2,4-二羟基苯甲酸辅助合成不同形貌二氧化铈及其在NH3-SCR中的应用
2018-08-01周诗健
苏 航 徐 蔓 周诗健 杨 福 孔 岩
(南京工业大学化工学院,材料化学工程国家重点实验室,南京 210009)
0 Introduction
In recentdecades,metaloxideshave been intensely applied in the fields of catalysis[1-2],sensor[3-4],lithium-ion battery[5-6],etc.Particularly,metal oxides with controlled shapes have positive effect in heterogeneous catalysis system.Some related properties,such as the specific architecture and high surface area have important influence on the anchoring of the active sites and controlling the diffusion of reactants.With the well-defined morphologies,the researchers can not only adjust the required physicochemical properties but also improve the catalytic performance[7].Therefore,it is of great significance to explore facile and controllable routes for designing the desired structures.
As well known,nitrogen oxides remain the major pollution source in the air,which could result in the formation of photochemical smog,ozone depletion and acid rain[8].These situations are directly harmful to ecological environment and human health.In order to address this dilemma,some effective approaches are expected.Fortunately,selective catalytic reduction of NO with NH3has been regarded as one of the considerable technologies for the abatement of NO(NH3-SCR),and the process can be described by the following equation:

Besides,as one of the metal oxides,ceria(CeO2)has been continually focused owing to the highly oxygen storage capacity,enhanced metal dispersion and facile stabilization of the support[9-10].Meantime,CeO2can also serve as an outstanding component in the field of selective catalytic reduction of NO with NH3[11].However,the CeO2-based materials have been significantly demonstrated that the morphologies are closely associated with the physicochemical properties.Typically,for the pure CeO2,hollow ceria nanosphere with multiple shells exhibited distinguishable photocatalytic activity in water oxidation[12].With regard to the hybrid CeO2-based materials,core-shell Pd@CeO2nanostructures were found to exhibit excellent catalytic activity in NO reduction[13].Recently,in the system of NH3-SCR,Li et al.reported that the novel MnOx-CeO2nanosphere showed superior activity than the non-structured MnOx-CeO2catalyst[14].Hybrid multi-shell hollow structured CeO2-MnOxwas designed and found that this material displayed excellent catalytic activity compared to the traditional CeO2-MnOxnanoparticles or single-shell hollow spheres[15].Although great efforts have been achieved,there is still room for controllable synthesis of CeO2with specific morphologies and apply to NH3-SCR.
To control the morphology of CeO2,some efficient methods,such as hydrothermal[16],polyol method[17],template method[18]and colloidal solution combustion[19]have been adopted.While,in consideration of the tunable reaction parameters,such as different temperatures and additives,the hydrothermal method could be considered as a promising route.Notably,benzoic acid compoundshavebeen achieved toprepare various polymers,pharmaceuticals and metal-organic framework particles (MOFs).Especially,Fan et al.[20]reported CuFe2O4@HKUST-1 heterostructureswith MOFs shell were constructed by 1,3,5-benzenetricarboxylic acid.Korpany et al.[21]explored a series of benzoic acid derivatives to fabricate surface functionalized iron oxide nanoparticles.Plentiful functional materials have opened a doorto discoverthe relationship between benzoic acid derivatives and nanomaterials.Therefore,choosing the appropriate method as wellas the suitable benzoic acid compounds play an important role in the synthesis of desirable nanoparticles.Whereas,to the best of our knowledge,it is still lack the research in controlling synthesis of nanocrystallines including CeO2by using benzoic acid derivatives.
Herein,the rod-like and sheet-like CeO2were successfully synthesized with the assistant of 2,4-dihydroxybenzonic acid.The selected 2,4-dihydroxybenzonic acid could be completely dissolved in the reaction system to form a homogeneous solution.Timedependentand temperature-dependentexperiments were carried out to study the growth mechanism of the CeO2.Besides,the physicochemical properties of the CeO2were investigated.In addition,the catalytic activity of the as-prepared CeO2was evaluated in the system of NH3-SCR.
1 Experimental
The rod-like CeO2was synthesized via the hydrothermalmethod and the calcination procedure.Typically,2 mmol of Ce(NO3)3·6H2O and 3.5 mmol of 2,4-dihydroxybenzoic acid (DHBA)were dissolved in the mixed solution of 15.0 mL ethanol and 30.0 mL deionized water.Then 5.0 mL of sodium acetate solution (0.5 mol·L-1)was added dropwise into the mixed solution with stirring.The obtained homogeneous solution was transferred into a Teflon-lined autoclave and heated at 180℃for 24 h in an electric oven.Moreover,the autoclave was cooled to room temperature,the precipitate wascentrifuged and washed with deionized water for four times and dried at 60℃for 6 h.Then the precursor(Ce-Pre-1)was calcined in air atmosphere at 500℃with a heat ramp rate of 2℃·min-1for 4 h,and the calcined products were named as Ce-Cal-1.
Instead,the sheet-like CeO2was obtained according to the similar process of rod-like CeO2by increasing the amount of DHBA to 5 mmol,and the hydrothermal products and the calcined products were labeled as Ce-Pre-2 and Ce-Cal-2,respectively.
Time-dependent experiments for the precursors of Ce-Cal-1 and Ce-Cal-2 were carried out at different hydrothermal intervals of 3,6,10 and 21 h without changing other reaction parameters.The temperaturedependent experiments of Ce-Cal-2 were performed at 120,140 and 160℃,respectively.
X-ray diffraction patterns (XRD)was used for characterizing the phase purity with a monochromatic Cu Kα radiation source (λ =0.154 178 nm)and operated at 40 kV and 100 mA in the range of 10°~80°.Field-emission scanning electron microscopy(FESEM)was performed on a Hitachi S4800 Field-Emission Scanning Electron Microscope and operated at 5 kV.High-resolution transmission electron microscopy(HRTEM)images were recorded on an EM-2010 EX microscope with the accelerating voltage at 200 kV.The N2adsorption-desorption isothermswere carried out in the relative pressure (P/P0)range from 0.01 to 0.99,and the surface area of samples were calculated by Brunauer-Emmet-Teller equation(BET).Temperature-programmed reduction under H2environment(H2-TPR)was carried out on a TP-5000 instrument.50 mg CeO2was pretreated under He-O2stream at 500℃for 1 h.After cooling down to room temperature,the catalyst was purged with 30 mL·min-1of He for 30 min to remove the excess O2.Then the flow of 5%H2-He was introduced into the sample with a flow rate of 30 mL·min-1and the temperature was raised to 950℃ at a rate of 10℃·min-1.The acidity of the CeO2was measured by NH3temperature programmed desorption (NH3-TPD)in the same instrument as the H2-TPR.Prior to TPD experiment,100 mg CeO2was pretreated at 300℃for 30 min and cooling to 50℃under argon flow.The sample was exposed to a flow of 2.500 g·L-1NH3/Ar(50 mL·min-1)at 100 ℃for 1 h,followed by argon purging for another 1 h.Then,the temperature was raised to 950℃in argon flow at the rate of 10℃·min-1.Thermogravimetry and differential scanning calorimetry (TG-DSC) was measured by a NETZSCH STA 409 instrument with a heating rate of 10℃·min-1under nitrogen atmosphere.Fourier transform infrared (FT-IR)spectra of the samples were obtained in the range of 4 000~500 cm-1with powders dispersed in KBr on Bruker VECTOR22 resolution.
The catalytic conversion of NO was measured via a fixed-bed reactor with 0.2 g pure CeO2(40~60 mesh)as catalyst.The feed gas contained 500 mg·L-1NH3,500 mg·L-1NO,5%(V/V)O2,5%(V/V)H2O,with N2as the balance gas.The total flow rate of the feed gas was 200 mL·min-1,corresponding to a space velocity of 60 L·g-1·h-1.The concentration of NO was detected by an onlin e Thermo fisher IS10 FTIR spectrometer equipped with a 2 m path-length gas cell(250 mL).The NO conversion can be calculated by NO conversion=(cNO,in-cNO,out)/cNO,in×100%.
2 Results and discussion
Fig.1(a)and Fig.1(e)show the X-ray diffraction patterns(XRD)of Ce-Cal-1 and Ce-Cal-2.The diffraction peaks at ca.28.5°,32.9°,47.3°,56.2°,59.1°,69.4°,76.5°,78.7°are well indexed to the facecentered cubic CeO2(fcc-CeO2,PDF No.34-0394),implying the samples are not amorphous.Fig.1(b)depicts the typical rod-morphology of Ce-Cal-1 with the average width of 100~300 nm and the average length of 500 nm~1.5 μm.The TEM image in Fig.1(c)also reveals the rod-like profile of CeO2.Fig.1(d)presents the corresponding HRTEM image of Ce-Cal-1.As shown in Fig.1(d),the lattice fringe spacing of 0.27 and 0.31 nm correspond to (200)and(111)diffraction planes of CeO2,respectively.On the other hand,Ce-Cal-2 displays the sheet morphology with the thickness below 80 nm and the length can reach to 700 nm(Fig.1(f)and Fig.1(g)).Theobvious lattice fringe spacing of 0.31 nm in Fig.1(h)matches well with (111)diffraction plane of CeO2.In addition,the SAED profiles manifest the typical single crystal,and some defects of the resulted CeO2could be discovered(marked as green rectangles).Therefore,the rod-like and sheet-like CeO2are successfully synthesized in this case.

Fig.1 XRD patterns(a,e),SEM images(b,f),TEM images(c,g),HRTEM images(d,h)with the corresponding SAED(inset)of Ce-Cal-1 and Ce-Cal-2,respectively
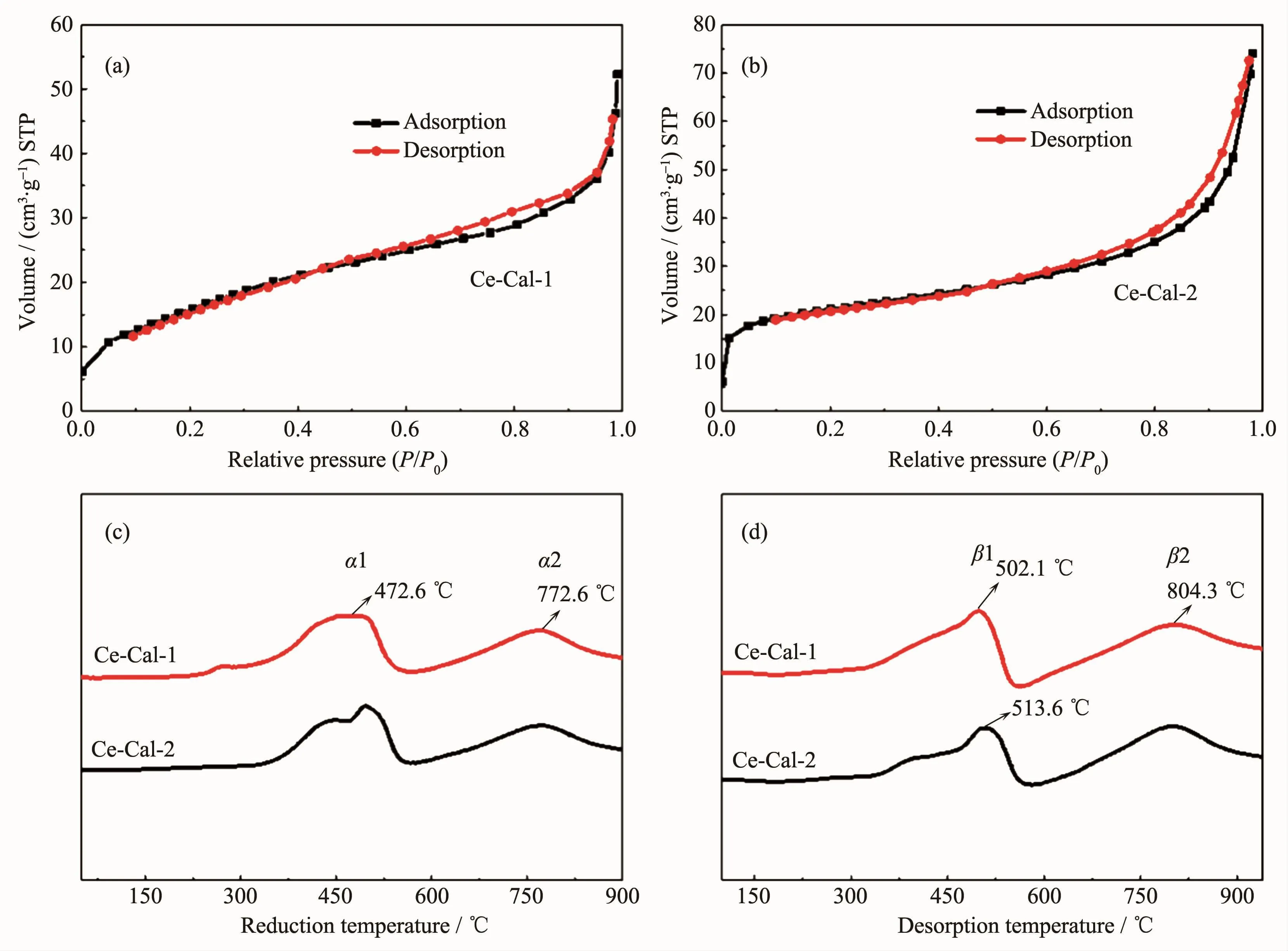
Fig.2 N2adsorption-desorption isotherms(a,b),H2-TPR(c)and NH3-TPD profiles(d)of Ce-Cal-1 and Ce-Cal-2
The N2adsorption-desorption isotherms of CeO2were measured and shown in Fig.2(a)and Fig.2(b).The BET surface areas of the Ce-Cal-1 and Ce-Cal-2 are calculated as about 61 m2·g-1and 68 m2·g-1,respectively.Temperature-programmed reduction under H2environment (H2-TPR)was tested to detect the redox property of the resulted CeO2(Fig.2(c)).Both the samples manifest the similar reduction peak positions,which are in good agreement with the pure ceria in other reports[22-23].To be specific,the α1 peak at the lower temperature between 250~600 ℃ could be attributed to the reduction of the absorbed surface oxygen species and the surface oxygen species of CeO2.The α2 peak in the range of 750~800 ℃ could be ascribed to the reduction of bulk oxygen.The H2consumption amount of Ce-Cal-1 at α1 is higher than that of Ce-Cal-2,which could be attributable to the abundant surface oxygen species in Ce-Cal-1(Table 1).Meantime,some differences of the bulk oxygen are also presented(α2),which may be connected with the different structures.Temperature-programmed desorption experiments of NH3(NH3-TPD)were examined to understand the acidity strength,and the results are presented in Fig.2(d).The desorbed β1 peak presents at the lower temperature of 300~570 ℃,corresponding to the desorption of physisorbed NH3and NH3at the weak acid sites[24].While the desorbed β2 peak ranging between 570 and 940℃is assigned to NH3absorbed at the strong acid sites[25].The desorbed peak positions of the acid sites are analogous with each other;however,the NH3amount of β1 and β2 in Ce-Cal-1 are higher than those in Ce-Cal-2,indicating that the Ce-Cal-1 could possess of more acid sites.Moreover,the acid amount of the strong acid sites in both Ce-Cal-1 and Ce-Cal-2 are higher than those in the weak acid sites.Therefore,the H2consumption and NH3desorption amount of Ce-Cal-1 are higher than those of Ce-Cal-2,possibly associating with the diverse shapes and differentexposed crystalline facets[26-27].Distinguishable physicochemical properties of the as-prepared rod-like and sheet-like CeO2can be discovered.
To reveal the crystal phase and morphology evolution for the precursors of Ce-Cal-1 and Ce-Cal-2,time-dependentexperimentswere investigated.As displayed for the precursors of Ce-Cal-1(Fig.3(a)),the diffraction peaks of the samples can be well indexed to pure orthorhombic phase of CeOHCO3(PDF No.41-0013).However,with regard to the precursors of Ce-Cal-2(Fig.3(b)),the resulted precursors show the gradual phase transformation behaviors from orthorhombic phase (initial period)to hexagonal phase(final period).As expected,those products can be completely transformed into hexagonal phase of CeOHCO3(PDF No.32-0189)with the longer reaction time(21 and 24 h).It is noticeable that the mixed phases of orthorhombicand hexagonalareinvolved in the intermediate stages.
Besides,representative SEM images of the precursors at different reaction intervals were examined.For the precursors of Ce-Cal-1,the SEM images display the simplex rod-like morphology from Fig.3(c)to Fig.3(g)without obvious morphology transformation.However,as depicted from Fig.3(h)to Fig.3(l),the product affords rod-like structure at initial 3 h,and then the rod particles partially dissolve and accompany with the presence of some apparently granular particles(6 and 10 h).Finally,more sheet-like particles emerge as the dominant state (21 and 24 h).It should be noted that when the reaction system is absence of DHBA,the hydrothermal product presents the pure phase of CeO2with irregular morphology (Fig.4).Those results indicate that the CeOHCO3with specific morphology fails to be obtained in this condition.
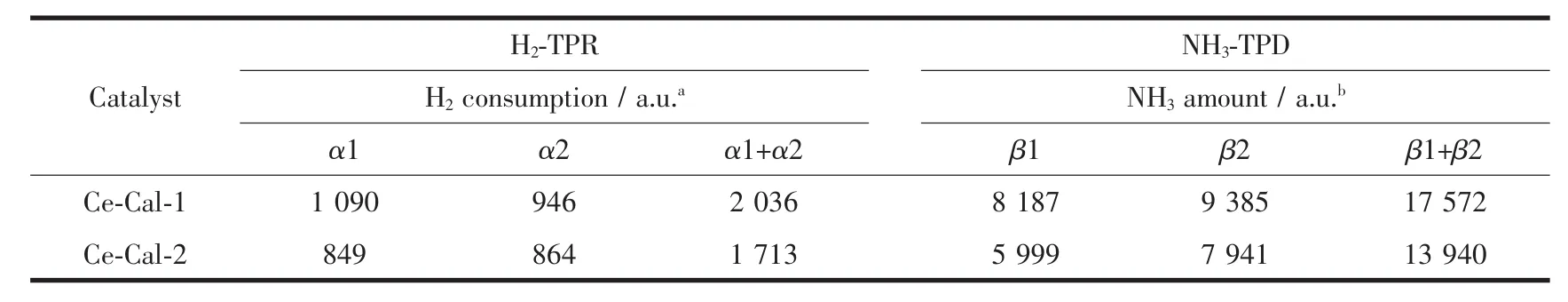
Table 1 Quantitative analysis of H2-TPR and NH3-TPD
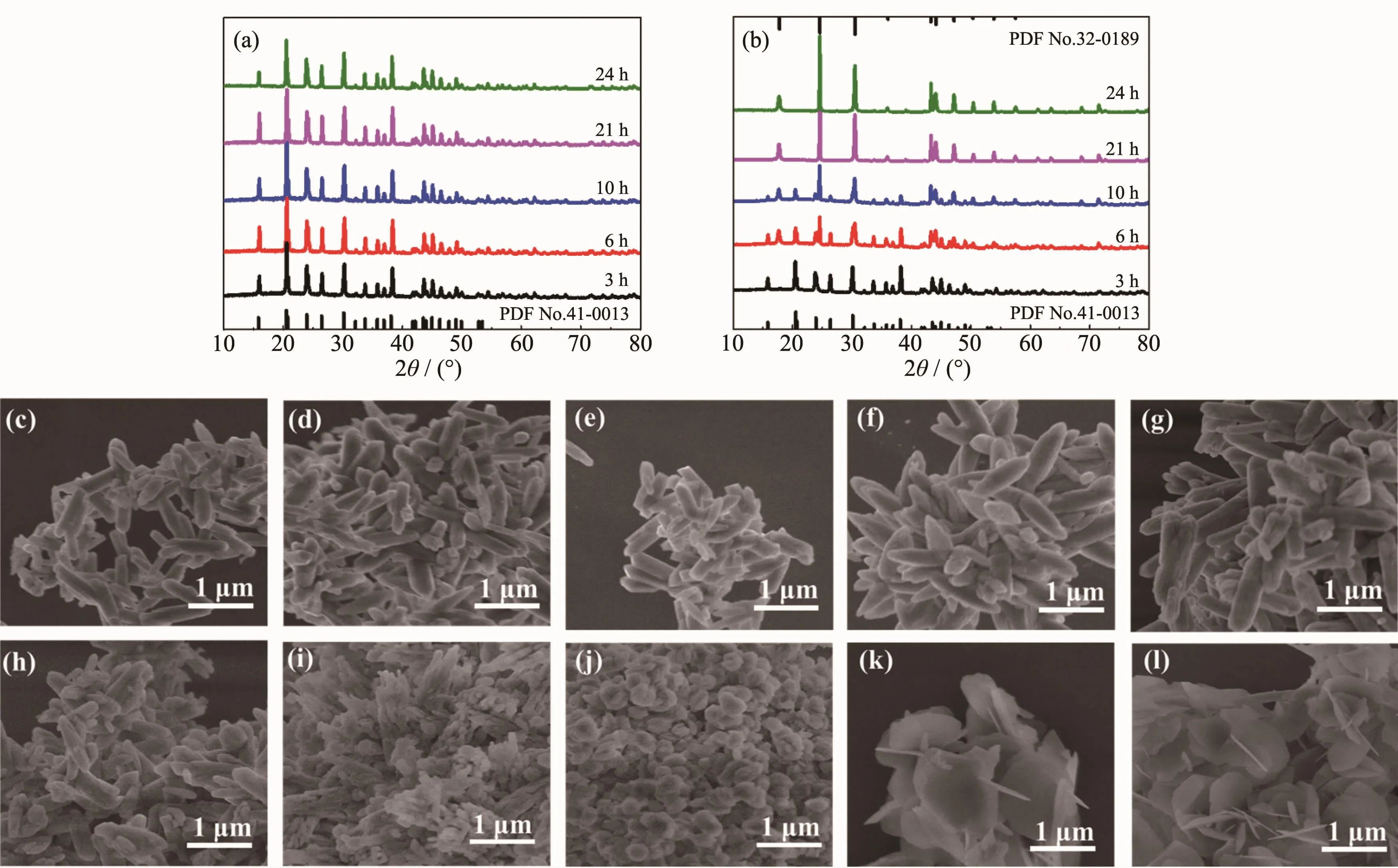
Fig.3 XRD patterns of the precursors Ce-Cal-1(a)and Ce-Cal-2(b)at different hydrothermal intervals;SEM images for the precursors of Ce-Cal-1(c~g)and Ce-Cal-2(h~l)at different reaction intervals of 3,6,10,21 and 24 h,respectively

Fig.4 XRD pattern(a)and SEM image(b)of the hydrothermal product synthesized without DHBA
Temperature-dependentexperimentsbased on Ce-Cal-2 were carried out and the SEM images are shown in Fig.5.The sample obtained at 120℃exhibits the rod-like structure with the average width of 300~500 nm and the length below 3 μm.Moreover,those particles present the highly decentralized state withoutsignificantaggregation.However,as the temperature up to 140 and 160℃,the products exhibit the rod-like morphology with the state of aggregation.Obviously,the typical sheet-like morphology ofthe productcan be observed asthe temperature reaching to 180℃(Ce-Pre-2).This phenomenon indicates that a morphology reconstruction process could be triggered with the high temperature.
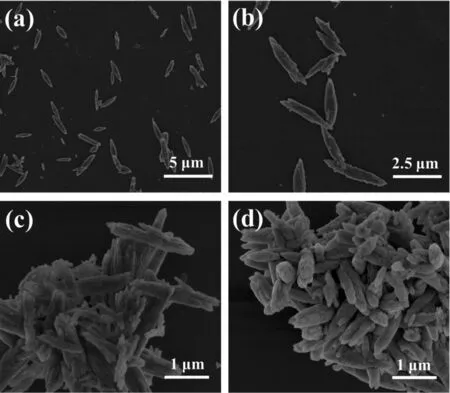
Fig.5 SEM images of the hydrothermal products at 120℃(a,b),140℃(c)and 160℃(d)
For investigating the inorganic species of Ce-Pre-1 and Ce-Pre-2,FT-IR was recorded and the results are displayed in Fig.6(a).The Ce-Pre-1 is taken as an example to illustrate.The peak at ca.3 461 cm-1could be due to the stretching vibration of O-H groups in the adsorbed water,and the bending mode of O-H at ca.1 638 cm-1could also be observed.The band at ca.1 561 cm-1should be attributable to the asymmetric stretching of CO2.Another sharp peak at ca.1 420 cm-1may be assigned to the stretching vibration of CO32-.Besides,in the region of 700~900 cm-1,the bands at ca.861 and ca.724 cm-1are correspondingly attributed to the deformation of CO32-and asymmetric vibration of CO2species,respectively.The peak at ca.594 cm-1could be ascribed to the Ce-O stretching band[28].Some of the characteristic peaks including the stretching vibration and bending mode of O-H,the asymmetric stretching of CO2and the stretching band of Ce-O in Ce-Pre-2 are similar to Ce-Pre-1,indicating the same component of the two samples(CeOHCO3).However,some difference can be found in the range of 1 400~1 500 cm-1and 700~900 cm-1,which could be due to the different crystal phases and morphologies of the CeOHCO3.In addition,the thermostability of Ce-Pre-1 and Ce-Pre-2 were analyzed by thermogravimetry and differential scanning calorimetry(TGDSC).As can be seen from Fig.6(b)and Fig.6(c),the curves manifest the weight loss between 240 and 300℃with the major exothermic peak.The weight loss of Ce-Pre-1 and Ce-Pre-2 are approximately 21.8%and 21.2%,respectively,which are close to the theoretical decomposition value of CeOHCO3to CeO2(20.7%).
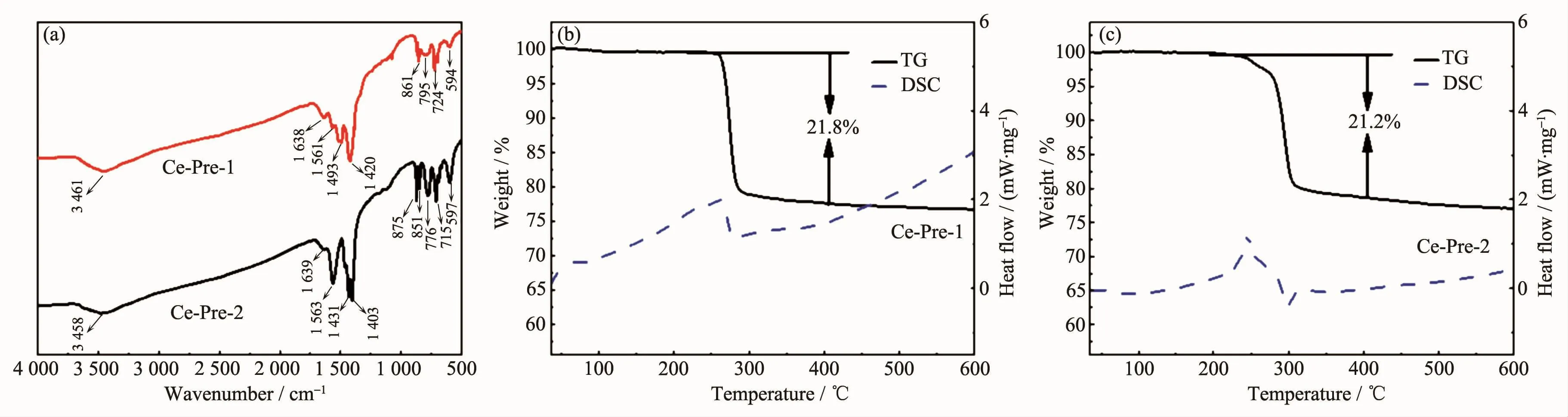
Fig.6 FT-IR spectra(a),TG-DSC curves(b,c)of Ce-Pre-1 and Ce-Pre-2
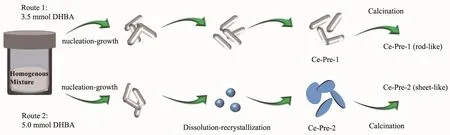
Fig.7 Illustration for the possible morphology evolution process for the Ce-Cal-1 and Ce-Cal-2
Based on the above-mentioned characterization and analysis,we tentatively propose that the DHBA could decompose into carbon species(CO32-).The OH-could also be produced by the hydrolysis of CO32-,CH3COO-under the hydrothermal condition.Thus,Ce3+could combine with OH-and CO32-to generate small granules with high surface energy.Meantime,the sustaining nucleation could be favorable to the growth of rod-like orthorhombic phase of CeOHCO3.It is noticeable that the rod-like CeOHCO3could exist as the stable product with 3.5 mmol of DHBA(Fig.7,Route 1).While theprocessofdissolution and recrystallization could be triggered with 5.0 mmol of DHBA,and rod-like CeOHCO3could dissolve and reconstruct to form the sheet-like hexagonal phase of CeOHCO3ultimately(Fig.7,Route 2).As reported,the defects of the crystals could induce the dissolution and recrystallization process for the formation of CeO2with nanosheet morphologies[29-30].However,in consideration of the different experiment conditions,the high content of the DHBA and high hydrothermal temperature could also play the important roles in this process.Furthermore,the as-prepared CeO2could preserve the rod-like and sheet-like morphologies after calcination.
In this work,Ce-Cal-1 and Ce-Cal-2 are used as catalysts for eliminating NO with NH3.As shown in Fig.8,both of the samples present the similar trend of NO conversion from 100 to 400℃.With increasing the temperature to 350℃,Ce-Cal-1 shows the higher conversion about 69.2% compared with Ce-Cal-2(50.9%).According to the results of H2-TPR and NH3-TPD,this phenomenon could be due to that Ce-Cal-1 possesses of the higher redox ability and more acid amount.The obtained CeO2particles exhibit structuredependent catalytic activity for the catalytic reduction of NO.Besides,both the NO conversion of Ce-Cal-1 and Ce-Cal-2 are higher than the reported pure CeO2,indicating high catalytic activity of Ce-Cal-1[31].On the account of the catalytic results,the rod-like CeO2could be viewed as the optimized structure for the reaction.
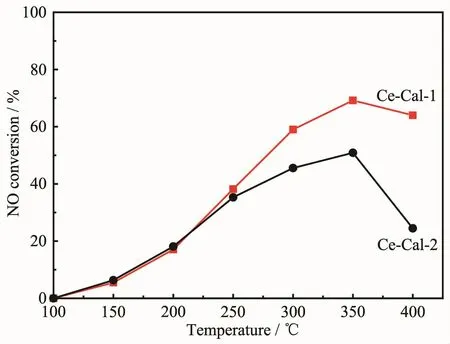
Fig.8 NO conversion of the Ce-Cal-1 and Ce-Cal-2
3 Conclusions
In summary,the rod-like and sheet-like CeOHCO3were successfully synthesized with the assistant of 2,4-dihydroxybenzonic acid.The rod-like orthorhombic phase of CeOHCO3was proposed as the stable product via the nucleation growth process,and the dissolution and recrystallization accompanied with the morphology evolution and phase transformation were supposed to the generation ofsheet-like hexagonalphase of CeOHCO3.The highly dispersed rod-like CeOHCO3could be obtained under the low hydrothermal temperature,while the state of aggregation and the transformation of morphology could be triggered by the high temperature.The obtained CeO2presented distinguishablestructure-dependentproperties,and the rod-like CeO2exhibited higher redox ability and moreacid amount.Moreover,the rod-like CeO2manifested the better catalytic activity in NH3-SCR.Furthermore,it is proposed that more benzoic acid compounds can be expected to fabricate metal oxides with desirable morphologies.
