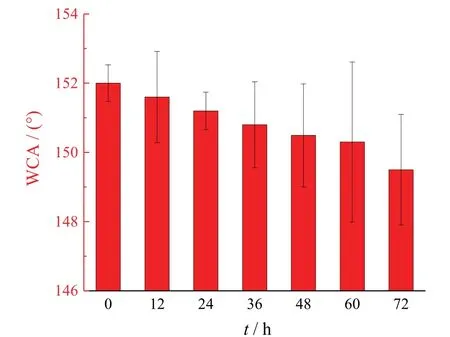
Figure 10 Variation of water contact angle of the superhydrophobic coating during immersion in DI water after 72 h图10 超疏水表面在去离子水中浸泡72 h内接触角的变化
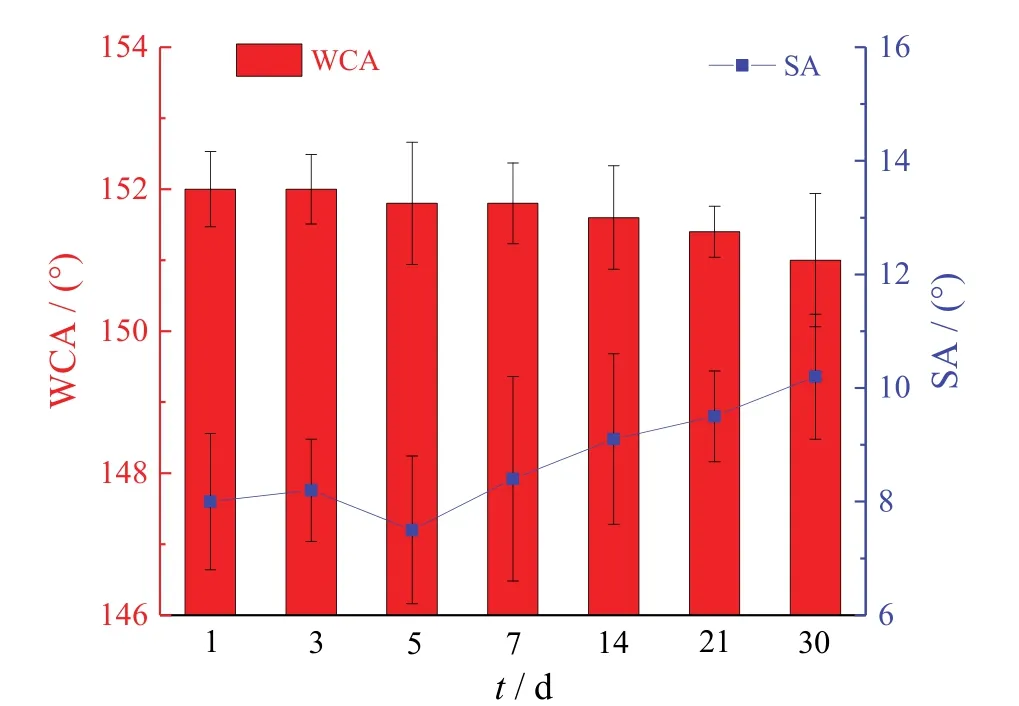
Figure 11 Variation of water contact and sliding angles of superhydrophobic glass during exposure to the ambient condition for a month图11 暴露在室温下一个月的超疏水表面的接触角和滚动角变化
When the superhydrophobic coating was damaged by an external force such as water jetting,its wettability would be changed.Figure12a showed a water jet impact test for evaluating the stability of superhydrophobic coating.A25mL syringe was utilized to jet aqueous Congo red solution onto the coating surface.The syringe was kept2cm above the coating surface,and the sample was exposed to water jet for20s(with a syringe pressing rate of~1mm/s),this procedure was repeated10times.The water jet directly reflects from the contact point at an angle of20°,without spreading on the surface.After being impacted by water jet for10min,the superhydrophobic coating was intact.In order to further confirm the excellent stability of the coating,tap water was jetted under a pressure of0.8MPa with a speed of20mL/s onto the coating surface for a while(see Figure12b).The coating still kept its superhydrophobicity.

Figure 12 Photos showing the progress of water jet test for the superhydrophobic glass图12 超疏水玻璃的喷水试验照片
3.6 Antifogging evaluation
In literature,it has been referred that the superhydrophilic surface exhibits a good antifogging performance that could broaden its practical application[42].The hydrophilic surfaces having an advancing contact angle less than40°is able to prevent water from condensing as droplets,and allows moisture to condense as a continuous thin film[43].Meanwhile,among recent reports on superhydrophobic coatings,more attention were paid to their antifogging property.Therefore,the antifogging performance of superhydrophobic and ordinary glasses was estimated experimentally by being removed from a−10°C freezer and placed in a humid laboratory environment(ca.50%RH).It could be seen from the Figure13that dense and numerous small water droplets were condensed immediately on the ordinary glass surface.However,on the surface of superhydrophobic glass,a thin layer of fog was formed and quickly disappeared,which was attributed to the smaller curvature radius of droplets on the superhydrophobic coating than on the bare glass.With a high evaporation rate,the water droplets could be easily sublimated on the superhydrophobic surface.Therefore,the superhydrophobic surface was also of good antifogging property.

Figure 13 Antifogging a experiments between test of bare glass (left) and superhydrophobic glass (right)图13 普通玻璃(左)和超疏水玻璃(右)的防雾性
4 Conclusion
In this study,CH3-SiO2@ZIF-8particles with a core–shell structure were successfully obtained by a facile sol–gel method and then sprayed on ordinary glass.A superhydrophobic coating was obtained due to the synergistic effectof micro-roughness and low surface energy.When the CH3-SiO2@ZIF-8particles with a rhombic dodecahedral shape were modified by0.2vol.%KH-550,the coating prepared therefrom featured a contact angle of(152±0.5)°and a sliding angle of(8±1.2)°.The transmittance of the coating reached90%in the wavelength range of visible light.Besides,the coating surface showed excellent self-cleaning,durability,and antifogging properties.This simple method should have a promising future on account of its easy application in large-scale productions.
Acknowledgment
This work was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
