One-pot synthesis of 5-hydroxymethylfurfural from glucose over zirconium doped mesoporous KIT-6☆
2018-08-31ChongwenJiangJundongZhuBingWangLuLiHongZhong
Chongwen Jiang *,Jundong Zhu ,Bing Wang ,Lu Li ,Hong Zhong ,2
1 College of Chemistry and Chemical Engineering,Central South University,Changsha 410083,China
2 Hunan Provincial Key Laboratory of Efficient and Clean Utilization of Manganese Resources,Central South University,Changsha 410083,China
Keywords:Glucose One-pot Dehydration 5-HMF Hydrothermal Mesoporous KIT-6
A B S T R A C T Zirconium doped mesoporous KIT-6 samples with different Si/Zr ratios were synthesized by the direct hydrothermal method.Various characterization techniques confirm that highly distributed Zr O2 nanoparticles and multicoordinated Zr4+species are incorporated in the mesoporous composites.One-pot synthesis of 5-hydroxymethylfurfura(HMF)from glucose was examined in the presence of Zr-KIT-6(20)the molar ratio of Si to Zr is 20 under aqueous system.The effects of temperature,reaction time,catalyst dosage and biphasic solvent system on the conversion of glucose and the HMF yield were investigated.It was found that the glucose conversion and the HMF yield have been improved from 54.8%to 79.0%and from 19.5%to 34.5%in the biphasic MIBK-water system,respectively.Both the acidity of Zr-KIT-6(20)and the biphasic MIBK-water system are responsible for the improved performance of glucose dehydration to HMF.
1.Introduction
Growing concerns over global energy shortage,as well as the desire to protect the environment,have renewed the urgency for developing renewable energy.The dehydration of biomass derivatives to 5-hydroxymethylfurfural(HMF),a possible platform substitute of fuels and fine chemicals,has attracted increasing attention[1].The abundance and low price of glucose,which is the main building block of biomass,increase the interests in the methods for effective glucose dehydration to HMF.The most widely described route of glucose to HMF consists of two steps:(1)isomerization of glucose to fructose and(2)dehydration of fructose to HMF.The isomerization of glucose is extremely difficult to happen,which limits the production efficiency of HMF[2–4].A variety of catalysts have been developed for production of HMF from glucose,including mineral acid catalysts[5],ionic liquids[6,7],metal chloride[8],metal oxide[9],and solid acid catalyst[10,11].There into,heterogeneous catalysts are more suitable for industrial application due to their separability and reusability.Morales et al.[12]found that yield of HMF at 23%can be achieved from 10%glucose in aqueous media catalyzed by a zirconium containing mesoporous MCM-41 at 170°C.The activity was fully recovered after calcination at 500°C during 2 h.Zhang et al.reported a novel mesoporous niobium phosphate was synthesized by a hydrothermal method and obtained a HMF yield of 33.3%and 39.3%in aqueous reaction and H2O/MIBK biphasic systems,respectively[13].Xu et al.demonstrated that the yield of HMF from glucose reached 70%at 110°C by Sn-MCM-41 in ionic liquid[14].As reported above,it is believed that the combination of heterogeneous catalysis with water as a solvent in the synthesis of HMF from glucose offers an environmentally friendly way.
Because of their good structural feature,high surface area and relatively large pore size,the ordered mesoporous molecular sieves have been widely studied in order to develop high-active catalysts suitable for some particular reactions.Morales et al.have prepared a series of solid acid catalysts based on mesoporous MCM-41 silica containing Zr[12]and Al[15],and examined their catalytic activity in a biphasic reaction system.They found that the effective dehydration of glucose was due to the existence of acid sites of high concentration.Pan et al.designed and synthesized a novel zirconium-incorporated KIT-6,and the catalyst was highly active for dehydration of various substrates due to its high Lew is acid sites[16].Further research found that Lew is acid sites are very important for the isomerization of glucose and appropriate Brönsted acid sites are beneficial to the production of higher HMF yield in hydrolysis of glucose[17,18].However,achieving a highly selective catalytic system with a high HMF yield for the conversion of glucose into HMF still remains a great challenge[19,20].Moreover,considering the complexity of polysaccharide degradation,the reactive solvent system[21]and possible by-product formation[22]also need to be carefully considered.
Herein,this paper focused on the one-pot synthesis of HMF from glucose over zirconic doped mesoporous KIT-6 catalyst.The preparation of KIT-6 catalyst was described and characterized,as well as these materials as an efficient,reusable,solid acid catalyst for dehydration of glucose into HMF in an aqueous system and abiphasic MIBK–water system.Furthermore,the effect of various factors such as reaction temperature,time and catalyst dosage on glucose conversion and HMF yield was investigated in detail.The present Zr-KIT-6 catalyst shows excellent catalytic performance and reusability for the hydrolysis of glucose to HMF.
2.Experimental
2.1.Materials
Glucose(99.5%),tetraethoxysilane(TEOS,99%),methanol(99.9%)and acetonitrile(99.9%)were purchased from Sinopharm Chemical Reagent Co.,Ltd.Pluronic P123,fructose(99.5%),and 5-HMF(99%)were purchased from Aladdin Chemistry Co.,Ltd.Methyl isobutyl ketone(MIBK,99%),ZrOCl2·8H2O(99.8%)and n-butanol(99%)were obtained from Tianjin Kemiou Chemical Reagent Factory.Other chemical reagents are all analytically pure and without further treatment.
2.2.Preparation of catalysts
Zirconium doped KIT-6 samples were prepared using Pluronic P123 and n-butanol as the structure-directing mixture,TEOS and ZrOCl2·8H2O as the sources of Si and Zr,respectively.In atypical synthesis process,4.0 g of Pluronic P123 was dissolved in 144 g of distilled water with 7.4 g of 37 wt%HCl under 35°C for 4 h.Subsequently,4 g n-butanol was added to the clear solution as co-solute and continued the stirring for 1 h.After that,8.6 g TEOS along with the required amount of ZrOCl2·8H2O was slowly dripped into the solution and stirred at 35 °C for 24 h.The synthesis was carried out in an autoclave lined with Teflon and then aged at 100°C for another 24 h without stirring.The precipitated product was filtered without washing and dried at 100°C for 12 h in air.The dried solid product was crushed to get fine powder and calcined at 550°C inflowing air for 6 h to remove the template.The samples were denoted as Zr-KIT-6(x),where x was the molar ratio of Si/Zr.
2.3.Catalyst characterization
Powder X-ray diffraction patterns(XRD)of the synthesized samples were recorded on a Rigaku D/Max-2500/pc powder diffraction system using Cu-KD radiation(40 k V and 250 m A)over the range 0.5°≤ 2θ ≤ 4°(low angle).N2adsorption–desorption isotherms were obtained on a Micromeritics ASAP 2020 Gas Adsorption Analyzer at−196 °C.Prior to measurement,the samples were degassed in vacuum at 200°C for 5 h.Surface area and total pore volume were determined by using the Brunauer–Emmett–Teller(BET)equation and the Barrett–Joyner–Halenda(BJH)method,respectively.The pore size distribution was conducted by analyzing the desorption branch of the isotherm using the BJH method.FT-IR spectra of the samples were collected using an AVATAR-360 spectrometer.The UV–vis diffuse reflectance spectra were evaluated in the range of 190–800 nm at room temperature by a Shimadzu UV-2450A double-beam digital spectrophotometer with BaSO4as reference.XPS analysis was performed on ESCALAB 250Xi type X-ray photoelectron spectrometer produced by Thermo Fisher-VG Scientific.The total acid density of catalysts was analyzed by Temperature-Programmed Desorption(NH3-TPD)operated at ChemBET Pulsar TPD apparatus equipped with a thermal conductivity detector(TCD),which were heated from 100 °C to 550 °C at a heating rate of 10 °C·min−1under a flow of He gas.After cooling,the adsorption of ammonia is at 100°C for 30 min.Subsequently the temperature was increased from 100 °C to 700 °C at a heating rate of 10 °C·min−1and the desorbed NH3was monitored,as reported in the literature[16].The types of acid sites on the catalyst surface were observed on Pyridine FT-IR spectra using a Nicolet 380 FT-IR spectrophotometer equipped with an OMNIC series operating software and with deuterated triglycine sulfate(DTGS)detector in the range from 400 cm−1to 4000 cm−1with a resolution of 4 cm−1.Before measurement,the self-supporting wafers of catalyst(10 mg,15 mm diameter)was degassed at 350°C for 4 h under vacuum.Subsequently,adsorbed pyridine vapor(99.8%,3.3 kPa)was introduced into the cell at room temperature for 5 min and evacuation at 100 °C,200 °C,300 °C,in total for 30 min.The distribution of Brönsted and Lew is acid sites was calculated according to the area of the peaks centered at 1700 cm−1and 1400 cm−1,respectively,as reported in the literature[23].
2.4.Dehydration of glucose
The dehydration reactions of glucose were performed in a 150 ml Teflon-lined stainless steel autoclave,which was placed into a preheated oil-bath container with magnetic stirring.In a typical run,the predetermined amounts of glucose and catalyst were added into selected solvent.And then,the reactor was kept at a certain temperature for a given time.The timer started when the temperature of the reactor reached the desired value.At the end of the reaction,the reactor was introduced in a bath with ice-cold water.The liquid samples were separated, filtered and diluted before HPLC measurement.All experimental runs were repeated two times and the average was employed.Glucose was monitored by HPLC(Shimadzu LC-10AD)with Inertsil NH2(150× 4.6 mm,5 μm)and a refractive index detector,while HMF was followed using C18(250 × 4.6 mm,5 μm)and a UV detector.Glucose conversion and HMF yield were calculated according to

3.Results and Discussion
3.1.Characterization of KIT-6 and Zr-KIT-6(x)materials
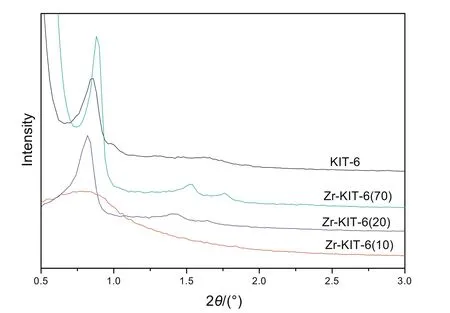
Fig.1.Low angle XRD patterns of KIT-6 and Zr-KIT-6 samples.
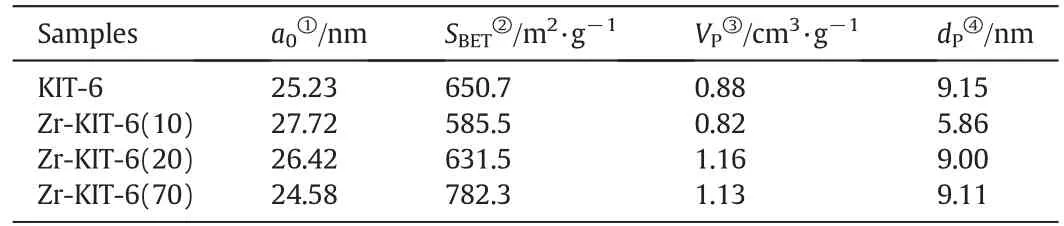
Table 1Physical properties of synthesized Zr-KIT-6 samples
Low angle XRD(2θ=0.5°–3°)patterns of KIT-6 and Zr-KIT-6 with three different Si/Ti ratios are shown in Fig.1.Most of the samples exhibit very similar patterns where a well-resolved intense peak around 0.8°–0.9°corresponds to the reflection of(211)planes,indicating that these samples possess a well-ordered three-dimensional mesoporous structure with cubic Ia3d symmetry[24].However,the intensity of the reflection peaks obviously decreases with the increase of the Zr loading.This might be due to the destructive interference of the mesoscopic order with increasing amount of Zr species.When the ratio of Si/Zr increases to 10,the XRD pattern has no recognizable diffraction peaks.The cubic unit cell parameter a0was calculated from the formula(see Table 1).When the ratio of Si/Zr decreases from 70 to 10,the unit cell parameter a0in creases from 24.58 to 27.72 nm due to the increase of Zr successfully incorporated on silica pore walls.
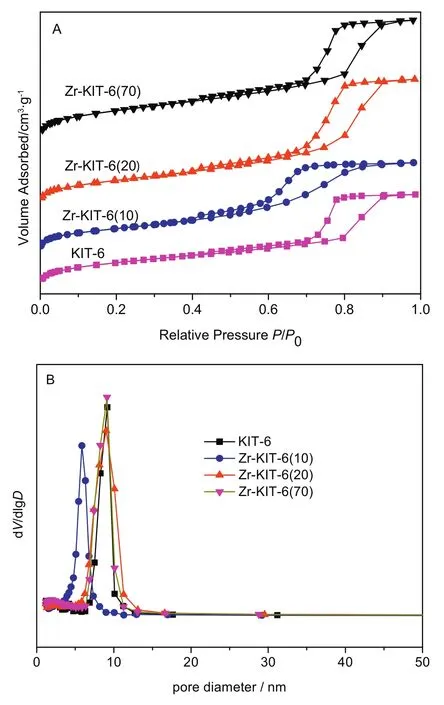
Fig.2.N2 sorption isotherms(A)and pore size distribution(B)of KIT-6 and Zr-KIT-6 samples.
N2sorption isotherms and pore sized istribution of Zr-KIT-6 samples are shown in Fig.2.The corresponding physical properties are also presented in Table 1.These isotherms are of type IV with H1 hysteresis loop according to the IUPAC classification,which is the obvious characteristic of mesoporous materials[25].The sharp steps of capillary condensation of N2are observed at high relative pressure(P/P0=0.7–0.9)in the isotherms of KIT-6,Zr-KIT-6(20)and Zr-KIT-6(70),indicating that these samples possess uniform pore size.Zr-KIT-6(10)displays a relatively broad hysteresis loop at P/P0=0.5–0.9,which presents a broad pore size distribution.The BJH desorption pore diameter of Zr-KIT-6 is reduced significantly at 10 of Si/Zr.It can be explained that excess Zr species are formed in the silica framework which cause the blockage of the pore and the decrease of pore size.All samples have a high surface area(585–782 m2·g−1)and large total pore volume(0.82–1.16 cm3·g−1).The BET surface area,total pore volume and pore diameter decrease as zirconium loading increases.This may be because the incorporation of Zr destroys the original highly ordered pore structure of KIT-6,which is consistent with the conclusion of XRD analysis.
Fig.3 presents FT-IR spectra of KIT-6 and Zr-KIT-6 with different Si/Zr ratios.Generally,the broad band at 3460 cm−1and narrow band at 1639 cm−1are attributed to stretching vibration and bending vibration of O--H in the sample surface water or hydroxyl groups.In pure silica KIT-6,the broad band around 1084 cm−1and narrow band at 960 cm−1are assigned to asymmetric Si--O--Si stretching mode and Si--OH group vibration,respectively[26].These bands broaden and merge together in the Zr-KIT-6 samples.The bending mode of Si--O--Si occurs at 460 cm−1,while 802 cm−1is due to symmetric Si--O--Si stretching mode.They also broaden and weaken with the increase of Zr loading.Do et al.reported similar results[27].This suggested that Zr is successfully incorporated within the framework of KIT-6.
The UV–vis DRS spectra of KIT-6 and Zr-KIT-6 samples are shown in Fig.4.According to previous researches[16,28],the absorption band in the 205–215 nm range is attributed to ligand-to-metal charge transfer(LMCT)from an O2−to an isolated Zr4+ion in a tetrahedral configuration.The broadening of the bands is noticed in all Zr-KIT-6 samples,and it is probably because of the presence of more than one type of Zr species.In Zr-KIT-6(20),a quite weak absorption band around 248 nm is observed,which may be attributed to the multi-coordinated Zr4+species[29].The broad absorption band of low intensity at 278 nm in all Zr-KIT-6 samples indicates that highly distributed ZrO2nanoparticles exist[16].
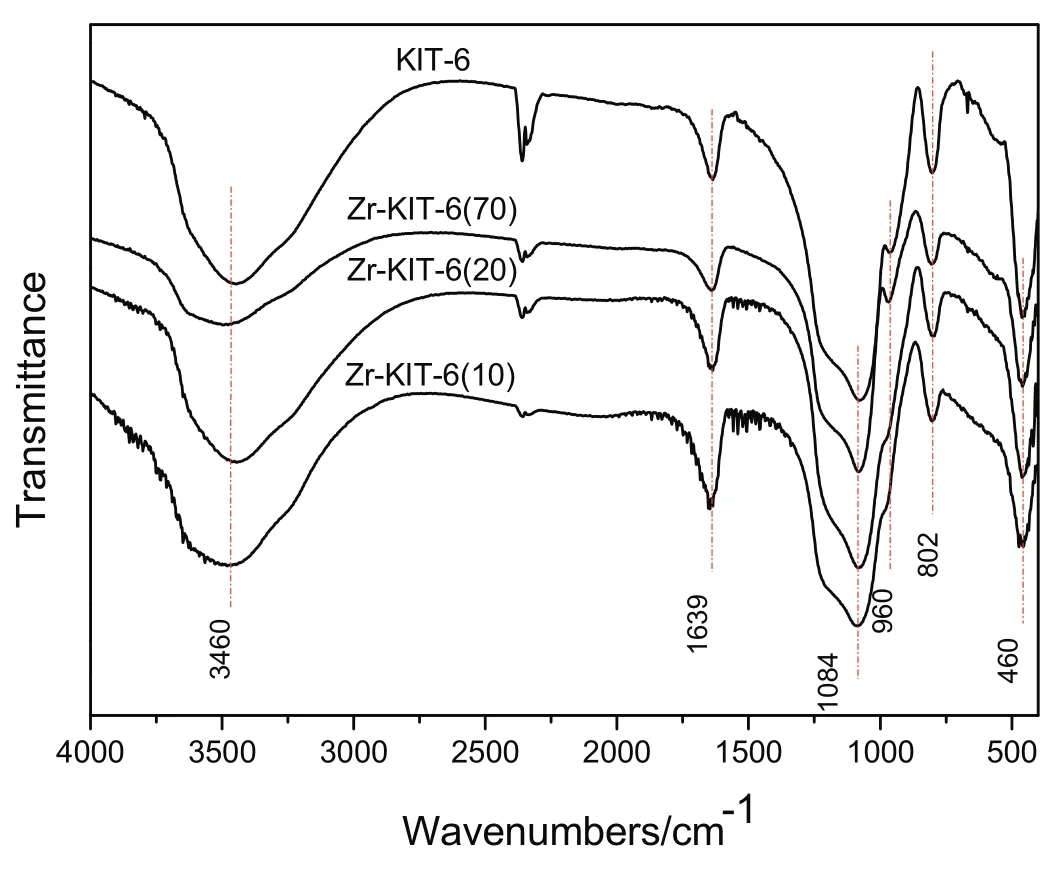
Fig.3.FT-IR spectra of KIT-6 and Zr-KIT-6 samples.
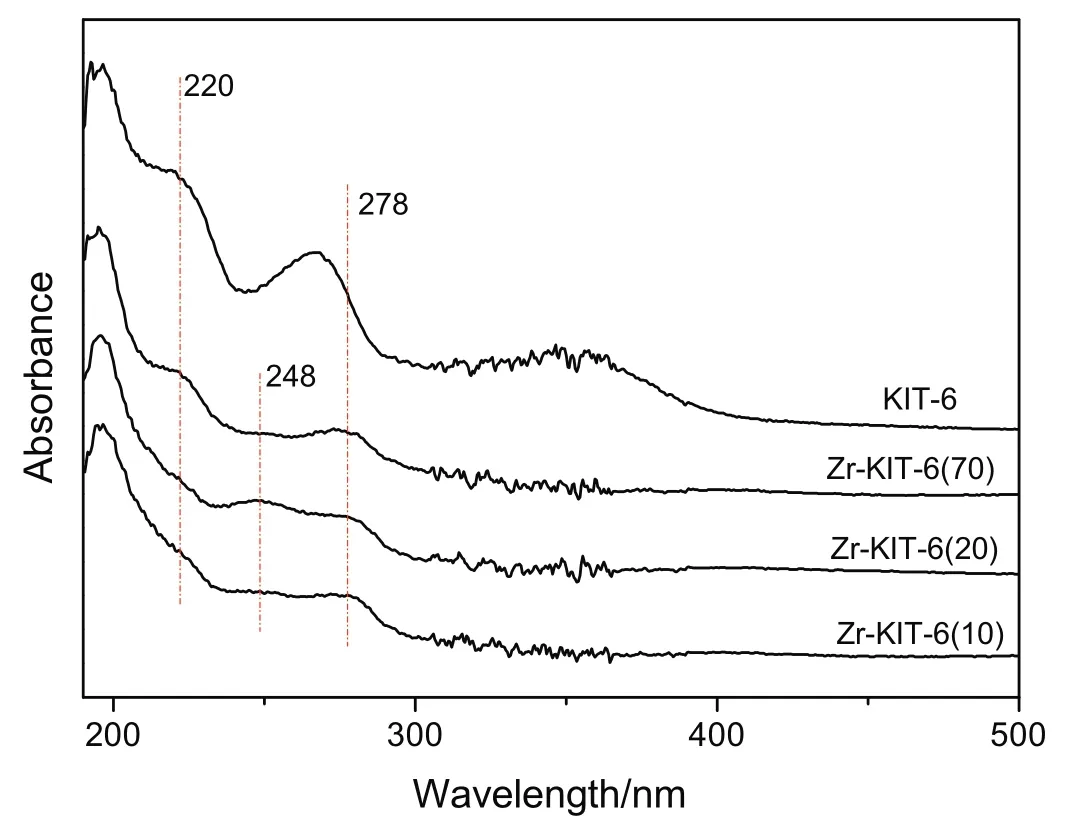
Fig.4.UV–vis DRS spectra of KIT-6 and Zr-KIT-6 samples.
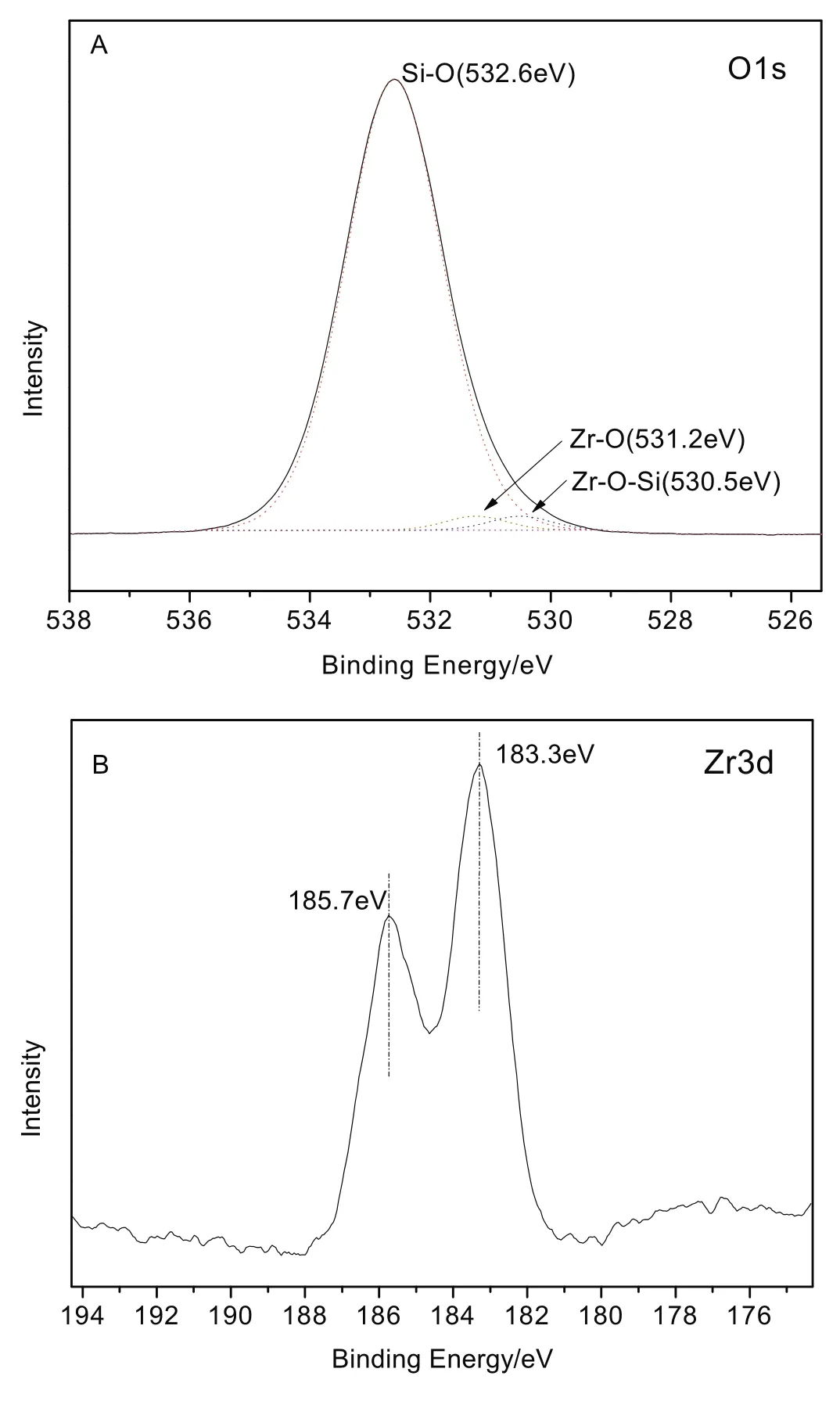
Fig.5.O1s XPS spectra(A)and Zr 3d XPS spectra(B)of Zr-KIT-6(20).
To further evaluate the different environments of zirconium species and the adjacent oxygen atoms,Zr-KIT-6(20)is measured by XPS as a representative sample.Fig.5 depicts the O1s XPS spectra(A)and Zr 3d XPS spectra(B)of Zr-KIT-6(20).As shown in Fig.5(A),the peak of O 1s spectrum demonstrates the presence of one main O environment,and the higher binding energy of O 1s peak is fitted into O--Si--O bond at 532.6 eV,which is assigned to the oxygen atoms existed as O2−species at the silicate framework[30,31].Besides,the lower binding energy originated from the formation of new bonds(Zr--O in pure Zr O2at 531.2 eV[29],Zr--O--Si at 530.5 eV[32]),which are mainly associated with mesoporous KIT-6 frame work because the Zr percentage is estimated to be 20%.In addition,with regard to Zr 3d XPS spectra in Fig.5(B)the peaks of Zr 3d at binding energies of 183.3 and 185.7 eV,corresponding to the Zr 3d5/2 and 3d3/2(resulted from the spin–orbit splitting),respectively[33,34],are well consistent with the binding energy values of Zr4+in ordered KIT-6 mesoporous molecule[35,36].All the results mentioned above indicated that the+4 oxidation state of Zr was successfully doped on the KIT-6 framework by the hydrothermal method.
3.2.Acid/base properties
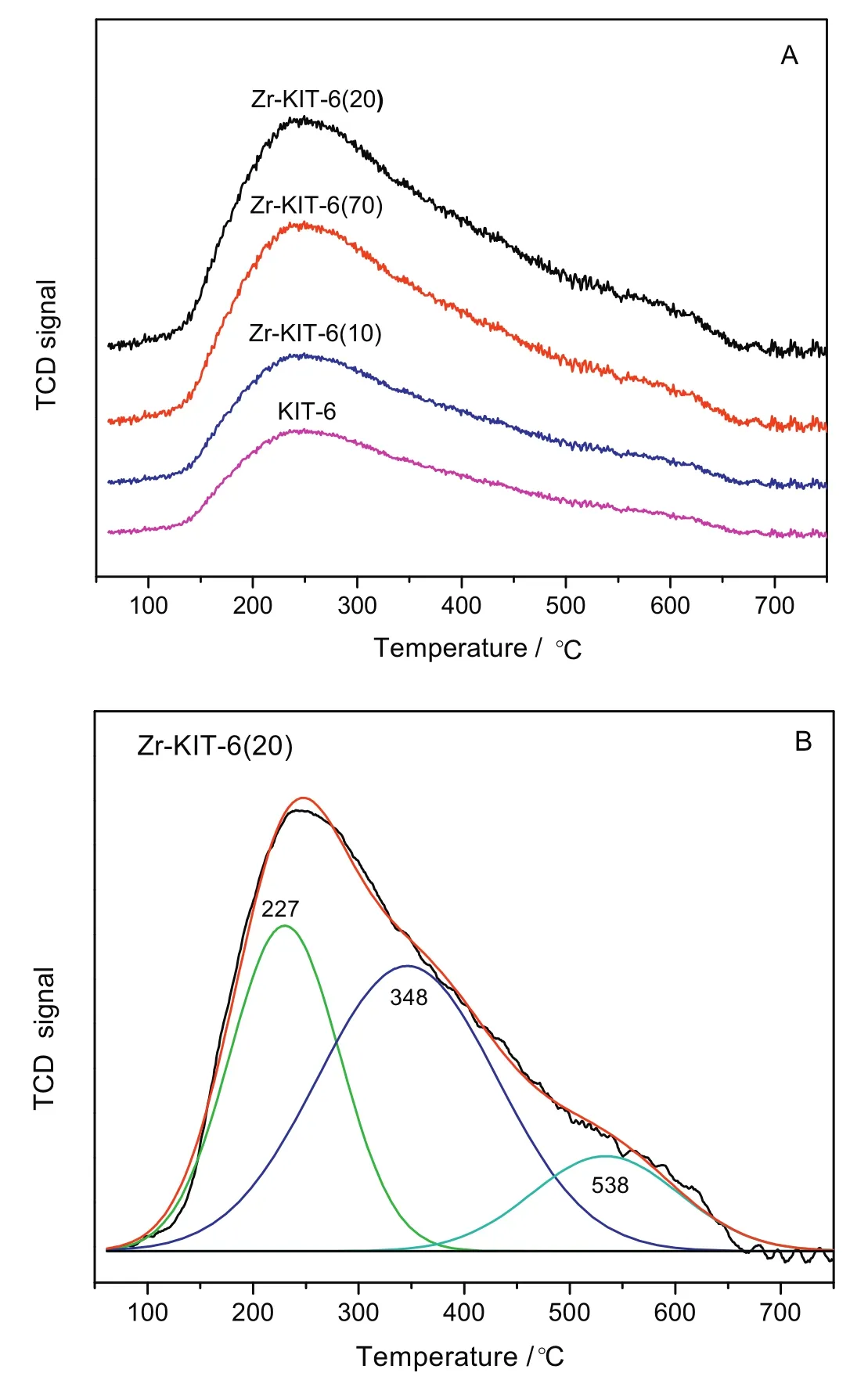
Fig.6.NH3-TPD of KIT-6,Zr-KIT-6(x)samples(A)and fitting curve(B)of Zr-KIT-6(20).
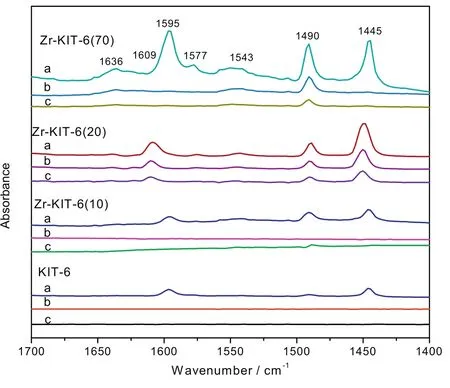
Fig.7.FTIR spectra of adsorbed pyridine over KIT-6 and Zr-KIT-6(x)samples at 100°C(a),200 °C(b)and 300 °C(c).
As we know,the acidity and acid species on solid acid catalysts play an important role in the hydrolysis of glucose.Therefore,NH3-TPD of KIT-6 and Zr-KIT-6(x)materials was conducted and the collected spectra are illustrated in Fig.6.Generally,these catalysts exhibit a combination of Brönsted and Lew is acid sites in the range of 150 °C and 300 °C[22],and total acidity enhancement increases and then decreases with the increase of the Zr content.Among all the catalysts,Zr-KIT-6(20)showed the highest total surface acidity.On the other hand,the peak in the NH3-TPD curve can be factored into different acid intensity peaks,as can be seen from Fig.6(B).The desorption of ammonia at different temperatures associated with acid sites with varying strengths.The peak at 227°C is associated with the NH4+adsorbed by the acid hydroxyl existence on the surface of the Zr-KIT-6(20),another two peaks at round 340 °C and 478 °C corresponded to the medium strong and very strong acid sites,respectively[23].This indicates that Zr-KIT-6(20)on the surface of the zirconium is a strong acid catalyst.
To gain a better understanding of these Brönsted and Lew is acid sites distributed,we studied these pyridine-adsorbed FTIR spectra of KIT-6 and Zr-KIT-6(x)materials at 1700–1400 cm−1(Fig.7).Normally,the weak absorption band at around 1490 cm−1and 1577 cm−1is attributed to the combination of Brönsted and Lew is(B+L)acid sites[23,35],while the peak at 1596 cm−1,1445 cm−1,1609 cm−1and 1543 cm−1,1686 cm−1assigned to Lew is acid(L)sites and Brönsted acid(B),respectively[35,36].In addition,the Lew is acid sites and Brönsted acid of catalysts decreased with an increase in the desorption temperature from 100 °C to 300 °C.With respect to pure KIT-6 catalyst,only two peaks correspond to Lew is acid sites.A band at 1636 cm−1and 1543 cm−1is observed for the Zr-KIT-6(20)and Zr-KIT-6(70),which are due to the strong interaction between Zr4+and molecular sieve frameworks[30,37].In addition,the blockage of channel for heavily Zr-doped KIT-6(10)results in deactivation of Lew is acid sites,being consistent with the observation from the NH3-TPD.
3.3.Catalytic activity of Zr-KIT-6
It is well established that many characteristics of Zr-KIT-6 catalysts,such as composition,crystalline phase,structure,and acid sites,would greatly influence the performance of glucose degradation to 5-HMF.On the basis of the above-mentioned analytical results,it can be concluded that the structure of Zr-KIT-6(20)containing higher acid densities as catalytic site and a larger specific surface area for proton transfusion was mainly responsible for its better catalytic performance in glucose hydrolysis.As illustrated in Fig.8,the Lew is acid site with an uncoordinated Zr4+defect center occupies a decisive position in glucose isomerization to fructose,and the Brönsted acid site from silanol groups on molecular sieve frameworks during the subsequent acid hydrolysis into more HMF[19,38,39].Moreover,these findings reveal an important new strategy for realizing the commercial production of HMF.Hence,Zr-KIT-6(20)was evaluated as an efficient acid catalyst for the conversion of glucose into HMF in the present catalytic study.
The reactions were mainly performed in the aqueous system.Firstly,the influence of reaction temperature on the catalytic reaction has been evaluated.Fig.9 shows the change trend of glucose conversion and HMF yield in a temperature range from 110 to 170°C.It is found that glucose conversion increases dramatically with the increase of temperature.HMF yield is also positively related to temperature.The maximum values of glucose conversion and HMF yield(49.0%and 16.3%,respectively)are achieved at 170°C.Higher temperature can promote the dehydration of glucose into HMF in the experiments.
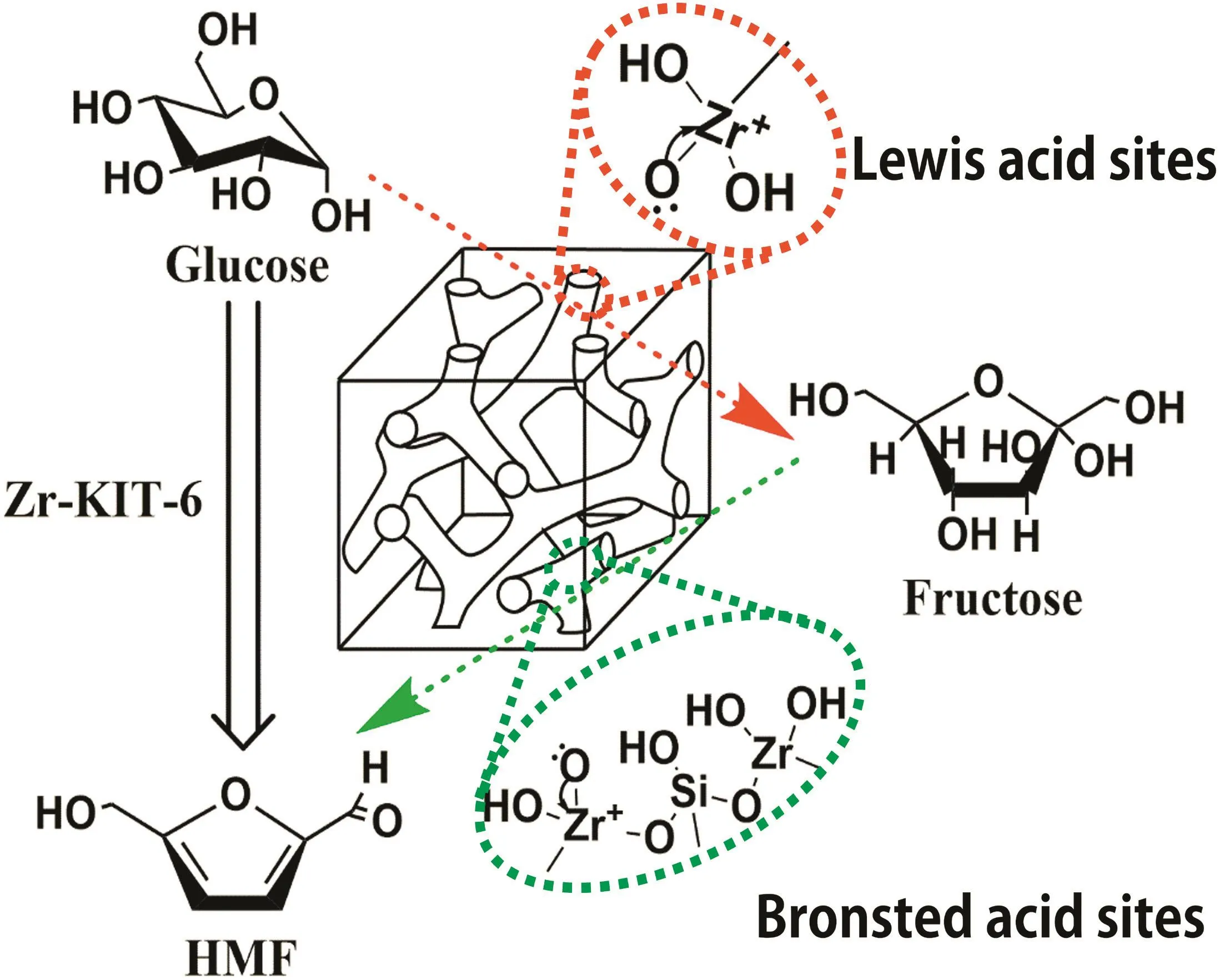
Fig.8.Plausible mechanism for the hydrolysis of glucose over Zr-KIT-6.
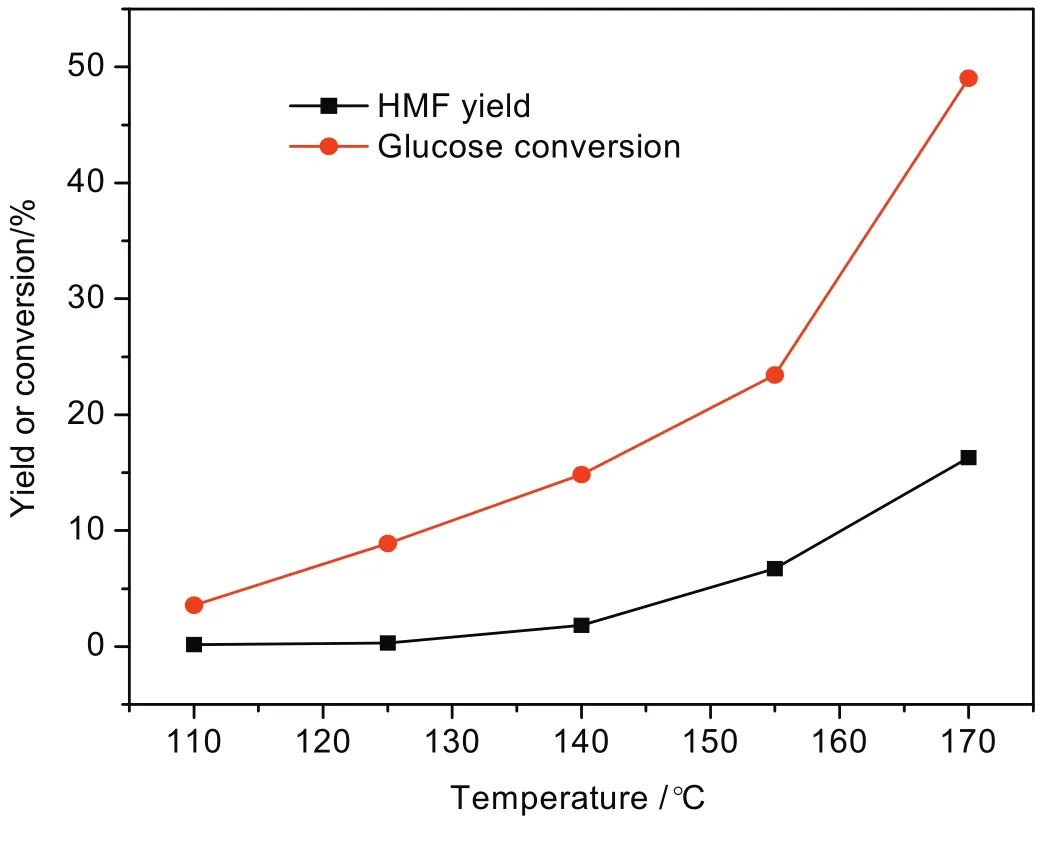
Fig.9.Effect of reaction temperature on glucose conversion and HMF yield.[Glucose:2 g,Zr-KIT-6(20):0.5 g,H2O:50 ml and reaction time:2.5 h.]
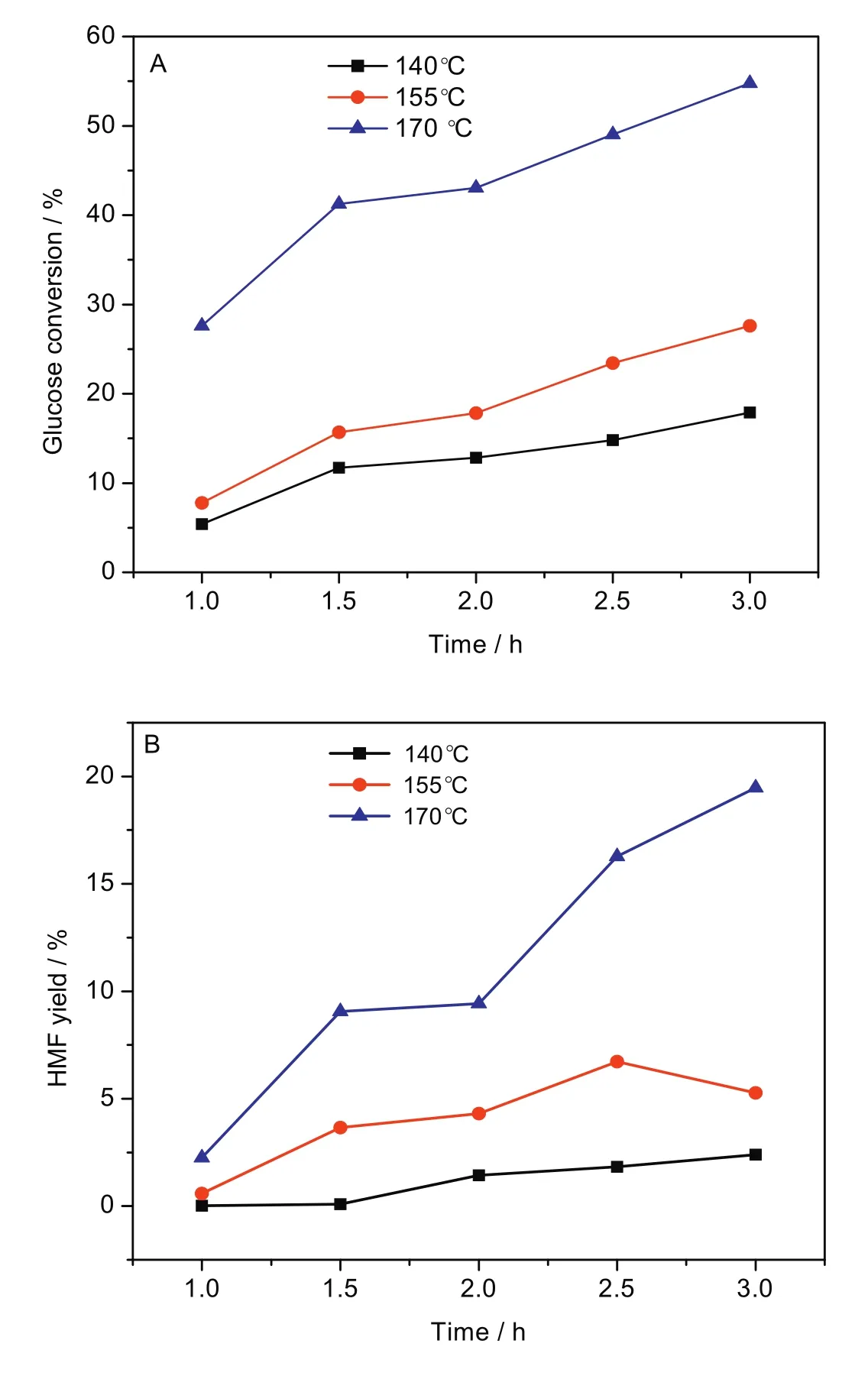
Fig.10.Effect of reaction time on glucose conversion(A)and HMF yield(B).[Glucose:2 g,Zr-KIT-6(20):0.5 g,H2O:50 ml.]
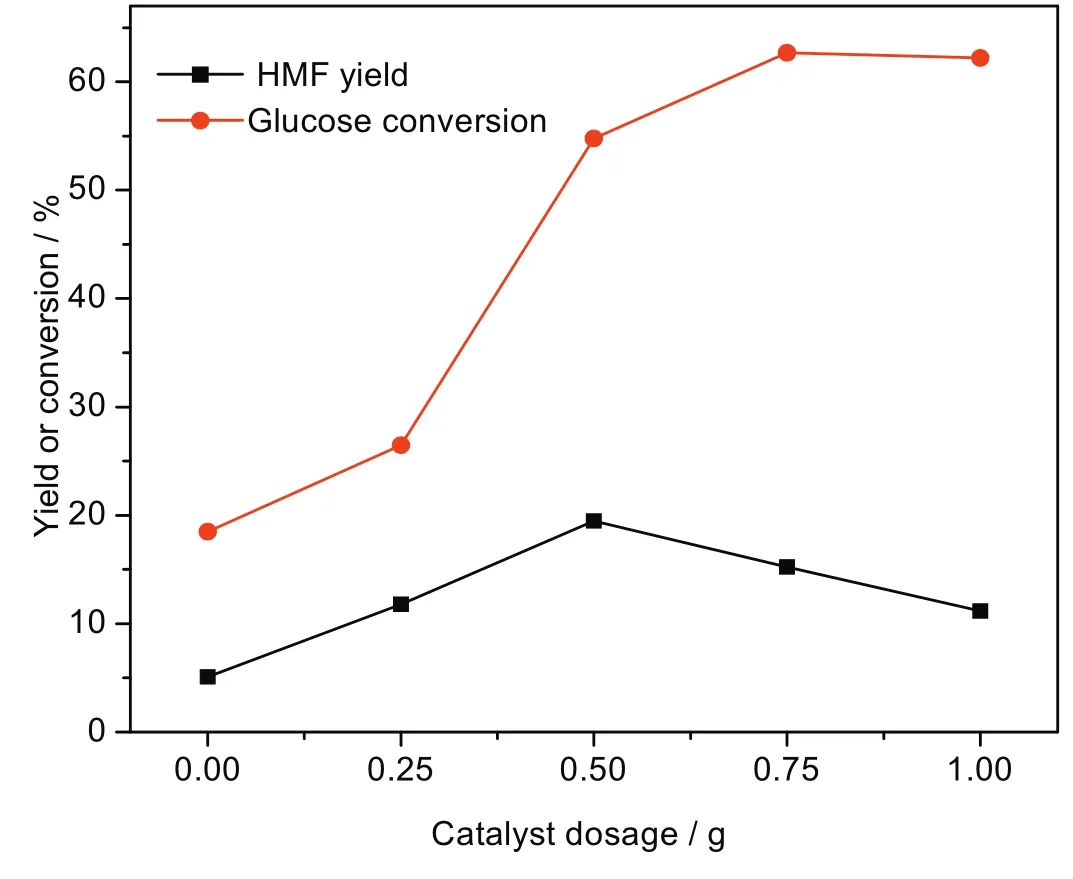
Fig.11.Effect of catalyst dosage on glucose conversion and HMF yield.[Glucose:2 g,Zr-KIT-6(20):0.5 g,H2O:50 ml reaction temperature:170°C and reaction time:3 h.]
Fig.10 shows the effect of reaction time on glucose conversion(Fig.10A)and HMF yield(Fig.10B).The catalytic performance of Zr-KIT-6(20)was further investigated at three different temperatures(140,155,170°C)with the reaction time(from 1.0 h to 3.0 h).The results reflect that glucose conversion rises with reaction time at the same temperature.Compared with the other two lower temperature,Zr-KIT-6(20)showed much higher catalytic activity at 170°C.Both glucose conversion and HMF yield are much more than the corresponding results at the same reaction time.The maximum glucose conversion(54.8%)and HMF yield(19.5%)are obtained at 170°C after 3 h.
Fig.11 demonstrates the effect of catalyst dosage on glucose conversion and HMF yield.Glucose conversion remarkably increases as catalyst dosage increases from 0 to 1.0 g.However,the HMF yield increases from catalyst dosage of 0 to 0.5 g and then decreases when it is more than 0.5 g.This could be explained by the formation of a higher amount of condensation products from the intermediates of glucose/fructose with HM For the acetalization of glucose with HMFin the aqueous medium[1].In addition,the humins formed in the aqueous medium may cover active sites of the catalyst,leading to its partial deactivation.
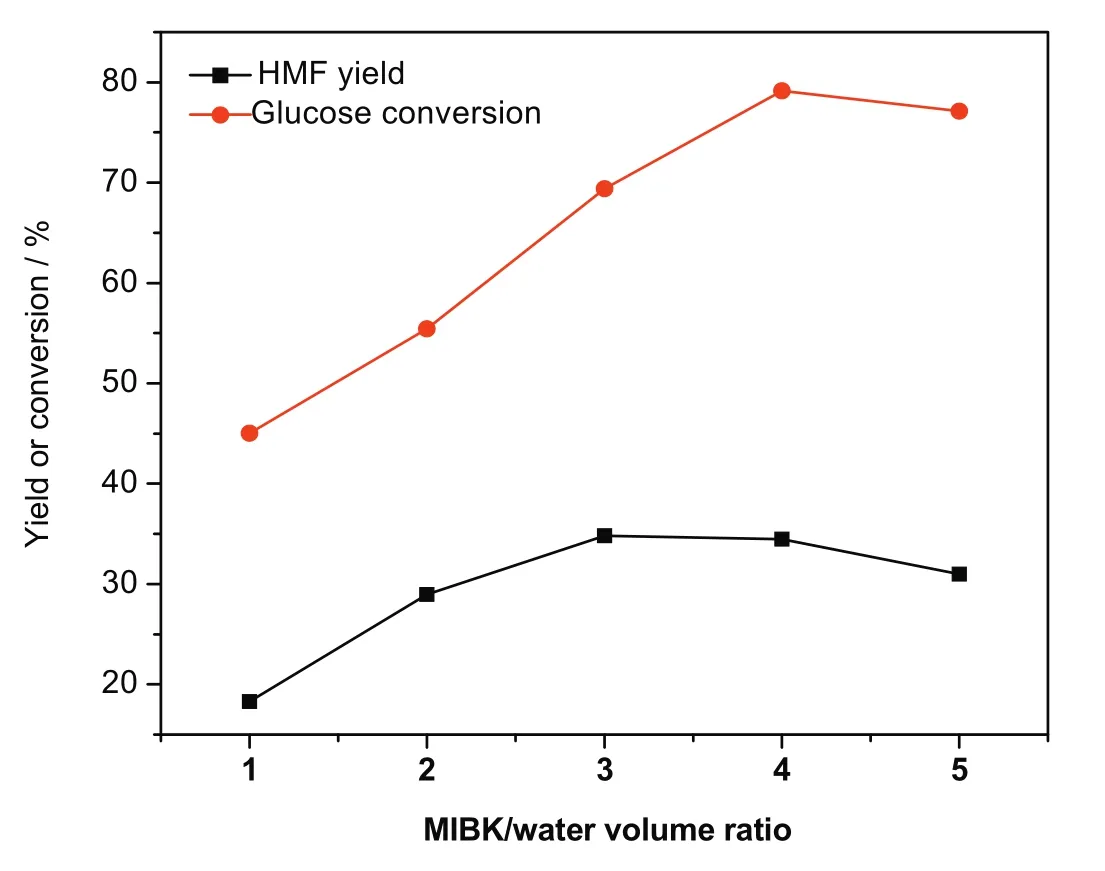
Fig.12.Effect of MIBK/water ratio on glucose conversion and HMF yield.[Glucose:2 g,Zr-KIT-6(20):0.5 g,H2O:50 ml reaction temperature:170°C and reaction time:3 h.]
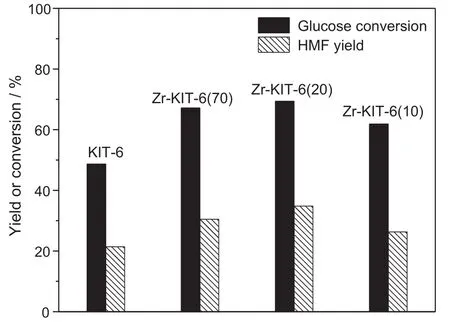
Fig.13.Effect of molar ratio of Si/Zr on glucose conversion and HMF yield.[Glucose:2 g,MIBK/water ratio:3,reaction temperature:170°C and reaction time:3 h.]
A biphasic solvent system,consisted of MIBK and water,was employed.MIBK was introduced to extract the HMF formed from the reactive aqueous phase,thereby suppressing the undesired side reactions to some extent.The whole volume of the reaction medium and glucose concentration were constant(60 ml,40 mg·ml−1).The effect of MIBK/water ratio on glucose conversion and HMF yield was investigated.As depicted in Fig.12,an increase in the MIBK/water ratio results in the increase of glucose conversion and HMF yield.Glucose conversion of 79.0%and HMF yield of 34.5%w ere achieved when the MIBK/water ratio was 4.Both glucose conversion and HMF yield slightly decrease as the MIBK/water ratio further increases.This could be explained by the fact that the small volume of glucose solution was dispersed into MIBK through vigorous stirring with shorter contact time between glucose and catalyst,which benefits from the selective dehydration of glucose to HMF.Moreover,excessive catalyst may be responsible for the less HMF yield.
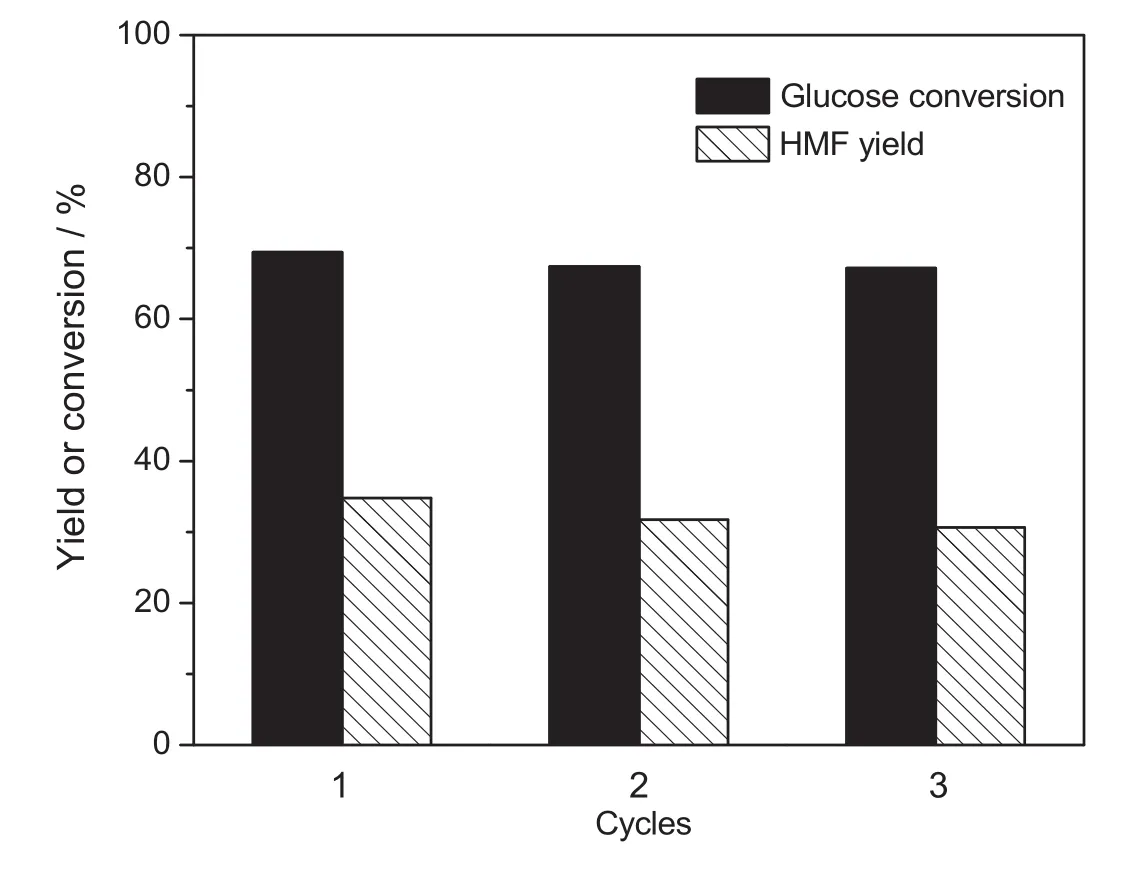
Fig.14.Reutilization of Zr-KIT-6(20).[Glucose:2 g,MIBK/water ratio:3,reaction temperature:170°C and reaction time:3 h.]
Fig.13 shows the effect of the addition of Zr amount on the dehydration of glucose to HMF.Both the glucose conversion and HMF yield increase if Zr is doped into the KIT-6 catalyst.Zr-KIT-6(20)presents the highest activity among all studied KIT-6 catalysts because Zr-KIT-6(20)has the strongest Lewis acid and Brönsted acid.However,it is interesting to note that Zr-KIT-6(70)shows the comparable HMF yield with Zr-KIT-6(20)in spite of lower conversion of glucose.Thepossible explanation for higher selectivity of HMF is that strong Brönsted acid in Zr-KIT-6(70)may benefit from the dehydration of fructose into HMF after the formation of fructose dehydrated from glucose.
Zr-KIT-6(20)as heterogeneous catalysts in this study can be easily recovered via simple filtration,followed by rinsing with water,drying and calcinating at 550°C for 3 h.The reusability of Zr-KIT-6(20)was also examined,and the result was shown in Fig.14.The stability of Zr-KIT-6(20)was assessed at a constant initial reaction condition.The recycling catalysts were found to maintain catalytic activities with little loss of activity after three runs.The results show that the catalytic activity of Zr-KIT-6(20)is basically stable and has good prospects for the dehydration of glucose into HMF.
4.Conclusions
Zirconium doped mesoporous KIT-6 catalyst has been prepared for the dehydration of glucose into HMF in a one-pot method.Highly distributed Zr O2nanoparticles and multi-coordinated Zr4+species in Zr-KIT-6 samples were confirmed through various characterizations.In the biphasic MIBK–water system,79.0%conversion of glucose and 34.5%HMF yield were optimally obtained in the presence of Zr-KIT-6(20)catalyst prepared in the mild condition.Both the acidity of Zr-KIT-6(20)and the biphasic system are responsible for the improved performance of glucose dehydration to HMF.The used catalyst could be easily generated by calcination at 550°C for 3 h,and was found to maintain catalytic activities after recycling.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Polyethoxylation and polypropoxylation reactions:Kinetics,mass transfer and industrial reactor design
- Inherently safer reactors and procedures to prevent reaction runaway☆
- Ag-Co3O4:Synthesis,characterization and evaluation of its photocatalytic activity towards degradation of rhodamine B dye in aqueous medium
- Selective propylene epoxidation in liquid phase using highly dispersed Nb catalysts incorporated in mesoporous silicates☆
- High-efficiency acetaldehyde removal during solid-state polycondensation of poly(ethylene terephthalate)assisted by supercritical carbon dioxide☆
- A phase inversion based sponge-like polysulfonamide/SiO2 composite separator for high performance lithium-ion batteries☆
