Synthesis and catalytic activity of SBA-15 supported catalysts for styrene oxidation
2018-08-31VasuChaudharySweta
Vasu Chaudhary,Sweta*
Department of Chemical Engineering and Technology,Indian Institute of Technology(BHU),Varanasi,Uttar Pradesh-221005,India
Keywords:SBA-15 Styrene Styrene oxide Benzaldehyde Oxidation Catalytic oxidation
A B S T R A C T Cu(II)and Mn(II)metals embedded on mesoporous SBA-15 were synthesized by co-precipitation technique.The support and catalysts were characterized by SEM–EDX,TEM,BET,XRD and ICP-AES methods.The catalytic activity of these catalysts was evaluated for styrene oxidation at various reaction conditions such as styrene to TBHP mole ratio,temperature,catalyst amount by using TBHP as an oxidizing agent.Major reaction products were styrene oxide and benzaldehyde and highest styrene conversion(97.3%)was observed at styrene to TBHP mole ratio of 1:4,temperature at 80°C and 20 mg of catalyst.Further,the recyclability of the catalysts was observed and found that they can be recycled three times without major loss in their activity and selectivity.©2017 The Chemical Industry and Engineering Society of China,and Chemical Industry Press.All rights reserved.
1.Introduction
The design of active and selective catalysts for the selective oxidation of alkenes is currently of great interest from the academic and industrial point of view for the synthesis of a variety of bulk and fine chemicals[1].
The liquid phase oxidation of styrene is a commercially important reaction.The main product obtained by oxidation of styrene is styrene oxide;which isavaluable intermediate for the synthesis of epoxy resins,perfumes,plasticizers,dyes and drugs[2,3].Conventionally styrene oxidation was carried out using organic peracids as oxidants or by a chlorohydrin process.How ever,these methods lead to poor selectivity for styrene oxide and generation of undesirable waste products.Therefore,the ever-increasing environment concern has compelled the researchers to consider environment-friendly technologies[4,5].
Various complexes of transition metals have been developed for styrene oxidation[6].Transition metals(Cu,Mn,Ni and Co)have the advantages of easy availability and low cost,among which Cu and Mnbased catalysts have shown high performance in the catalytic oxidation of alkenes.Gupta et al.showed polymer supported Schiff base complexes as catalysts for various alkenes and reported that Mn(III),Ru(III),Cu(II),Fe(III),Co(II),and Ni(II)were active metals for oxidation reactions[7].Chang et al.studied the cyclohexene oxidation using O2as oxidant catalyzed by chloromethylated polystyrene supported tridentate Schiff base Cu(II),Ni(II),Co(II),and Mn(II)complexes,among which Cu(II)complex was found to be most active(52%conversion)[8].Li et al.reviewed the toluene oxidation over Cu and Mn based catalysts and showed that bimetallic catalyst has higher activity than monometallic catalysts[9].Therefore,Cu and Mn based catalysts have shown great attention of the researchers for oxidation reaction.
A large variety of catalytic supports have been developed for oxidation reactions i.e.silica,alumina,molecular sieves,polymers,and zeolites.Nowadays porous materials are attracting wide concern because of their uniform surface area,narrow pore size distribution and exceptionally good dispersion of metal particles.Earlier microporous materials such as zeolites were used as catalyst support owing to their high surfacearea,strong acidic sites,uniform microporous channels,high hydrothermal stability and excellent shape selectivity[10,11].How ever,due to narrow pore size(0.4–1.2 nm)of zeolites,their active sites become inaccessible when large reactant molecules diffuse into their structure.To overcome this problem,in recent years extensive research has been carried out on ordered mesoporous materials i.e.MCM-41,MCM-48,MCM-50,HMS,SBA-15 and SBA-16[12,13].SBA-15 is most commonly used material among all mesoporous materials since it has a high specific surface area(400–900 m2·g−1),large pore size(4–30 nm)and pore volume,more wall thickness(3.2–6.4 nm)and high thermal and hydrothermal stability[14–19].Fan et al.reviewed Cu-Mn/CeO2/SBA-15 for advanced oxidation process and proved that support SBA-15 is suitable for oxidation reactions[20].Sareen et al.showed that Cu O/SBA-15 is highly effective and attractive for oxidation of cyclohexane[21].Cruz et al.reported that Copper(II)complexes supported over mesoporous SBA-15 are efficient for selective oxidation of benzyl alcohol with high stability[22].
In the current work,we describe the synthesis and characterization of Cu(II)and Mn(II)catalysts supported over mesoporous SBA-15.These catalysts were investigated for selective oxidation of styrene using TBHPasthe oxidant.All the catalysts have shown very good activity and selectivity for styrene oxide.
2.Experimental
2.1.Chemicals required
Pluronic P123(99%purity,Sigma-Aldrich),tetra ethyl ortho silicate(99%purity,Alfa Aesar),copper(II)nitrate trihydrate(99%purity,Merck Specialities Private Limited),manganese(II)nitrate tetrahydrate(>97%purity,Sigma-Aldrich)were used as obtained.Other chemicals and solvents were of analytical grade.
2.2.Synthesis of SBA-15
The mesoporous material SBA-15 was synthesized as reported by Zhao et al.[23].In a typical synthesis,4 g of Pluronic P123(EO20PO70EO20,M w=5800)was dissolved in 30 g of distilled water and 120 g of 2 mol·L−1HCl.After 3 h of stirring(600–700 r·min−1)at 40°C,8.5 g of TEOS was added drop wise and continued to stir at the same temperature for 24 h.A solid product was obtained after aging at 80°C for another 48 h without stirring and then recovered by filtration,washed with distilled water and ethanol(ethanol:water=1:1).Thereafter sample was air dried at room temperature for 6 h followed by drying in an oven at 80°C for 6 h and finally calcined at 550°C for 4 h.
2.3.Catalyst preparation
Cu-Mn/SBA-15 catalyst was synthesized by co-precipitation method by incorporating copper and manganese metals into mesoporous SBA-15 using aqueous solutions of Cu(NO3)2·3H2O and Mn(NO3)2·4H2O as precursors.For 20 wt%metal loading,the calculated amounts of metal precursors were dissolved in 50 ml HPLC grade water.After 30 min,0.5 g SBA-15 support was added and temperature of the mixture was raised to 70 °C and pH was adjusted to 2.After 3 h,0.5 mol·L−1NaOH was added drop wise until pH value reached to 10 followed by aging for 6 h.Subsequently,washing and filtration were done with HPLC grade water.The resulting material was dried at 80°C for 6 h and finally calcined at 450°C for 4 h.Cu/SBA-15 and Mn/SBA-15 catalysts(20 wt%)were prepared in the similar way.
2.4.Catalysts characterization
The BET surface area was determined by nitrogen adsorption and desorption isotherms at 77 K using ASAP 2020(Micromeritics,USA).Prior to the adsorption measurements,the samples were degassed at 473 K for 6 h.The metal contents(Cu and Mn)in each sample were examined by inductively coupled plasma-atom emission spectroscopy(ICP-AES)on ICP spectrometer(ICAP6000 SERIES;Thermo Fisher Scientific).Typically,0.1 g of the catalyst sample was dissolved into 10 ml of HNO3and then solution was diluted up to 50 ml by distilled.The morphological properties of the samples were observed by Scanning Electron Microscopy(SEM)method and the samples were sputtered with a thin gold film to decrease the charging property using SEM:EVO 18-20-45 system.Porous structure and arrangement of pores were analyzed by Transmission Electron Microscopy(TEM)images were recorded using TECNAI 20G system.The structural behavior of the samples was analyzed by X-Ray Diffraction(XRD),using X-ray diffractometer(Ultima IV,Rigaku)with CuKαradiation.
2.5.Test of catalytic activity
A magnetically stirred three-necked round bottom flask(50 ml)integrated with athermometer and are flux water condenser was used for the catalytic oxidation of styrene in the liquid phase.In a typical reaction mechanism,20 mg of catalyst was swelled for 30 min in 10 ml acetonitrile followed by addition of 1.04 g styrene(10 mmol)and 0.90 g TBHP(10 mmol).This reaction mixture was heated for 7 h at 60°C.The samples were withdrawn at every 1-h time interval and were analyzed by gas chromatography instrument(Centurian-5800,India)with a SGE capillary column(length 30 m,i.d.0.25 mm, film thickness 0.25 μm)and a FID detector.The samples were analyzed by gas chromatography(GC)while is o-octane was used as the internal standard.On the basis of mole percent of styrene conversion,selectivity and yield were calculated.
3.Results and Discussion
The BET surface area,pore diameter and pore volume of support and catalysts are summarized in Table 1.The support shows a high surface area of 723 m2·g−1and pore volume of 0.801 cm3·g−1.BET study confirmed that when metals were co-precipitated onto the support,a decrease in surface area was observed in the metal complexes,which may be due to pore blocking by metal ions.The pore sizes were found in the range 2.20–6.20 nm and pore volumes were 0.119–0.801 cm3·g−1.The metal content of all the synthesized catalysts were determined by ICP-AES,(Table 1).For all the catalysts,ICP value is lower than the theoretical one.However for Cu/SBA-15,it is close to the theoretical one,while for Mn/SBA-15 and Cu-Mn/SBA-15 catalysts,major changes in textural properties were observed,due to which lower metal loading was observed.
3.1.Scanning Electron Microscopy(SEM)
Surface morphology and topology of support and different catalysts synthesized by co-precipitation method are considered by SEM as shown in Fig.1.Curved cylindrical configuration of support SBA-15 is shown in Fig.1(A)and this morphology has remained unaltered by the incorporation of Cu(II)and Mn(II)metals as shown in Fig.1(B),(C),(D)which verify the preservation of physical structure and texture of mesoporous support SBA-15.
Compositional analysis of synthesized SBA-15 support and catalysts was done using Energy Dispersive X-Ray Analysis(EDX).EDX results of SBA-15(Fig.1(A))successfully revealed the presence of silica in the synthesized sample.The EDX spectra of catalyst materials Cu/SBA-15(Fig.1(B)),Mn/SBA-15(Fig.2(C))and Cu-Mn/SBA-15(Fig.1(D))shown the presence of copper and manganese metals,thus confirming the metal impregnation.The silica percentage presented in the EDX analysis was performed on a small portion of sample,assuming a uniform composition throughout the sample.

Table 1Textural and chemical properties of the support and catalysts

Fig.1.Scanning Electron Microscopy(SEM)&EDX analysis of(A)SBA-15,(B)Cu/SBA-15,(C)Mn/SBA-15 and(D)Cu-Mn/SBA-15.
A
B
C
D

Fig.2.Transmission Electron Microscopy(TEM)analysis of(A)SBA-15,(B)Cu/SBA-15,(C)Mn/SBA-15 and(D)Cu-Mn/SBA-15.
A
B
C
D
3.2.Transmission Electron Microscopy(TEM)
Fig.2.shows the TEM images of SBA-15 support and all the catalysts.TEM image of SBA-15 support(Fig.2(A))confirms the hexagonal arrangement of homogeneous pores which is the distinctive property of the SBA-15.Dark spots within the mesoporous channels show the good distribution of the metal ionsin the interior of the channels as confirmed by the Fig.2(B),(C)and(D).This confirmed the introduction of Cu and Mn metals inside the pores of SBA-15.

Fig.3.XRD pattern of(A)Cu/SBA-15,(B)Mn/SBA-15,and(C)Cu-Mn/SBA-15.The inset shows the low angle XRD pattern of SBA-15 support.
3.3.XRD analysis
XRD patterns of Cu/SBA-15,Mn/SBA-15,and Cu-Mn/SBA-15 are shown in Fig.3.The inset shows the low angle XRD pattern of mesoporous SBA-15 support.The low-range diffraction pattern shows indication of one deflection peak at a 2θ value between 0.5°and 1°,comprising one strong peak(100),conforming a highly ordered hexagonal mesoporous silica structure.The wide range XRD pattern of catalysts demonstrated no diffraction peak of support at a 2θ value between 5°and 60°because peaks of SBA-15 were undetectable by XRD in this range and shows only the presence of copper and manganese.
For Cu/SBA-15 catalyst,only one strong peak was observed at 2θ value of 24°(210;face centered cubic),characteristic of copper metal.For Mn/SBA-15 catalyst,diffraction peaks were observed at 24.39°(111)and 42.23°(101),conforming body centered cubic and tetragonal structure,respectively.Therefore crystalline pattern of SBA-15 as well as catalysts confirm the presence of Cu and Mn metals and their crystalline structure.
3.4.Catalytic oxidation of styrene
Styrene selective catalytic oxidation was carried out using Cu–Mn/SBA-15 as catalyst and TBHP as the oxidant.Product distribution of the following oxidation reaction is shown in Fig.4.The effect of various reaction parameters such as styrene to TBHP mole ratio,temperature and catalyst amount was quantified in terms of styrene conversion,selectivity,and yield of styrene oxide and benzaldehyde.
3.5.Effect of styrene to TBHP mole ratio

Fig.4.Product distribution from styrene oxidation.
The effect of styrene to TBHP mole ratio on the styrene conversion and product yield was studied by varying the mole ratios from 1:1 to 1:4 at 60°C.The experiments showed that for all the mole ratios of styrene to TBHP,styrene conversion increases with time.Overall styrene conversion was increased from 61.54%(1:1)to 97.3%(1:4)after 7 h of reaction as shown in Fig.5.There was no significant change in styrene conversion from 1:3 to 1:4,how ever,there was a major change when the ratio was changed from 1:2 to 1:3.In the beginning,benzaldehyde was found as the major product and the yield of benzaldehyde tend to decrease with increase in TBHP ratio and reaction time.After 7 h,styrene oxide was found as a major reaction product with a maximum yield of 80.0%at 1:4 mole ratio.Therefore,all other catalytic parameters were evaluated at the styrene to TBHP mole ratio of 1:4.
3.6.Effect of temperature

Fig.5.Effect of styrene to TBHP mole ratio on styrene conversion at 60°C and 20 mg catalyst.

Fig.6.Effect of temperature on styrene conversion at 1:4 styrene to TBHP mole ratio and 20 mg catalyst.
The temperature of the reaction is a significant factor because of which there are considerable changes in reactant conversion and products yield.Very low conversion(<40%)was obtained below 60 °C reaction temperature.Hence the influence of reaction temperature on styrene oxidation was studied in a temperature range of 60-80°Cat styrene to TBHP mole ratio 1:4 for Cu–Mn/SBA-15 catalyst.The results showed that conversion of styrene increased as temperature increased with time.Conversion of styrene increased from 85.58%(60°C)to 97.3%(80 °C)as shown in Fig.6.At 80 °C,maximum conversion of styrene was obtained as 97.3%and maximum yield of styrene oxide is achieved as80.0%and that of benzaldehyde is 20.0%.Thus80°C temperature was selected for the further studies.
The estimated value of activation energy for Cu–Mn/SBA-15 catalyst was determined from the graph of ln(K)vs(1/T)(Fig.7).The value of activation energy for the present reaction was found to be 27.36 k J·mol−1i.e.the reaction rate is governed by chemical step.

Fig.7.Arrhenius plot for Cu-Mn/SBA-15 catalyst.

Fig.8.Effect of catalyst amount on styrene conversion at 80°C and 1:4 styrene to TBHP mole ratio.
3.7.Effect of catalyst amount
The effect of catalyst amount of styrene oxidation was explored by varying the catalyst amount from 10 to 30 mg,keeping the styrene to TBHP moleratio at 1:4 and temperature 80°C.At 10 mgcatalyst conversion was 96.23%and it was slightly increased to 97.3%at 20 mg and again decreased to 93.69%at 30 mg as shown in Fig.8.The probable reason for the decrease in styrene conversion at catalyst amount>20 mg can be explained by the decrease in effective reactant(styrene and TBHP)concentration on a catalyst particle for the higher catalyst amount i.e.30 mg.This decrease in reactant concentration on catalyst particle,where the reaction is actually taking place,may decrease the rate of reaction,which results in lower conversion i.e.92.27%[7].And the maximum yield of styrene oxide was 80.0%at 20 mg catalyst amount.Thus 20 mg was taken as the optimum value of catalyst for styrene oxidation.

Fig.9.Yields of styrene oxide and benzaldehyde at 80°C,20 mg catalyst amount and 1:4 styrene to TBHP mole ratio.

Table 2Optimized reaction conditions for styrene oxidation(comparative study of all catalysts)
Thus optimum reaction conditions for styrene oxidation have been found at styrene to TBHP mole ratio 1:4,reaction temperature 80°C and catalyst amount 20 mg.At these reaction conditions,maximum styrene conversion was97.3%and yield of styreneoxide and benzaldehyde were 80.0%and 20.0%respectively in 7 h.At optimum reaction conditions,the yield of styrene oxide(desired product)and benzaldehyde is shown in Fig.9.At 80°C,20 mg catalyst and 1:4 styrene to TBHP mole ratio,the yield of styrene oxide increased whereas benzaldehyde yield decreased with time.
Under these optimized conditions,Cu/SBA-15 and Mn/SBA-15 catalysts were also analyzed for oxidation of styrene and their results are summarized in Table 2.All the catalysts showed very high catalytic activity.The Cu metal exposed a prominent out come in the styreneoxidation in comparison with Mn metal,because of difference in oxidation states of Cu(+1,+2)and Mn(+2,+3,+4,+6,+7),therefore because of variable valences,Cu/SBA-15(more electronegative)is more proficient than Mn/SBA-15 in styrene oxidation.Cu-Mn/SBA-15 bimetallic catalyst showed higher catalytic activity than monometallic catalysts because of synergetic interactions between Cu and Mn metals.This synergism effect of bimetallic catalyst speeds up the catalytic rate and hence shown greater catalytic activity than monometallic catalysts[24].
3.8.Catalyst recycling
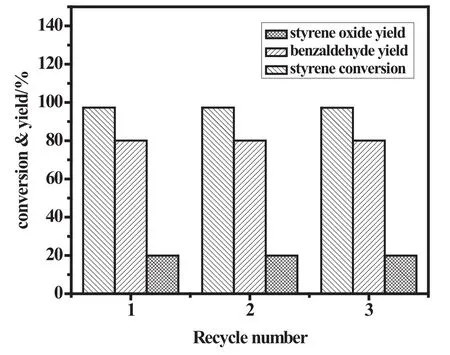
Fig.10.Conversion and yield dependency on recycle number.

Table 3Comparison of catalytic activity of catalysts deposited on different supports towards styrene oxidation
Recyclability of the heterogeneous catalyst is the main advantage and a key parameter that is necessary for the estimation of its catalytic performance and it can be done by studying the reusability of the catalyst.To confirm the recovery and reusability,Cu-Mn/SBA-15 catalyst after the experiment was separated from the reaction mixture by simple filtration and repeatedly washed with hot acetonitrile to remove adsorbed organic products followed by drying at 70°C.The recyclability of catalyst was assessed by carrying out three consecutive runs(Fig.10).It was found that catalyst could be used three times with slight decrease in conversion and yield.The recycling study implied the stability of synthesized catalyst and shown that catalyst could be reused several times without any significant loss in catalytic activity.
3.9.Comparison with other catalysts reported in literature
The catalytic activity of Cu-Mn/SBA-15 catalyst for the oxidation of styrene was compared with those reported in the literature and the results are summarized in Table 3.It was observed that the conversion of styrene using this catalyst is comparable to those reported in the literature.How ever,the selectivity of styrene oxide using present catalyst is much higher than any other reported catalysts.
4.Conclusions
The Cu/SBA-15,Mn/SBA-15,and Cu-Mn/SBA-15 were synthesized by co-precipitation method and characterized by BET,SEM-EDX,TEM,ICPAES and XRD techniques.These catalysts were used to catalyze the oxidation of styrene at various reaction conditions.At optimized reaction conditions,Cu-Mn/SBA-15 catalyst was found to be highly selective among all the catalysts with 97.3%conversion&80.0%styrene oxideyield and recyclability for this catalyst was three.Additionally,the activation energy of the oxidation reaction was calculated i.e.27.36 kJ·mol−1,which showed that the rate is truly governed by the chemical step.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Polyethoxylation and polypropoxylation reactions:Kinetics,mass transfer and industrial reactor design
- Inherently safer reactors and procedures to prevent reaction runaway☆
- Ag-Co3O4:Synthesis,characterization and evaluation of its photocatalytic activity towards degradation of rhodamine B dye in aqueous medium
- One-pot synthesis of 5-hydroxymethylfurfural from glucose over zirconium doped mesoporous KIT-6☆
- Selective propylene epoxidation in liquid phase using highly dispersed Nb catalysts incorporated in mesoporous silicates☆
- High-efficiency acetaldehyde removal during solid-state polycondensation of poly(ethylene terephthalate)assisted by supercritical carbon dioxide☆
