Systematic Review and Meta-Analysis of Randomized Controlled Trials of Xianling Gubao Capsule as Adjuvant Treatment of Osteoporotic Fracture
2018-07-26WangGuiqian王桂倩LiaoXing廖星LiuFumei刘福梅andXieYanming谢雁鸣
Wang Guiqian (王桂倩), Liao Xing (廖星), Liu Fumei (刘福梅), and Xie Yanming (谢雁鸣)
Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing 100700, China
ABSTRACT OBJECTIVE: To assess the effectiveness and safety of Xianling Gubao (XLGB) Capsule in the treatment of osteoporotic fracture (OF). METHODS: Published articles from eight Chinese and English databases were searched from inception to August 2017. Clinical trials were selected according to inclusion criteria and exclusion criteria. Risk evaluation tool was used to assess the quality of studies. Data was extracted and meta-analysis was performed by RevMan 5.3 software. RESULTS: A total of 229 articles were searched, and 27 were included for study with 2292 samples,including 1152 cases in the experiment group and 1140 cases in the control group. For patients with four limbs OF,XLGB Capsule combined with manipulation reposition with plaster external fixation can shorten healing time of fracture[MD=–0.77, 95% CI (–1.25, –0.30)], increase bone mineral density (BMD) [MD=0.17, 95% CI (0.10, 0.25)], blood calcium content [MD=0.52, 95% CI (0.38, 0.64)], phosphorous content in serum [MD=0.28, 95% CI (0.21, 0.35)], and alkaline phosphatase content [SMD=–5.24, 95% CI (–3.45, –2.42)]. In the treatment of spine OF, XLGB Capsule had no significant effects on reducing re-fracture rate of non-surgical vertebral body, but it could postpone the interval time of refracture of non-surgical vertebral bodies [RR=0.71, 95% CI (0.23, 2.18)]. Combined treatment with XLGB Capsule could improve clinical effective rate [RR=1.17, 95% CI (1.11, 1.23)]. Both alone and combined application of XLGB Capsule could increase BMD and calcitonin content of patients with spine OF. Combined application of calcium or medicine that regulating calcium metabolism was faster in reducing Cobb angle of centrum kyphosis than application alone [MD=–2.68,95% CI (–3.72, –2.12)]. Alone or combined application showed no significant effects on osteoporotic pain. The adverse reactions reported in the study were mainly the digestive system damage. CONCLUSIONS: XLGB Capsule alone or combine with other western medicine had better efficacy in treating OF than western medicine treatment alone. However,there was potential bias in the included studies, so the conclusion still needed further high quality randomized controlled trials to improve the evidence level.
KEYWORDS: Xianling Gubao Capsule; Osteoporotic fracture; Randomized controlled trial; Systematic review;Meta-analysis
Osteoporosis (OP) is a degenerative skeletal disease characterized by the decrease of bone strength and the increase of fracture risk1. With the growth of age, the risk increases gradually. Osteoporosis fracture (OF),which is also known as fragility fracture, is the serious consequence of OP. Fracture can occur in minor trauma or daily activities2. As is shown in an epidemiologic study in North America, the risks of common OF in white women over the age of 50 were 17.5% of hip fracture, 15.6%diagnostic vertebral fracture, and the rate for male was 6%and 5% respectively3. In recent years, the incidence rate of OF has also shown a significant increasing trend in our country. With great harm, OF could increase the fatality rate and mortality rate. Take hip fracture as an example,the mortality rate is about 20%-25% in a year and more than 50% of the survivors may suffer disability, which significantly decline the quality of life.
There are many kinds of anti-osteoporosis drugs. According to The clinician's for prevention and treatment of osteoporosis (2014)4, U.S. Food and Drug Administration (FDA) authorized drugs for osteoporosis fracture treatment, including bisphosphonates(Alendronate sodium, Alendronate sodium +Vitamin D),calcitonin, hormone receptor modulators (Raloxifene),estrogen, selective estrogen compound, PTH 1-34 and RANKL McAb. Chinese medicine has a good clinical effects and characteristic advantages in the treatment of OP. Xianling Gubao (XLGB) Capsule is a famous Chinese patent medicine, which is a new ethnic drug developed by modern scientific research methods based on folk prescription of Miao nationality. Professor Shi Guangda, who had collected, sorted out and screened folk prescriptions of Miao nationality, developed this prescription, whose compatibility of medicines include Herba Epimedii, Radix Dipsaci, Fructus Psoraleae, Radix Rehmanniae, Rhizoma Salviae Miltiorrhizae, Rhizoma Anemarrhenae and other 3 herbs. XLGB Capsule is used to treat osteoporosis, fracture, osteoarthritis and aseptic necrosis of bone5. At present, XLGB Capsule is an exclusive production of Guizhou Tongjitang Pharmaceutical Co., Ltd. (SFDA approval number:Z20025337). In addition, it is selected into The Catalog of National Essential Drugs 2012 as the first choice for the prevention of OP6. There are four systematic review articles of this drug at present. One article performed Meta-analysis of efficacy and safety of XLGB Capsule in the adjunctive treatment of osteoporotic pain. Another article conducted systematic review on the treatment of knee osteoarthritis.The results of the two studies7,8both showed the treatment group was better than control group in effective rate and cure rate, but no systematic review was conducted for the outcome indicator of primary OF. Thus, this study aims at conducting systematic review on clinical efficacy outcome evaluation of XLGB Capsule in treating OF.
MATERIALS AND METHODS
Inclusion criteria
There are no special requirements on languages in randomized controlled trials, whether using blind method or not. Subjects of study: patients with certain diagnosis of osteoporotic fracture, without limit on age,sex, race, protopathy, and clinical stages. Intervention measures: ① Treatment group: XLGB Capsule (dosage,usage and course of treatment are unlimited), Control group: conventional therapy. ② Treatment group:XLGB Capsule (dosage, usage and course of treatment are unlimited) + conventional therapy, Control group:conventional therapy.
Exclusion criteria
The control group was treated with XLGB Capsule;Full-text article cannot be obtained; Repeated publications.
Bibliographic retrieval
A comprehensive search strategy was carried out including searching Cochrane Library (1993 to August 2017), PubMed (1997 to August 2017), EMbase (1974 to August 2017), the Web of Science (1997 to August 2017),and Clinical trials.gov (Aug.2017) database, with English key searching terms of "xianlinggubao", "xian ling gu bao" and "xianlinggubao capsule", and with Chinese key searching terms of "仙灵骨葆 (XLGB)" or "仙灵骨葆胶囊 (XLGB Capsule)", "骨质疏松 (osteoporosis)", and"骨折 (fracture)" in titles and abstracts of CNKI (1978 to August 2017), CMB (1979 to August 2017), VIP(1989 to August 2017), Wanfang (1998 to August 2017).Based on all the selected articles, search was made again for the randomized controlled trials with the treatment of osteoporotic fracture. Correlated conference papers,academic dissertations, and grey literatures were within the search range. There was no restrict of language,publication date and publication status.
Data extraction and quality evaluation
Data extraction
Two staff (Guiqian Wang and Fumei Liu) conducted data extraction independently from included literature using a predefined literature information data table.Discrepancies were resolved through group discussion or referring to the third researcher (Xing Liao or Yanming Xie). The contents of data extraction included information such as general condition, therapeutic criteria,intervention measures and outcome indicators (existing traditional clinical research, most researchers prefer effective rate, manage cure, excellence, effectiveness and invalid according to dichotomy outcome indicator. Regard cure, excellent, effectiveness as effectiveness).
Quality assessment
All the data analysis was processed by systematic review management software Revman 5.3. "The risk of bias" tool in Cochrane Handbook 5.1.0 was applied to assess the following 7 domains: ① sequence generation;② allocation concealment; ③ blinding of participants and personnel; ④ blinding of outcome assessment; ⑤incomplete outcome data; ⑥ selective outcome reporting;⑦ other bias. Finally, three levels of "low risk", "high risk", or "unclear risk" were the quality appraisal category.
Statistical analysis
The RevMan 5.3 software provided by the Cochrane Collaboration was used for data analysis. Dichotomous data were expressed as odds ratio (OR); continuous data were presented as mean difference (MD); and 95%confidence intervals (CI) were calculated for both. The heterogeneity test adopted Homogeneity test (Q test).When there was statistical homogeneity among the studies(P>0.10, I2<50% ), the fixed effect model was used for meta-analysis. On the contrary, if there was statistical heterogeneity between the studies (P<0.10, I2>50%),the heterogeneity source was analyzed, and subgroup analysis was performed for the possible heterogeneity factors. If there was a statistical heterogeneity between the two groups with small clinical heterogeneity, the stochastic effect model was used. If heterogeneity arose from low quality research, sensitivity analysis was carried out. If the heterogeneity was too large or cannot find the data source, the meta-analysis was given up, and only descriptive analysis was carried out. Funnel plot was used for the index with the maximum number of literatures(10) to detect the possibility of publication bias.
RESULTS
Selection of included studies
Initially, 229 articles were retrieved. With NoteExpress literature management software, duplicate articles were excluded. After reading titles and abstracts,and further the full text, 27 papers were selected according to inclusion criteria and exclusion criteria,including 27 Chinese articles and no English articles. The screening process was shown in Figure 1.
Characteristics of included studies
The included 27 studies were carried out in mainland of China, including 2292 patients, with 1152 cases in experiment group and 1140 cases in control groups. The smallest sample size was 36 cases; the largest sample size was 124 cases. Three studies did not report the gender of the patient9-11, and the remaining 24 papers reported. The included 27 literatures included 6 articles of limb fractures and 21 articles of spinal fractures (Table 1).
Quality assessment of included study

Figure 1. Flow diagram of literature screening and selection process
Among the included 27 studies, 9 studies applied random number table to divide groups12-16,24,25,29,34,and the sequence generation of 2 studies was based on the order of admission9,18, with 1 study based on the patient's willing31. All the 27 studies did not mention the allocation concealment and whether or not double blinding for the personnel and participants. Two studies reported loss to follow-up and withdraw27,28. It was not clear whether selective reporting bias existed because of the lack of study protocol. Methodological quality assessment of included studies was performed according to Cochrane handbook "assessment tools for the bias risks of randomized controlled trials (Version 5.1.0)".The results were all of RCTs of low quality (grade B or C) (Figure 2).
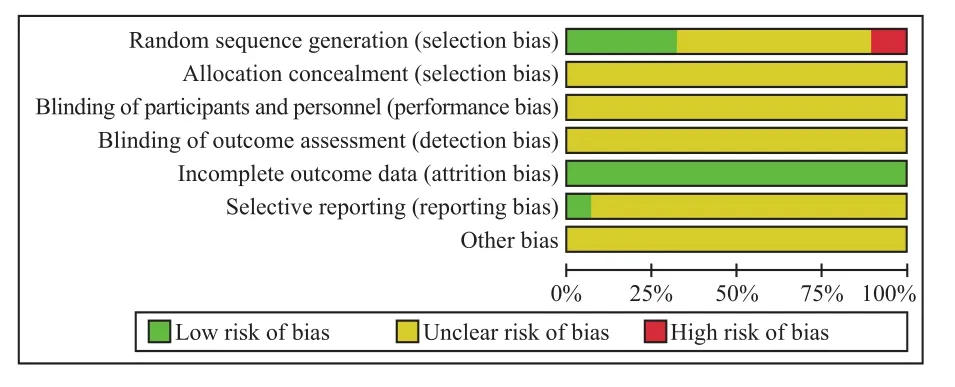
Figure 2. Risk of bias graph. Each item was evaluated as a percentage of the literature, and the quality of the selected literature was evaluated
Results of efficacy analysis
Limb osteoporosis fracture
In limb osteoporosis fracture, 2 studies were senile osteoporosis distal radial fracture14,19; 2 studies were senile femoral intertrochanteric fracture21,24; 1 study was postmenopausal osteoporotic hip fracture27; 1 study was osteoporotic humerus neck fracture34.
Six interventions for the treatment of limb osteoporotic fracture were all adjuvant therapies, whichwas combined application based on the conventional western therapeutic measures.
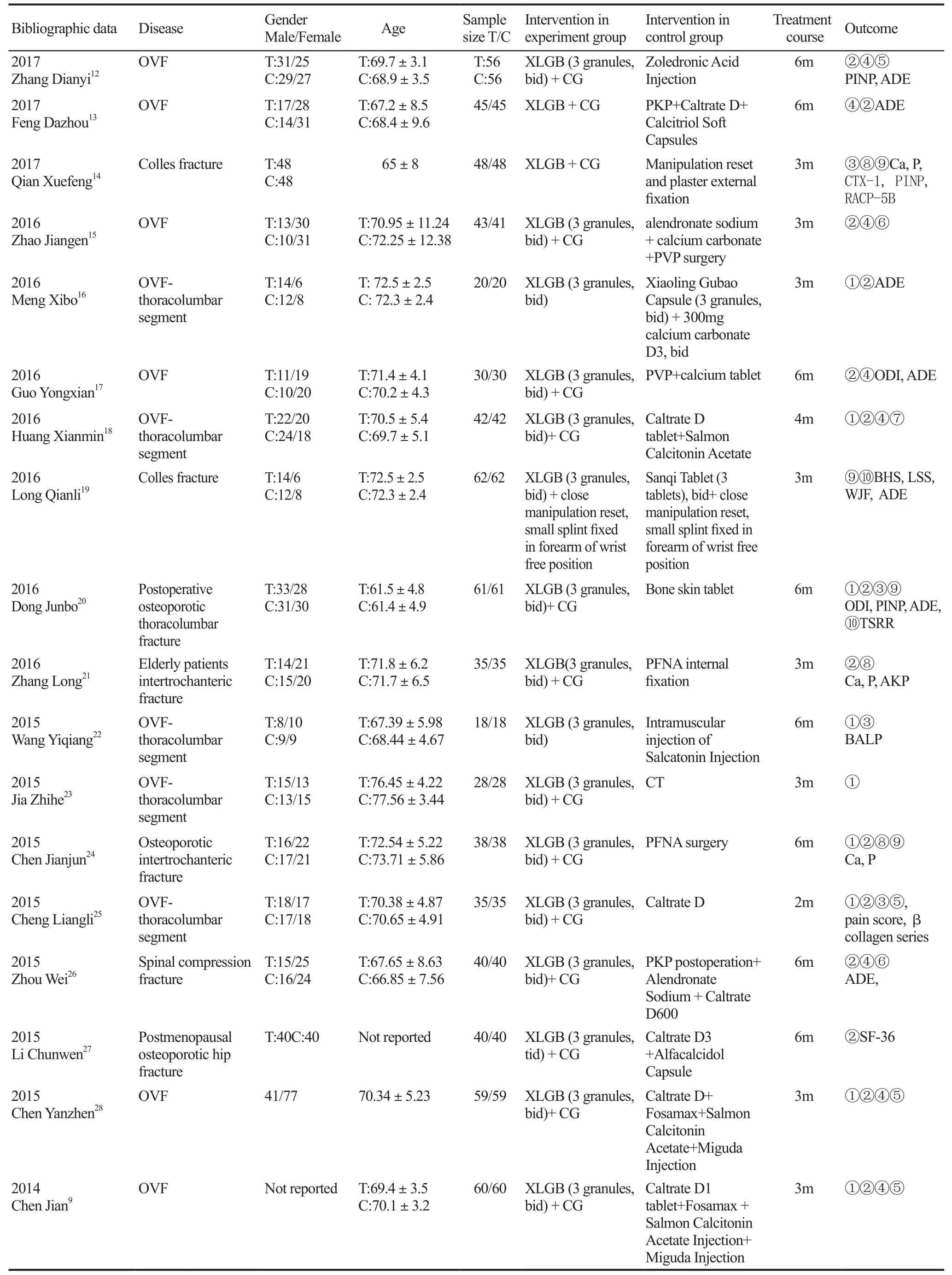
Table 1. The characteristics of included studies
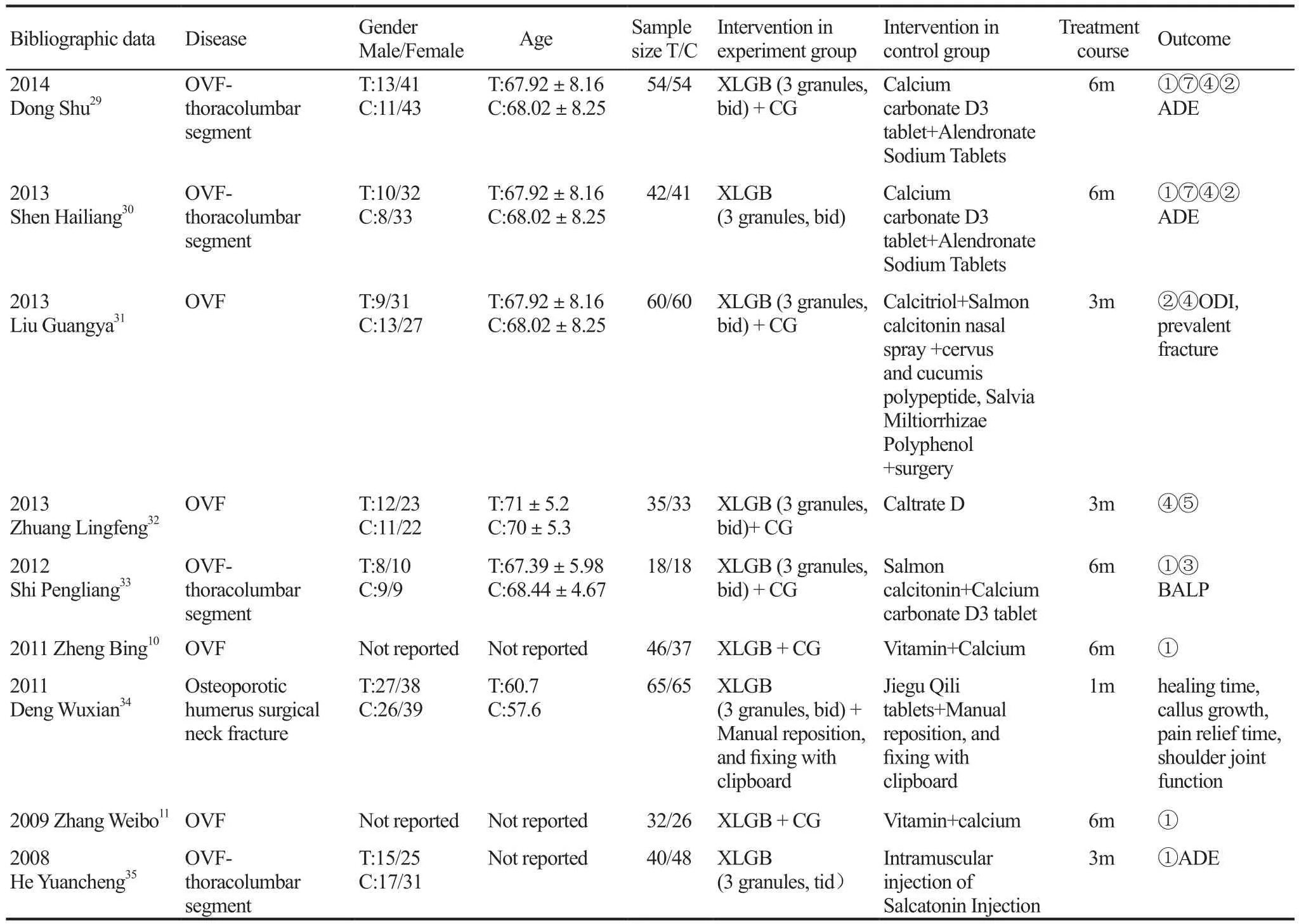
(Continued)
Primary outcome indicators
One study reported a new fracture in experiment group and control group, and did not elaborate the nature and location of the fracture27.
Secondary outcome indicators
Fracture-healing time: Three studies reported three different fracture-healing time14,24,34, so the mean standard deviation was adopted to calculate. 95% CI of the study did not coincide with the other studies14, so the random affects model was used for combined analysis. And the results were I2=61%, Z=3.19, P=0.001. XLGB Capsule combined with manipulation reposition with plaster external fixation can shorten healing time of fracture(Figure 3).
Bone mineral density (BMD): Three studies reported BMD21,24,27. The study was excludedbecause 95%CI did not coincide with the other studies27. There were 73 cases in experiment group and control group after combining the other two studies. MD=0.17, 95% CI (0.10,0.25), I2=0%, Z=4.37, P<0.0001 (Figure 4).
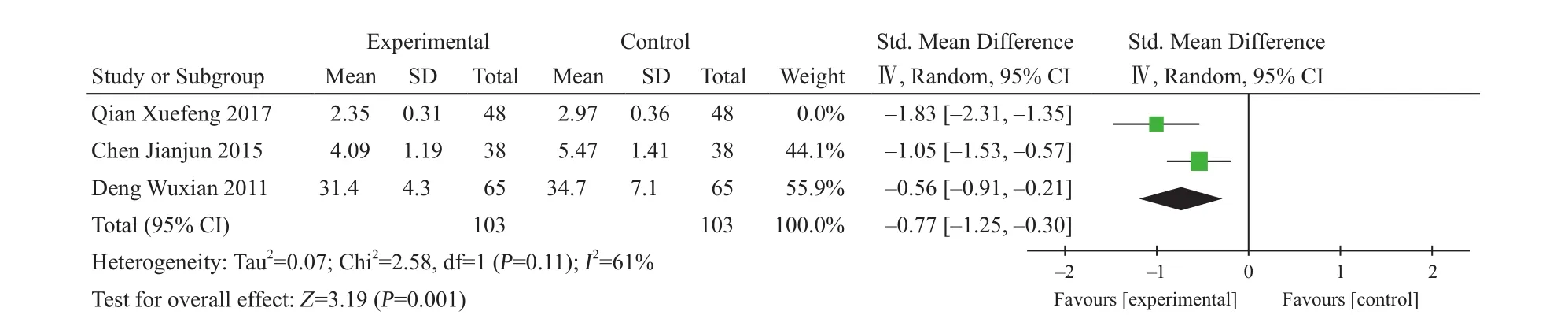
Figure 3. Fracture-healing time of limb osteoporosis fracture by the adjunctive treatment of XLGB Capsule
Serum calcium (Ca) content: Three studies reported serum calcium14,21,24. There were 121 cases in experiment group and control group. I2=0%, MD=0.52, 95% CI (0.38,0.64), Z=9.93, P<0.00001. Serum calcium content can be improved by the adjunctive treatment of XLGB Capsule(Figure 5).
Serum phosphorus (P) content: Three studies reported serum phosphorus (P) content14,21,24. There were 121 cases in experiment group and control group. MD=0.28, 95% CI (0.21, 0.35), I2=0%, Z=7.84,P<0.00001. XLGB Capsule combined with manipulation reposition with plaster external fixation can improve serum phosphorus (P) content (Figure 6).
Alkaline phosphatase (ALP): Four studies reported ALP14,19,21,24. The normal value of ALP for females is 50-135U/L. However, the study of experiment group was 316.76±35.64, twice as much as normal value14, which was not consistent with clinic logic, and its CI was not coincidence in the other three studies. So it was excluded.Based on manipulation reposition and splint fixation,the study combined with XLGB Capsule in experiment group and Sanqi Tablet in control group19, which was not coincidence with the other interventions. Therefore, the other two studies were combined. There were 73 cases in both experiment group and control group. SMD=–5.24,95% CI (–3.45, –2.42), I2=0%, Z=14.71, P<0.00001(Figure 7).
In conclusion, XLGB Capsule combined with manipulation reposition and splint fixation can shorten fracture-healing time, improve BMD, Ca, P, ALP content in the treatment of limb osteoporosis fracture.
Spine osteoporosis fracture
Primary outcome indicators
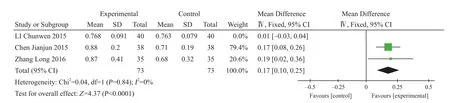
Figure 4. BMD of limb osteoporosis fracture by the adjunctive treatment of XLGB Capsule
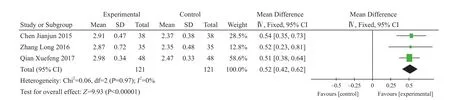
Figure 5. Serum Ca content of limb osteoporosis fracture by the adjunctive treatment of XLGB Capsule

Figure 6. Serum phosphorus (P) content of limb osteoporosis fracture by the adjunctive treatment of XLGB Capsule

Figure 7. Serum ALP content of limb osteoporosis fracture by the adjunctive treatment of XLGB Capsule
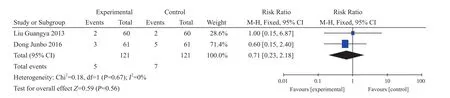
Figure 8. Rate of spine osteoporosis re-fractures treated by XLGB Capsule
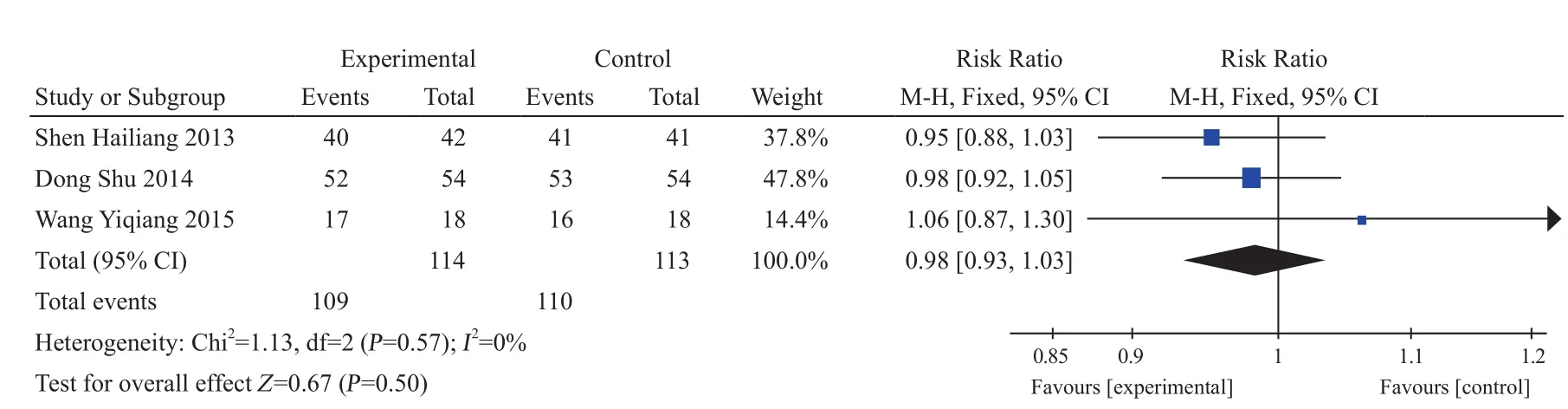
Figure 9. Clinical effective rate of single use of XLGB Capsule in the treatment of spine oste
Two studies reported the cases of spine osteoporosis re-fracture20,31. There were 121 cases in experiment group and control group. RR=0.71, 95% CI (0.23,2.18), I2=0%, Z=0.59, P=0.56 (Figure 8). There was no statistical difference between the two groups. After 8 months' follow-up, study reported that 3 cases occurred thoracolumbar spine re-fracture in observation group with 4.9% of the rate of thoracolumbar spine re-fracture; and 5 cases occurred thoracolumbar spine re-fracture in control group with 8.2% of the rate of thoracolumbar spine refracture20. Study reported that 2 cases occurred re-fracture in the treatment group with 153 days of average time of vertebral re-fracture; and 2 cases occurred re-fracture in control group with 132 days of average time of refracture31. There was no significant effect on the reduction of the re-fracture rate of the non-surgical centrums by taking XLGB Capsule, but it could delay the interval of the non-surgical vertebral fracture.
Secondary outcome indicators
Clinical effective rate: Seventeen studies reported clinical effective rate9-12.18,20,22,23,25,28-30,32,35,36. Four of them only took XLGB Capsule in experiment group22,29,30,36,and the other 13 studies received combined treatment in experiment group. Analyzing from the determination standard, one study was determined by pain intensity35,and the determination standards of other 16 studies followed curative effects of Clinical Research of New Chinese Medicine Guidelines, and The Treatment of Osteoporotic Vertebral Compression Fractures Guidelines.And the degree of improvement of waist pain, BMD content and the healing degree of fracture were generally analyzed.
Clinical effective rate of the single use of XLGB Capsule:
Four studies only applied XLGB Capsule in the treatment of spine osteoporosis fracture22,29,30,36,and all were enumeration data. There were 109 cases in experiment group and 110 cases in control group.RR=0.98, 95% CI (0.93, 1.03), I2=0%, Z=0.67, P=0.50.There was no statistical significance (Figure 9).
Clinical effects of combination use of XLGB Capsule were as follows:
Combined analysis was conducted to the 16 studies of the coincidence of clinical efficacy criteria in combining use of XLGB Capsule. All was enumeration data. There were 471 cases in experiment group and 389 cases in control group. Heterogeneity test showed I2=78%, so random effect model was used to combine data. RR=1.17, 95% CI (1.11, 1.23), Z=6.0, P<0.00001,which was of statistical significance. Combined use of XLGB Capsule based on conventional treatment can improve clinical efficacy in the treatment of spine osteoporosis fracture (Figure 10).
Bone mineral density (BMD)
Single use of XLGB Capsule:
Two studies reported BMD29,30. There were 96 cases in experiment group and 95 cases in control group. After heterogeneity test, I2=78%, so random effect model was adopted. MD=–0.42, 95% CI (–0.82, –0.02), P=0.04,which had statistical significance (Figure 11).
Combination use of XLGB Capsule:
Eleven studies reported BMD content in the treatment of spine osteoporosis fracture with combination use of XLGB Capsule9,12,13,15-18,20,25,26,28. There were 376 cases in experiment group and374 cases in control group. After heterogeneity test, I2=86%, so random effect model was adopted. SMD=2.28, 95% CI (1.78, 2.78),P<0.00001, which had statistical significance (Figure 12).
Visual Analogue Scale/Score (VAS)
Single use of XLGB Capsule:
Two studies reported of VAS29,30. There were 96 cases in experiment group and 95 cases in control group.After heterogeneity test, I2=0, so fixed effect model was adopted. MD=–0.01 (–0.26, 0.24), P=0.93, which had not statistical significance (Figure 13).
XLGB Capsule combined with other treatment therapies:
Ten studies reported changes of VAS by the combination use of XLGB Capsule with other treatment therapies9,12,13,15,17,18,25,26,28,32. There were 445 cases in experiment group and 95 cases in control group. After heterogeneity test, I2= 95%, and the heterogeneity was great. Except two studies13,26, the experiment group of the other eight studies were better than the control group.XLGB Capsule combined with other treatment therapies can improve VAS score, which needed further verification of clinical trials.
Bone gla protein (BGP)
Single use of XLGB Capsule:

Figure 10. Clinical effective rate of combined treatment of XLGB Capsule in the treatment of spine osteoporosis fracture
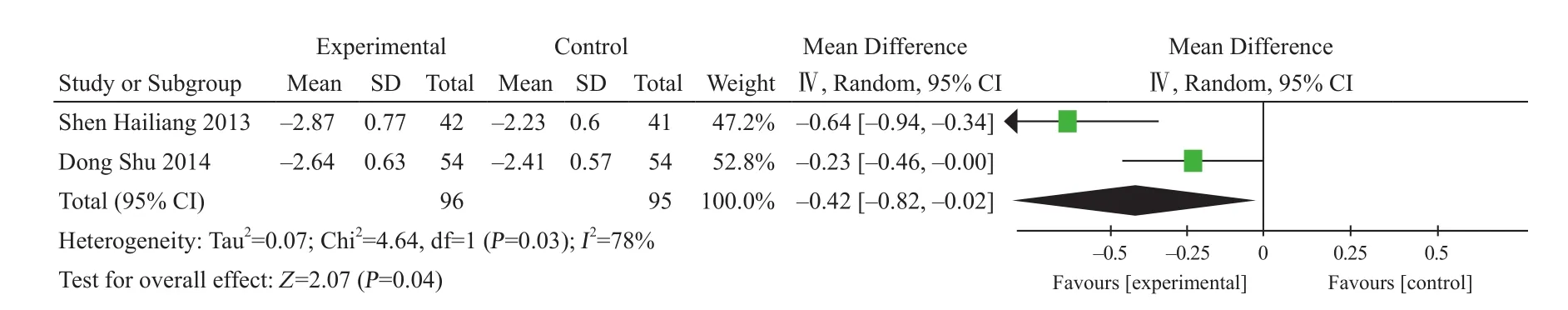
Figure 11. BMD changes of single use of XLGB Capsule in the treatment of spine osteoporosis fracture
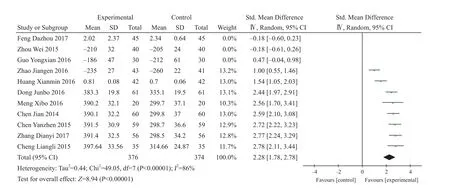
Figure 12. BMD changes of combination use of XLGB Capsule in the treatment of spine osteoporosis fracture

Figure 13. Improvement of VAS of single use of XLGB Capsule in the treatment of spine osteoporosis fracture

Figure 14. The improvement of BGP with combination use of XLGB Capsule in the treatment of spine osteoporosis fracture
One study reported BGP36. There were 18 cases in experiment group and control group. P=0.03, it had statistical significance. Single use of XLGB Capsule was better than Salmon calcitonin and oral calcium carbonate in the improvement of BGP.
Combination use of XLGB Capsule:
Three studies reported BGP20, 25, 33. There were 114 cases in experiment group and control group. After heterogeneity test, I2=58%, so random effect model was adopted. MD=1.72, 95% CI (0.95, 2.49), P<0.0001,which had statistical significance. Combination use of XLGB Capsule with calcium was better than single use of calcium in improving serum BGP (Figure 14).
Both single use and combined application of XLGB Capsule had good curative effect in improving BGP.
BLAP
Single use of XLGB Capsule:
One study reported BLAP36. There were 18 cases in experiment group and control group. P=0.03, and it had statistical significance. Single use of XLGB Capsule was better than Salmon calcitonin and oral calcium carbonate treatment in the improvement of BLAP.
Combination use of XLGB Capsule:
Two studies reported BLAP20,33. After heterogeneity test, I2=84%, so descriptive analysis was adopted only.The study had 61 cases in experiment group and control group20. After 6 months treatment, experiment group:5.6±1.4, and control group: 4.3±1. Another study had 18 cases in experiment group and control group33. After treatment, experiment group: 90.44±13.13, and control group: 99.24±10.59.
Cobb angle of centrum kyphosis
Five studies reported Cobb angle of centrum kyphosis with combination of XLGB Capsule9,12,25,28,32.After heterogeneity test, I2=87%. The study was excluded25because 95% CI did not coincide with the other studies,and the other studies were combined. After heterogeneity test results, I2=44%, MD=–2.68, 95 CI% (–3.72, –2.12),P<0.00001, which had statistical significance. XLGB Capsule combined with calcium and drugs of regulating calcium metabolism was better than single use of them in accelerating the reduction of Cobb angle of centrum kyphosis (Figure 15).
Adverse reactions
Seven studies reported adverse reactions12,17,19,26,29,30,35.All together 17 cases of digestive system damage were reported. Study reported 1 case of gastrointestinal reaction, 3 cases of hyperpyrexia and 2 cases of hypocalcemia12; Study also reported 2 cases of bloating and anorexia26. Study reported 4 cases of gastrointestinal reaction29. Study reported 4 cases of constipation, which disappeared after using aperient35(Figure 16).
Publication bias
The BMD and effective rate of the patients with osteoporotic fractures of more than 10 patients were analyzed for publication bias (Figure 17). As can be seen from the funnel plot, it was asymmetric on both sides of the study, indicating publication bias existed. This could be caused by all sorts of reasons, for example studies with positive results were easily published, and several studies with a small sample with low quality.
DISCUSSION
A total of 27 studies included in this systematic review were randomized controlled trials on the treatment of osteoporotic fractures of XLGB Capsules. The intervention methods for experimental group and control group mainly included calcitriol, calcitonin nasal spray,the cervus and cucumis polypeptide and operation. The shortest course of treatment was 1 month and the longest was 6 months.

Figure 16. Funnel plot of effective rate of osteoporosis fracture patients
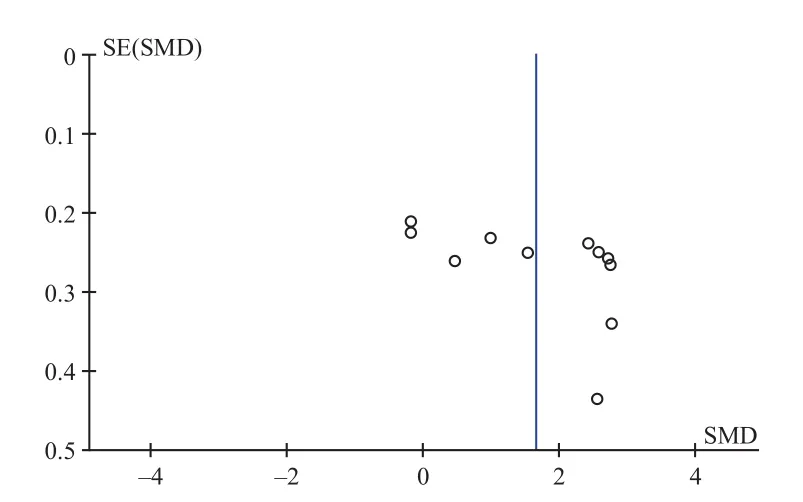
Figure 17. Funnel plot of BMD of osteoporosis fracture patients
Summary of evidence
In the 27 studies included, 9 studies were using random number table to divide groups, and the sequence generation of 2 studies was based on the order of admission, with 1 study based on the patient's will. All the 27 studies did not mention the allocation concealment and whether or not double blinding for the personnel and participants. Two studies reported loss to follow-up and withdraw
Selective reporting may occur because no studies submitted research proposal and conducted clinical registration, affecting the authenticity of the conclusions.Two studies reported the number of loss to followup and withdraw cases, and no studies mentioned ITT analysis. Because no studies submitted research proposal and conducted clinical registration, which may generate selective reporting, and further affected the authenticity of the study conclusion. There also existed a retrospective registration and outcome report bias.
Significance
A larger number of domestic clinical studies were often restricted by factors such as funding, research cycle, leading to misconducts of blind pursuit of"positive results", and shortening the research cycle,which reduced the test efficiency. As a result, the outcomes of many studies were lack of real reliability analysis. For studies with small sample size, and similar factors of intervention measures, outcome indicators, the types of study design, meta-analysis could be considered. The efficacy of examination can be improved through the quantitative pooled analysis, and thus to find the best evidence.
The package insert of XLGB Capsule indicates the actions of the drug to nourish the liver and kidney,invigorate the blood circulation and unblock the collaterals, strong muscles and bones. It is mainly used in the treatment of osteoporosis, fractures, bone arthritis,bone aseptic necrosis. Studies have shown that hormones that affect the formation of bone tissue are GH, thyroid hormone and calcitonin and so on. And local growth factors affecting the formation of bone tissue mainly are TGF-β1, bone morphogenetic protein, insulin-like growth factor and so on. In the process of bone formation,hormonal regulation, growth factors and nerve system jointly form the nerve - endocrine - local growth factor regulation network, which participates in the whole regulation process of the formation of bone tissue.Animal experiments showed that the XLGB Capsule could increase the animal serum GH concentration, and TGF-β1 were positive staining at 14th and 21st day of the fracture treatment. Therefore, in the process of fracture healing, XLGB Capsule promotes bone formation regulation process involved in the mechanism of action of fracture healing by increasing the concentration of the hormone GH and affecting the formation of bone tissue by enhancing local growth factors affecting the formation of bone tissue expression of TGF-β137,38. In addition,calcium salt deposition is a key step in fracture healing process, and bone tissue repair must be completed by matrix calcification process. The results showed that the time of fracture healing was significantly shortened after the application of XLGB Capsule, which was related to the increase of calcium phosphorus deposition39.Based on this, XLGB Capsule is used in the treatment of osteoporosis fracture.
Limitations
In the aspect of diagnostic criteria, the diagnoses and treatment guidelines of osteoporosis and bone mineral salt disease from Chinese Medical Association of Osteoporosis and Bone mineral salt Disease Branch recommends the WHO recommended diagnostic criteria,which is that based on determination method of dualenergy X-ray absorptiometry (DXA), bone density decreased by 1-2.4 standard deviations is low bone mass(osteopenia), and the decrease degree2.5 standard deviations is osteoporosis. The diagnostic criteria of the original studies included in the study were inconsistent,which reduces the persuasiveness and will not be conducive to being internationally recognized.
In the study design, the random methods of most studies included in this systematic review were inadequate or incorrect, without allocation concealment,or blinding methods. Therefore, there was the possibility of selective bias, performance bias and measurement bias, thus exaggerating the study outcomes. In addition,although the authors (name, unit), year of the publication,and interventions of two papers were different, the measured values of outcome indicators were completely consistent, which exposed that the scientific literacy of some researchers were remaining to improve, and the attitudes to scientific conclusion were not rigorous and careful enough.
猜你喜欢
杂志排行
World Journal of Integrated Traditional and Western Medicine的其它文章
- Therapeutic Effects of "Soothing the Liver Method" on Stress-related Non-alcoholic Fatty Liver on Neuroendocrine-metabolic Level
- New Year's Message
- Analysis on the Pharmacists Intervention Results of the Problems from 2000 Prescriptions of Chinese Herbal Pieces
- Probing the Definition of Yin and Yang in Our Body
- A Life System Tuning Model
- A Systematic Review and Meta-Analysis of Herb-Partitioned Moxibustion in the Treatment of Primary Dysmenorrhea
