Molecular cytogenetic of the Amoy croaker,Argyrosomus amoyensis(Teleostei, Sciaenidae)*
2018-07-11LIAOMengxiang廖梦香ZHENGJiao郑娇WANGZhiyong王志勇WANGYilei王艺磊ZHANGJing张静CAIMingyi蔡明夷
LIAO Mengxiang (廖梦香), ZHENG Jiao (郑娇), WANG Zhiyong (王志勇),WANG Yilei (王艺磊), ZHANG Jing (张静), CAI Mingyi (蔡明夷)
Key Laboratory of Healthy Mariculture for the East China Sea,Ministry of Agriculture,Fisheries College,Jimei University,Xiamen 361021,China
AbstractThe family Sciaenidae is remarkable for its species richness and economic importance. However,the cytogenetic data available in this fi sh group are still limited, especially those obtained using fluorescence in situ hybridization (FISH). In the present study, the chromosome characteristics of a sciaenid species,Argyrosomus amoyensis, were examined with several cytogenetic methods, including dual-FISH with 18S and 5S rDNA probes, and a self-genomic in situ hybridization procedure (Self-GISH). The karyotype ofA.amoyensiscomprised 2n=48 acrocentric chromosomes. A single pair of nucleolar organizer regions (NORs)was located at the proximal position of chromosome 1, which was positive for silver nitrate impregnation(AgNO3) staining and denaturation-propidium iodide (DPI) staining but negative for Giemsa staining and 4',6-diamidino-2-phenylindole (DAPI) staining, and was confi rmed by FISH with 18S rDNA probes. The 5S rDNA sites were located at the centromeric region of chromosome 3. Telomeric FISH signals were detected at all chromosome ends with different intensities, but internal telomeric sequences (ITSs) were not found.Self-GISH resulted in strong signals distributed at the centromeric regions of all chromosomes. C-banding revealed not only centromeric heterochromatin, but also heterochromatin that located on NORs, in interstitial and distal telomeric regions of Specific chromosomes. These results suggest that the karyotype of Amoy croaker was relatively conserved and primitive. By comparison with the reported cytogenetic data of other sciaenids, it can be deduced that although the karyotypic macrostructure and chromosomal localization of 18S rDNA are conserved, the distribution of 5S rDNA varies dynamically among sciaenid species. Thus,the 5S rDNA sites may have different evolutionary dynamics in relation to other chromosomal regions, and have the potential to be effective cytotaxonomic markers in Sciaenidae.
Keyword: Argyrosomus amoyensis; fluorescence in situ hybridization; genomic DNA; rDNA; telomere
1 INTRODUCTION
Sciaenidae is one of the most species-rich families in the order Perciformes, with approximately 67 genera and 283 fi sh species (Nelson et al., 2016).Sciaenids are mainly distributed in the Indian, Pacific and Atlantic Oceans (Longhurst and Pauly, 1987;Sasaki, 1996), and are important fishery resources worldwide, as well as candidates for aquaculture.Although Sciaenidae is considered a monophyletic group, great diversity in body shape and mouth position relative to different feeding patterns and life histories has been observed in several species (Chao and Musick, 1977).
Despite its economic importance and great species diversity, few cytogenetic studies have been reported in this fi sh group, and most used conventional Giemsa staining (Arai, 2011). However, the available cytogenetic data suggest that the karyotypic macrostructure is remarkably conserved in the family(Accioly and Molina, 2008). Karyotype stability is a common trend in marine fishes, but some hypervariable chromosomal regions have been found, particularly those represented by ribosomal sites or positions adjacent to them (de Mello Affonso and Galetti,2005). Thus, the chromosomal mapping of ribosomal sequences has received increasing attention as a way to identify and quantify chromosome dynamics(Calado et al., 2014).
The Amoy croaker (Argyrosomusamoyensis)(Bleeker, 1863; Perciformes, Sciaenidae), once namedNibeamiichthioides, is a carnivorous sciaenid native to the Indo-West Pacific (Sasaki, 2001). It has become a popular mariculture species along the southeastern coast of China because ofits delicious taste and strong disease resistance (Meng, 1996; Jian et al., 2013). The karyotype of the Amoy croaker has been described only once, by Wang et al. (2002) with Giemsa staining. Therefore, in the present study, the chromosomal distribution of the nucleolar organizer regions (NORs) was examined in the Amoy croaker using Giemsa, silver nitrate impregnation (AgNO3),denaturation and propidium iodide (DPI), and 4',6-diamidino-2-phenylindole (DAPI) staining.Moreover, fluorescence in situ hybridization (FISH)with 18S (major) rDNAs, 5S (minor) rDNAs, and TTAGGGn sequences (a general telomere sequence for vertebrates) was performed, as well as a selfgenomic in situ hybridization (self-GISH) procedure.These results were compared with the available cytogenetic data of other Sciaenid species to explore whether the fi ne structure of the karyotype was as stable as the macrostructure in this family, and to screen out candidate cytogenetic markers for cytotaxonomic and phylogenetic studies.
2 MATERIAL AND METHOD
2.1 Specimen collection and chromosome preparations
Five male and five female Amoy croaker individuals used in the present study were obtained alive from the breeding base of Jimei University in Ningde, Fujian,China. Mitotic chromosome preparations were made from the head kidneys as described previously (Wang et al., 2002). Additionally, part of a fi n from each fi sh was cut out and fi xed in absolute ethyl alcohol to extract genomic DNA.
2.2 Chromosome staining and banding
The chromosomes were examined by Giemsa,DPI, Ag-, and DAPI staining. In the Giemsa staining,the chromosomes were stained with 10% Giemsa in phosphate buffer (pH 6.8) for 25 min and then washed with running water. Ag-staining was performed as described by Howell and Black (1980). DPI staining was performed as described by Rab et al. (1996). For DAPI staining, the chromosomes were stained with 1 μg/mL DAPI in phosphate buffered saline (PBS, pH 7.0) for 10 min. C-banding was carried out according to Sumner’s description (Sumner, 1972).
2.3 FISH and self-GISH procedures
Genomic DNA was extracted from the fi xed fi n tissue using a Genomic DNA Kit (Generay, Shanghai,China). A partial coding region of the 18S rDNA ofA.amoyensiswas amplified using the primers F(5′-CGCGCAAATTACCCACTCCC-3′) and R(5′-CTGAACGCCACTTGTCCCT-3′), which were designed from a conserved region of the 18S rDNA from several fi sh species. The PCR reaction was performed in a total volume of 20 μL containing double distilled water, 1×PCR buffer, 0.2 mmol/L dNTPs, 0.5 mmol/L of each primer, 1 U of EasyTaq polymerase (TaKaRa, Shiga, Japan) and 50 ng genomic DNA. The PCR cycling conditions were an initial denaturation step at 94°C for 4 min, followed by 30 cycles of 94°C for 30 s, 54°C for 1 min, 72°C for 1 min, and a fi nal extension step of 72°C for 5 min.The amplified products were visualized after electrophoresis on a 1% agarose gel and sequenced for verifi cation by a custom service (Shenggong,Shanghai, China). The whole coding and nontranscribed region of the 5S rDNA was obtained by PCR amplifi cation with the primers F(5′-GTCAGGCCTGGTTAGTACTTGGAT-3′) and R(5′- GGGCGCATTCAGGGTGGTAT-3′), which were designed from the coding sequence of the 5S rDNA from several fi sh species. PCR was performed as described above. The obtained PCR product was cloned into the pEASY-T1 vector (TransGen Biotech,Beijing, China) and sequenced for verifi cation by a custom service (Shenggong). Telomeric repeats(TTAGGG)nwere amplified by PCR without a template using (TTAGGG)5and (TAACCC)5primers(Ijdo et al., 1991). The extracted genomic DNA was used as probe in self-GISH experiments.

Fig.1 Karyotype of A. amoyensisa. self-GISH; b. C-banding. Bars represent 5 μm.
FISH and self-GISH were performed as described previously (Fujiwara et al., 1997; Cai et al., 2013).The 18S rDNA, 5S rDNA and genomic DNA probes were labeled with digoxigenin-11-dUTP or biotin-11-dUTP by nick translation according to the manufacturer’s instructions (Roche, Basel,Switzerland). Chromosomes slides were denatured for 2 min in 70°C 70% formamide/2× SSC, and then dehydrated in an ethanol series. Hybridization was performed with a mixture containing 2 ng/μL denatured probes without blocking DNAs, 50%deionized formamide, 10% dextran sulfate, 2×SSC,and double-deionized water at 37°C for 8-16 h in a moist chamber. Post-hybridization washes were performed in 50% formamide/2×SSC at 37°C for 20 min, 2×SSC and 1×SSC at room temperature for 20 min each, and 4×SSC at room temperature for 5 min. The digoxigenated and biotinylated probes were detected with anti-digoxigenin-rhodamine(Roche) and avidin-Alexa fl uor-488 (Invitrogen,Carlsbad, CA, USA), respectively. Then, the chromosomes were counterstained with DAPI or PI in Antifade solution (Vector Laboratories, Burlingame,CA, USA).
2.4 Microscopy and image analyses
Good chromosome samples at metaphase were examined and photographed using an Olympus BX53 fluorescence microscope (Olympus, Tokyo, Japan)and Olympus DP 80 digital image capture system(Olympus). Ten good metaphase plates without overlapping were analyzed. The relative length of each chromosome was measured with the Image-Pro Plus (IPP) 6.0 software, and the data were analyzed statistically as described previously (Cao et al., 2015).The chromosomes were classified according to Levan et al. (1964).
3 RESULT
In all 10 individuals examined, the Amoy croaker karyotype consisted of 48 acrocentric chromosomes.The chromosome complements were paired, and then ranked according to their average relative lengths(Fig.1). The relative lengths were calculated based on data from 10 well-spread metaphases after self-GISH,which showed a continuous distribution. No obvious difference was found between the karyotypes of two sexes. After self-GISH, strong signals were present at the centromeric regions of all chromosomes (Fig.1a).
C-banding revealed that the heterochromatic blocks not only located in the centromeric region, but also on NORs and in interstitial and telomeric regions of Specific chromosomes. In addition, a greater variation in heterochromatin content was observed among chromosome pairs (Fig.1b).
After Giemsa staining, the largest chromosome pair was easily distinguished by Specific secondary constrictions at the proximal region, and was assigned to chromosome 1 (Fig.2a). With AgNO3staining, a single pair of active nucleolus organizer regions (Ag-NORs) was mapped to a position similar to the secondary constrictions (Fig.2b). The NORs were also differentiated by DPI and DAPI staining (Fig.2c,d), presenting as positive and negative bands (DPI+and DAPI−), respectively. Size heteromorphism of the NORs between homologous chromosomes was observed with the above methods.

Fig.2 Metaphase of A. amoyensisa. Giemsa staining, arrows indicate the secondary constriction; b. silver staining, arrows indicate AgNORs; c. DPI staining, arrows indicate positive areas; d.DAPI staining, arrows indicate negative bands; e. dual-color FISH with 18S rDNA (red) and 5S rDNA (green) probes; f. FISH with the telomeric sequence,arrows indicate chromosomes with a weak signal. Bars represent 5 μm.
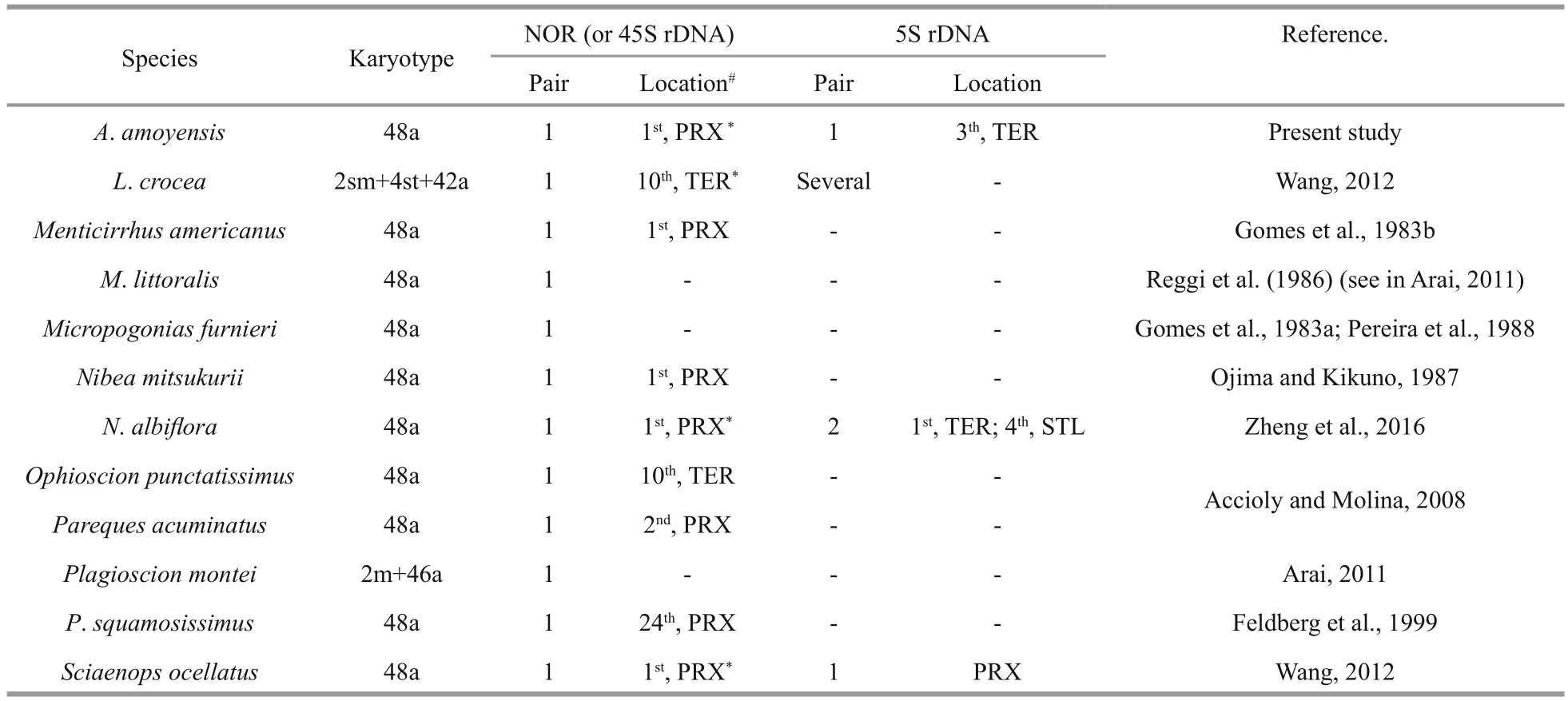
Table 1 Chromosomal organization of rDNA in Sciaenidae
Dual-color FISH clearly showed that the 18S (red)and 5S (green) rDNA sites were located in different chromosome pairs (Fig.2e). The 18S rDNA probes revealed two clusters corresponding to the Ag-NORs.The 5S rDNA sites were localized to the proximal region of chromosome 3. Size heteromorphism for both rDNA sites between homologous chromosomes was common in the observed metaphases. FISH with a telomere probe showed signals at both ends of all chromosomes, although some signals were very weak. Interstitial telomere signals were not found(Fig.2f).
4 DISCUSSION
The family Sciaenidae is remarkable for its chromosomal stability (Accioly and Molina, 2008).The representatives of this family share several symplesiomorphic cytogenetic features, such as 2n=48, fundamental number (FN)=48, a typical symmetrical karyotype, single and pericentromeric nucleolar organizer regions (NORs) and few conspicuous heterochromatic blocks (Accioly and Molina, 2008). This trend of chromosomal conservation, both numerical and structural, was corroborated by the present results obtained in the sciaenidA.amoyensis.
The karyotype ofA.amoyensiscomprised 2n=48 acrocentric chromosomes. This karyotype is a synapomorphy for most studied Sciaenids, expect a few species vary in chromosome numbers or fundamental numbers, such asCynoscionarenarius(2n=48, FN=50),Corvinanigra(2n=46, FN=48)(Arai, 2011). A 48 acrocentric chromosome karyotype,also widespread in other families of Perciformes, is regarded as the primitive condition for this fi sh group(Galetti et al., 2000; Junior et al., 2006) and a synapomorphy for modern teleosteans (Brum and Galetti, 1997).
InA.amoyensis, the NORs were located at the proximal position of chromosome 1, which was confi rmed by FISH with 18S rDNA probes. Interstitial NOR sites in a single acrocentric pair are also present in other sciaenid species (Table 1), and represent a conserved condition for this family, as well as for Perciformes (Aguilar and Galetti, 1997). Besides being interstitially located, NORs are also located in terminal regions in a few sciaenids, such asLarimichthyscroceaandOphioscionpunctatissimus(Table 1). These variation of NOR location, together with the changes of FN in some sciaenids, indicate that pericentromeric rearrangements may have occurred occasionally with low frequency during the evolutionary process of sciaenids.
The 5S rDNA sites have been mapped in only four sciaenid species. Contrary to the stability of numbers of NORs, high levels ofinterSpecific variation have been observed for 5S rDNA sites (Table 1). High dynamism of 5S rDNA sites is also present in other fi sh groups. For example, in the genusGymnotus(Gymnotiformes, Gymnotidae), the 5S rDNA sites show different topologies and frequencies (from one pair inG.sylviusup to 15 inG.cf.carapo), although the 18S rDNA sites are conserved in both number and location (Scacchetti et al., 2011). The diversity of 5S rDNA together with relative stability of 18S rDNA in the karyotypes of sciaenids reinforces the hypothesis that they evolve independently and are subject to individual selective pressure (Martins and Galetti,1999). Unequal exchange and transposition mediated by transposons were proposed as possible mechanisms for the high dynamism of rDNA (Coen and Dover,1983; Da Silva et al., 2011; Symonová et al., 2013).Although the exact mechanism underlying the variability in the 5S rDNA sites of sciaenids is still unknown, a terminal distribution, as observed inA.amoyensis,S.ocellatusandL.crocea, may contribute to the dispersion of 5S rDNA sites. The dynamic character of 5S rDNA sites makes them effective cytotaxonomic markers, particularly under the background of low karyotypic diversity among sciaenids.
Dual-FISH showed that the 18S and 5S rDNA sites were located on distinct chromosome pairs inA.amoyensis. Divergent locations for the 18S and the 5S rDNA loci appear to be the most common situation in fi sh, and even in higher eukaryotes (Drouin and de Sá, 1995). The separate locations of the two rDNA clusters enable them to evolve independently, and avoid disruptive interference such as translocation between the 45S and 5S rDNA arrays. However, a syntenic organization for both rDNA sites has also been found in species in different groups, indicating that the conversion between synteny and divergence of 45S and 5S rDNA sites may have occurred independently many times during the evolutionary process of organisms (Martins and Galetti, 1999). For example, one of the two detected 5S rDNA sites were syntenic with the 45S rDNA inN.albif l ora(Zheng et al., 2016).
Self-GISH is a modified GISH procedure in which self-genomic DNA probes free of blocking DNA are hybridized to the chromosomes. The procedure has been used to produce signal bands in plants as well as in fi sh (She et al., 2007; Markova and Vysko, 2009;Zheng et al., 2016). The signal pattern of self-GISH was demonstrated to be in accordance with that obtained from FISH withC0t-1 DNA in rice,suggesting that the signal intensity of self-GISH may reflect theC0t values of DNA probes (She et al.,2007). Hence, the self-GISH procedure has been used to survey the distribution of repetitive DNAs in chromosome complements and to assist chromosome identifi cation (She et al., 2007; Zheng et al., 2016). In the present study, remarkably strong signals produced by self-GISH were distributed at all centromeric regions. However, the distribution of self-GISH signals is different from the C-bands, the regions of constitutive heterochromatin. Although the mechanism of GISH banding remains to be elucidated,the stable fluorescence bands obtained by the procedure can serve as useful markers for chromosome identifi cation. It may be useful for sciaenids whose chromosomes are diffcult to be distinguished for small size, similar shape and continuous distribution of quantitative characteristics (i.e. length).
The chromosomal distribution of the telomeric DNA sequences has been examined with FISH in approximately 80 fi sh species to investigate chromosomal rearrangements (Ocalewicz, 2013). A lack ofinterstitial telomere signals indicates chromosome rearrangement, inversion and fusion events may not have occurred during the evolutionary history ofA.amoyensis, which reinforces de chromosomal conservation in this species (Slijepcevic,1998; Ruiz-Herrera et al., 2008). The variability of signal intensity among the telomeres may reflect different telomere lengths among the chromosomes(Canela et al., 2007).
5 CONCLUSION
In the present study, we updated the information on the karyotype of Amoy croaker. The karyotype of Amoy croaker was relatively conserved and primitive,considering its chromosome composition (2n=48a),single 18S rDNA at proximal region, no synteny between 18S and 5S rDNA sites, and no interstitial telomere. In comparison with the available cytogenetic data of other sciaenids, it can be deduced that although the karyotypic macrostructure and chromosomal location of 18S rDNA is predominantly conserved,sciaenids show some cytogenetic variability at the microstructural level. Therefore, critical analysis of the fi ne structures of the karyotypes with more markers in more sciaenid species is needed to investigate the dynamics of genes and repetitive sequences and to answer evolutionary and ecological questions.
猜你喜欢
杂志排行
Journal of Oceanology and Limnology的其它文章
- Response of the North Pacific Oscillation to global warming in the models of the Intergovernmental Panel on Climate Change Fourth Assessment Report*
- Effect of mesoscale wind stress-SST coupling on the Kuroshio extension jet*
- Surface diurnal warming in the East China Sea derived from satellite remote sensing*
- Cross-shelf transport induced by coastal trapped waves along the coast of East China Sea*
- Observations of near-inertial waves induced by parametric subharmonic instability*
- Seasonal variation and modal content ofinternal tides in the northern South China Sea*
