基于End-On叠氮桥联的混合价Co(Ⅱ/Ⅲ)和一维Cu(Ⅱ)链-席夫碱化合物的合成、结构及磁学性质
2018-03-14田菊梅张景萍
田菊梅 张景萍
(1厦门医学院口腔医学系,厦门 361023)
(2东北师范大学化学学院,长春 130024)
The design of molecule-based magnetic materials is one of the hot topics owing to their potential applications including high-density information storage,quantum information computing technology,spintronic and magnetocaloric refrigeration[1-13].Magnetic materials can display attractive architecture and magnetic exchange coupling due to their tunable characteristics by employing well established design principle and synthetic procedure[14-17].There are many factors influencing the target magnetic complexes during the course of synthesis,such as well-designed polydentate ligands,bridging ligands,transition metal ions,solvent,temp-erature,stoichiometric ratio,pH value,crystallization mechanism and the order of addition.Our present work has focused on investigating the influence of bridging ligands on the molecular structure dimension and magnetic properties of the resulting system,and exploring the magneto-structural correlations.
According to the reported literature,we find that the ligand diazine Schiff base N,N′-bis(salicylidene)hydrazine (H2salhn)is especially useful in forming mono-/dinuclear metal complexes,e.g.,dinuclear 3d metal complexes M2(salhn)3(M=Fe,Co,Mn)[18-21],dinuclear zinc and copper complex M2(salhn)2(H2O)4(M=Zn,Cu)[22],mononuclear ruthenium complex[RuH(CO)(PPh3)2(salhn)][23],series of di-ruthenium complexes[24],dirhodium [(CO)2Rh(salhn)Rh (CO)2][25].The N2O2-donor Schiff base H2salhn provides the=N-N=fragment,which acts not only as chelating sites,but also as bridging unit between the two metal ions[20].Therefore,H2salhn is inclined to act as a role of chelating ligands,and thus to form two nuclear clusters.This allows us to consider employing other bridging ligands,which may be helpful to assemble extended system (from 0D to 1D or 2D or 3D)and to investigate the related magnetic properties of the resulting novel systems.
Therefore,a suitable bridging ligand plays a crucial role in assembling extended magnetic materials of the Schiff base H2salhn system.In our previous work[26-27],our group has assembled magnetic materials by selecting carboxylate bridging ligands to bind 3d transition-metal ions into polynuclear aggregates.As the expansion of this line,the azido ligand is another suitable choice as bridging ligand for the construction of magnetic materials due to their extremely versatile coordination modes and effectively mediating magnetic exchange interactions[28-38].Herein,we have successfully synthesized and studied two novel complexes[CoⅡCoⅢ4(salhn)4(N3)6(CH3OH)2(H2O)2]·4CH3OH·2H2O (1)and[Cu2(salhn)(N3)2]n(2).Complex 1 consists of a mixed-valence penta-nuclear cobalt cluster.Compared with the previous Co2(salhn)3diamagnetic system[20],complex 1 shows antiferromagnetic behaviors according to the magnetic measurements.Complex 2 consists of a 1D copper chain,in which the azido groups successfully replace coordinated water molecules to form extended network by using different synthetical method as compared to the reported Cu2(salhn)2(H2O)4system[22].In these two complexes,azido groups both adopt end-on (EO,μ-1,1)coordination mode.The magnetic measurements indicate complex 2 shows antiferromagnetic behavior.Here,we have reported the synthesis,X-ray singlecrystal structure,and magnetic properties of these two complexes.
1 Experimental
1.1 Reagents and physical measurements
Caution! The reported azideispotentially explosive.Only small amounts of the material should be handled,and with care.
Solvents and starting materials were purchased commercially and used as received without further purification unless otherwise noted.The Schiff base H2salhn was prepared in~95%yield by condensation reaction of hydrazine and salicylaldehyde in a 1∶2 molar ratio in methanol according to the reported method[19].The resulted yellow precipitate followed by recrystallization from dimethylformamide (DMF)to form yellow single crystal of H2salhn suited for X-ray diffraction analysis, which crystallizes in the monoclinic space group P21/n with the crystallographic parameter:a=8.526 0(19)nm,b=6.319 0(14)nm,c=11.823(3)nm,β=107.846(3)°.
The elemental analyses (C,H,and N)were performed on a Perkin-Elmer Model 240C elemental analyzer.The Cu and Co elemental analyses were determined by the Leaman inductively coupled plasma(ICP)spectrometer.Infrared spectra (400~4 000 cm-1)were measured on a Perkin-Elmer Fourier transform infrared (FTIR)spectrophotometer using KBr pellets.Powder X-ray diffraction (PXRD)measurements were assessed on a Siemens D5005 diffractometer with Cu Kα (λ=0.154 184 nm)with a step size of 0.1°in 2θ range of 5°~50°at room temperature.The XRD accelerating voltage and emission current were 40 kV and 30 mA,respectively.
1.2 Syntheses of complexes 1~2
1.2.1 Synthesis of[CoⅡCoⅢ4(salhn)4(N3)6(CH3OH)2

H2salhn (0.24 g,1 mmol)and KOH (0.11 g,2 mmol)were dissolved in methanol solvent (20 mL),forming a clear yellow solution,which was magnetically stirred at 40~50 ℃ for 30 min.Then,a methanol solution (20 mL)of CoCl2·6H2O (0.71 g,3 mmol)was added to the hot yellow solution.After 15 min,an aqueous solution (1 mL)of sodium azide (0.20 g,3 mmol)was added under continuous stirring for 1 h.Black-colored,needle-like crystals of complex 1 were obtained from the filtrate after a few days.Yield:65%(based on Co ions).Anal.Calcd.for C62H72Co5N26O18(% ):Co,16.70;C,42.21;H,4.11;N,20.64.Found(%):Co,17.10;C,41.82;H,4.28;N,20.14.IR (KBr,cm-1):2 077 and 2 023 for the azido groups,1 603 for ν(C=N).
1.2.2 Synthesis of[Cu2(salhn)(N3)2]n(2)
An aqueous solution (1 mL)of sodium azide(0.039 g,0.6 mmol)was added to a methanol solution(20 mL)of Cu(NO3)2·3H2O (0.097 0.4 mmol).After stirring for 20 min,a hot DMF solution (2 mL)of H2salhn (0.048 g,0.2 mmol)and triethylamine (0.040 g,0.4 mmol)was added to the resulting mixture with continuous stirring for 3 h.Black-colored,block crystals of 2 were obtained from the filtrate after a few days.Yield:60% (based on Cu ions).Anal.Calcd.for C14H10Cu2N8O2(%):Cu,28.28;C,37.42;H,2.24;N,24.94.Found(%):Cu,28.34;C,37.12;H,2.29;N,24.44.IR (KBr,cm-1):2 046 and 2 101 for the azido groups,1 604 for ν(C=N).
1.3 Details of X-ray crystallography
Single-crystal X-ray diffraction data sets for 1 and 2 were obtained on a Bruker SMART APEX CCD area detector diffractometer using graphite monochromated Mo Kα radiation (λ=0.071 073 nm)at 195 K.Structure solution (direct methods)and the refinement of full-matrix least-squares were carried out using the SHELXTL software package[39].All the non-hydrogen atoms were refined with anisotropic thermal parameters.The hydrogen atoms attached to carbon and nitrogen atoms were placed in geometrically calculated positions.Crystallographic and refinement details for all compounds are summarized in Table 1.Selected bond lengthsand anglesare listed in TableS1~S2(Supporting information).
CCDC:869426,1;869427,2.
1.4 Magnetic measurements
The magnetic susceptibility measurementsonpolycrystalline samples of 1 and 2 were conducted on Quantum Design SQUID MPMS XL-5 instruments in the temperature range of 300~2 K and under the applied magnetic field of 1 and 5 kOe,respectively.The field dependent magnetizations for 1 and 2 were measured at 2 K in the field range of 0~5 T.The temperature dependence of the field-cooled (FC),zerofield-cooled (ZFC)for 2 was assessed under a dc field of 100 Oe.

Table 1 Crystallographic data and structure refinement summary for complexes 1~2
2 Results and discussion
2.1 Synthesis
Complex 1 has been synthesized by reacting CoCl2·6H2O,H2salhn,KOH,and NaN3in 3 ∶1 ∶2 ∶3 molar ratio in the mixture solvent(41 mL)of methanol and water (40 ∶1,V/V).The trivalent cobalt may be resulted from the oxidation of Co(Ⅱ) to Coバ by atmospheric oxygen gas under basic condition.Excess of metal material may be responsible to the presence of divalent cobalt center in complex 1,compared to the full trivalent metal system Co2(salhn)3assembling from CoCl2·6H2O,H2salhn,KOH in 2∶3∶6 molar ratio[20].
A mixture solvent(methanol 20 mL,DMF 2 mL and water 1 mL)of Cu(NO3)2·3H2O,H2salhn,NaN3,and triethylamine in 2 ∶1 ∶3 ∶2 molar ratio yields block crystals of 2.
2.2 X-ray crystal structures
2.2.1 Structural analyses of 1

Fig.1 Structure of complex 1:(a)ellipsoid of the subunit and the numbering scheme with probability of 30%;(b)penta-nuclear cobalt cluster and the numbering scheme for cobalt ions viewing from the same direction as (a);(c)zigzag-like cluster along a axis
Single-crystal X-ray diffraction analysis indicates that complex 1 crystallizes in the monoclinic space group P21/n.The selected bond distances and bond angles are given in Table S1.The molecule is a neutral zigzag-like penta-nuclear cobalt cluster.And four CH3OH molecules and two H2O molecules are located in the crystal lattice.The asymmetric unit consists of three Co ions,two deprotonated ligands(salhn2-),two end-on azido groups,one terminal azido group,one coordinated H2O molecule and CH3OH molecule (Fig.1a).Co(1)is located on an inversion centre and exhibits a N2O4coordination polyhedron with octahedral geometry.Two nitrogen atoms from two azido groups are situated at axial positions.The equatorial positions are occupied by four oxygen atoms belonging to two coordinated H2O molecules and CH3OH molecules,respectively.The azido group acts as the only bridge between Co(1)and Co(2)with the bond angleCo(1)-N(11)-Co(2), 131.89° and the Co(1)…Co(2)distance,0.374 7 nm.The two phenolate oxygens of H2salhn are deprotonated acting as a quadridentate (N2O2)dianion containing two nitrogen atoms from diazine (=N-N=)binding two metal ions Co(2)and Co(3).The other two sites for Co(2)and Co (3)have been taken up by nitrogen atoms from azido anions.It is worth noting that a reference of charge balance as well as an analysis of the Co-N and Co-O bond lengths,which are longer for Co(1)(with 0.213 5 and 0.207 2~0.207 9 nm,respectively)than for Co(2)and Co(3)(with 0.191 4~0.196 7 nm and 0.187 9~0.189 8 nm,respectively),clearly indicate the presence of mixed valence cobalt ions.The+2 valence state is assigned to Co(1),while the+3 valence state is assigned to Co(2)/Co(3)and their symmetry related Co(2)#1/Co(3)#1,which are also confirmed by bond valence sum (BVS)calculations[20,40-41].
2.2.2 Structural analyses of 2
Complex 2 crystallizes in the monoclinic space group P21/n,and the asymmetric unit consists of two crystallographically independent Cu atoms,one salhn2-ligand,and two N3-groups (Fig.2a).Ligand H2salhn acts as a quadridentate (N2O2)bianion (salhn2-)linked through two imine N atoms and two deprotonated phenoxide O atoms.In the dimer unit,each Cu ion is penta-coordinate to form a distorted square pyramidal coordination environment.The Addison parameter (τ)is 0.06 for Cu(1)and 0.22 for Cu(2)(τ is equal to zero for a perfect square-pyramidal geometry,while it is one for an ideal trigonal bipyramidal geometry)[42].Each Cu ion is coordinated by two N atoms from two different azido groups,one N atom from one salhn2-ligand,and two oxygen atoms from two different salhn2-ligands.Within the dimer,a triplicate bridge between Cu(1)and Cu(2)is made by two phenoxide O atoms(O(1),O(2))from two salhn2-ligands and one EO(μ-1,1)azido N atom N(6).The two phenoxide-O (O(1),O(2))show different bridge angles (∠Cu(1)-O(1)-Cu(2)=83.48°and ∠Cu(1)-O(2)-Cu(2)=79.11°).The bridge anglethrough azido N atom (Cu (1)-N (6)-Cu (2))is 89.77°.The Cu(1)…Cu(2)distance within the dimer is 0.285 4(6)nm,but the inter-dimer Cu…Cu distance is 0.336 9 nm.The Cu-O and Cu-N bond distances lie in the range of 0.192 0(2)~0.234 1(2)nm and 0.194 8(3)~0.202 9(3)nm, respectively. The selected bond distances and bond angles are given in Table S2.

Fig.2 Structure of complex 2:ellipsoid of the dimer and the numbering scheme with probability of 50%(a);1D chain along a axis (b),or c axis (c)
Inspection of the structure of the 1D chain shows a scissor-shaped structure running along a axis(Fig.2b and 2c).The adjacent chains are packing along a axis through C(3)-H(3)…O(2)and C(14)-H(14)… N(5)hydrogen bonding interactions (Fig.3).The hydrogen bond C(3)-H(3)… O(2)involves C-H fragment of a benzene ring and the O-atom of phenolate with C(3)…O(2)distance of 0.343 1 nm and C(3)-H(3)… O (2)angle of 135.61°.The another hydrogen bond C (14)-H(14)…N(5)involves C-H fragment of methylene group and the N-atom of azido group with C(14)…N(5)distance of 0.345 9 nm and C(14)-H(14)…N(5)angle of 177.0°.
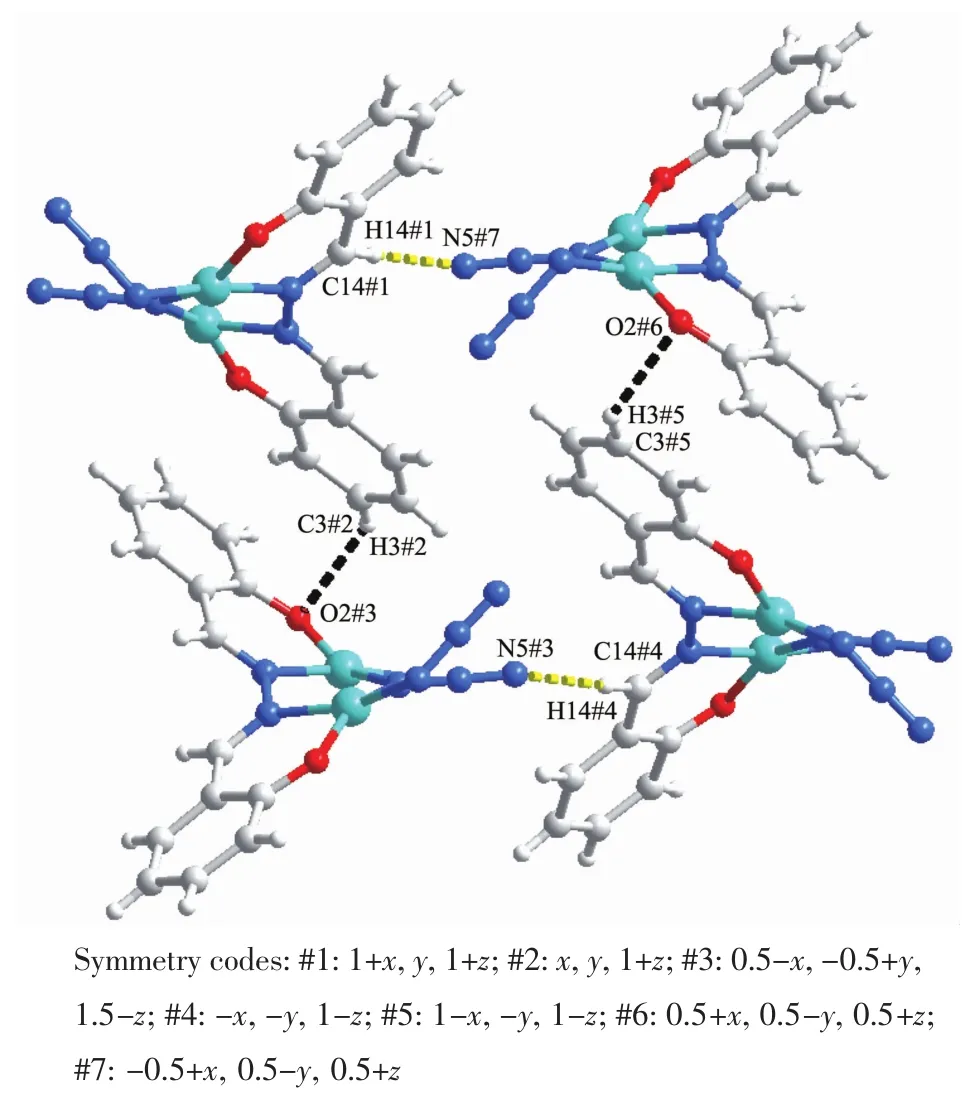
Fig.3 Packing view of 2 formed by interchain hydrogen bond C(3)-H(3)…O(2)and C(14)-H(14)…N(5)along a axis
2.2 IR spectroscopy and PXRD properties
As depicted in Fig.S1,a strong stretching band at 1 625 cm-1indicates the presence of imine group C=N stretching for ligand H2salhn,which is in agreement with the crystalstructure.However,the imine stretching appears at 1 603 cm-1for 1 and 1 604 cm-1for 2,which may be resulted from the effect of N atom coordination with metal.The similar trend can be found in the reported other metal coordination complexes possessing the same ligand[19-20].The characteristic azido stretching bands are found at 2 023 and 2 077 cm-1for 1 as well as 2 101 and 2 046 cm-1for 2,which are typical for EO(μ-1,1)azido bridging[32,43].The purity of bulky samples of 1 (Fig.S2)and 2 (Fig.S3)were corroborated by the similarities between simulated and experimental PXRD patterns.
2.4 Magnet behaviour
Variable temperature dc susceptibility measurements of 1 and 2 were collected in the temperature range of 300~2 K under an applied field of 1 000 and 5 000 Oe,respectively.From a magnetic point of view,complex 1 is effectively mononuclear Co(Ⅱ)system because the Coバsites are diamagnetic.As shown in Fig.4a,the χmT value at room temperature for 1 is 2.97 cm3·mol-1·K,much higher than the spin-only high spin Co(Ⅱ) (S=3/2)value of 1.875 cm3·mol-1·K with g=2.0.This is probably induced by the presence of orbital contribution in octahedral Co(Ⅱ)ion[44-45].Upon cooling,the χmT value first slightly decreases to a minimum value of 2.15 cm3·mol-1·K at 22 K.Below 22 K,χmT value increases slightly to reach a maximum of 2.19 cm3·mol-1·K at 14 K,and secondly drops abruptly to a minimum value of 1.57 cm3·mol-1·K at 2 K.Fitting the data above 100 K with the Curie-Weiss law gives the Curie constant C=3.23 cm3·mol-1·K and Weiss temperature θ=-22.35 K (line in Fig.4a).The large negative Weiss constant value and the first decreaseofχmT above22 K both indicatethe occurrence of significant spin-orbital coupling and zero field splitting (ZFS)of the anisotropic HS Co(Ⅱ)ion[46-48].The sharp increase between 22 and 14 K could be due to the presence of impurity and the second decrease of χmT below 14 K suggests the intermolecular antiferromagnetic interaction. The magnetization versus field plot at 2 K also confirms the observed antiferromagnetism (Fig.4b).At 5 T,the magnetization amounts to 1.68Nβ,which is not saturated and is away from the theoretical value of 3Nβ assuming g=2.

Fig.4 (a)χmT vs T and χmvs T plots in the temperature range of 2~300 K under a 1 000 Oe applied field;(b)Magnetization vs field plot at 2.0 K among 0~7 T for 1
The χmT value of complex 2 at room temperature is 0.99 cm3·mol-1·K,which is larger than the spinonly value of 0.75 cm3·mol-1·K calculated for two Cu(Ⅱ) ions with g=2 (Fig.5a).As the temperature is decreased,the χmT value shows a slight decrease to a value of 0.93 cm3·mol-1·K at 100 K,before an abrupt decrease to reach a value of ca.0.046 cm3·mol-1·K at 2.0 K.A fitting of the data above 100 K to Curie-Weiss law gives C=1.03 cm3·mol-1·K and θ=-11.34 K(line in the lower part of Fig.5a).The negative Weiss temperature and the decrease of χmT both indicate a predominant antiferromagnetic interaction.Inspection of the structure suggests two different Cu…Cu interactions combining with two different Cu…NEO…Cu angles (detailed describing in the structure part).We try to use two models:(i)alternating S=1/2 antiferromagnetic chain; (ii)regular S=1/2 antiferromagnetic chain model[42,49].Unfortunately,these two models are not able to reproduce the magnetic properties adequately.Finally we have fitted the magnetic properties to the Ising model for 1D chain[49](line in the upper part top of Fig.5a).This model reproduces the magnetic property of 2 extremely well with g=2.32 and J=-23.49 cm-1.The field dependence of the magnetization for 2 is displayed in Fig.5b.The magnetization data does not reach to the saturation value of 2.0Nβ at 5 T,but equals 0.15Nβ,which further confirms the strong antiferromagnetic interaction.Divergence of the ZFC and FC susceptibilities was observed below 50 K indicating a possible long-range ordered (Fig.6).

Fig.5 (a)χmT vs T and χmvs T plots in the temperature range of 2~300 K under a 5 000 Oe applied field;(b)Magnetization vs field plot at 2.0 K among 0~7 T for 2

Fig.6 ZFC and FC as function of temperature at H=100 Oe for 2
For copper(Ⅱ)azido complexes,the different azido bridged fashion may mediate the different magnetic interaction.There are two cases for end-on azido bridged[29,34,36,50]:(i)the Cu-NEO-Cu angle is lower than 104°(theory)or 108°(experiment),which prop-agates ferromagnetic;otherwise,it is antiferromag-netic. (ii)The longer bond distance of Cu-N3(EO)may lead to the weaker ferromagnetic interaction.In complex 2,there are two different transition angle (∠Cu(1)-N(6)-Cu(2)=89.77°and ∠Cu(1)-N(3)-Cu(2)#1=117.02°),which may propagate ferromagnetic and antiferromagnetic coupling,respectively.But the long bond distance (Cu(1)-N(6)0.201 5 nm and Cu(2)-N(6)0.202 9 nm)may weaken the ferromagnetic coupling.Therefore,the whole 1D chain behaves antiferromagnetic interaction.
3 Conclusions
In conclusion,we used azide as bridging group and successfully assembled two EO azido bridged complexes 1 and 2,which both show antiferromagnetic behaviors.Compared with the reported metal-H2salhn complexes[20,22],the azido groups within the complex successfully bridge the metalions to form the extended structure and mediate the magnetic interaction.Further investigating other bridging ligand,such as cyano,oxalate,nitride,etc,which mediate magnetic interaction of other metal Schiff base complexes,is in progress in our group.
Supporting information is available at http://www.wjhxxb.cn
[1]Mazzanti M.Nat.Chem.,2011,3:426-427
[2]Li B,Zhang J P,Zhang Y,et al.CrystEngComm,2011,13:418-420
[3]Mougel V,Chatelain L,Pécaut J,et al.Nat.Chem.,2012,4:1011-1017
[4]Mills D P,Moro F,McMaster J,et al.Nat.Chem.,2011,3:454-460
[5]Das A,Gieb K,Krupskaya Y,et al.J.Am.Chem.Soc.,2011,133:3433-3443
[6]Sanvito S.Chem.Soc.Rev.,2011,40:3336-3355
[7]Peng J B,Zhang Q C,Kong X J,et al.Angew.Chem.Int.Ed.,2011,50:10649-10652
[8]Peng J B,Zhang Q C,Kong X J,et al.J.Am.Chem.Soc.,2012,134:3314-3317
[9]Coronado E,Giménez-Marqués M,Espallargas G M,et al.Nat.Commun.,2012,3:828-835
[10]Yoshida H,Yamaura J,Isobe M,et al.Nat.Commun.,2012,3:860-864
[11]Choubey S,Bhar K,Chattopadhyay S,et al.Dalton Trans.,2012,41:11551-11554
[12]CHEN Yan-Min(陈延民),JIANG Yan(姜岩),HONG Si-Yu(洪 思 雨),et al.Chinese J.Inorg.Chem.(无 机 化 学 学 报),2017,33(6):1023-1029
[13]Seki H,Hosaka Y,Saito T,et al.Angew.Chem.Int.Ed.,2016,55:1360-1363
[14]Talham D R,Meisel M W.Chem.Soc.Rev.,2011,40:3356-3365
[15]HU Peng(胡 鹏),XIAO Feng-Ping(肖 凤 屏 ),ZHI Zhong-Qiang(植中强),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(7):1273-1279
[16]Li Z Y,Ohtsu H,Kojima T,et al.Angew.Chem.Int.Ed.,2016,55:5184-5189
[17]Friedländer S,Liu J,Addicoat M,et al.Angew.Chem.Int.Ed.,2016,55:12683-12687
[18]Jian F F,Zhu C Y,Xiao H L,et al.Z.Anorg.Allg.Chem.,2005,631:769-772
[19]Saroja J,Manivannan V,Chakraborty P,et al.Inorg.Chem.,1995,34:3099-3101
[20]Sreerama S G,Pal S.Inorg.Chem.,2005,44:6299-6307
[21]Mo H,Fang C J,Duan C Y,et al.Dalton Trans.,2003:1229-1234
[22]Aggarwal R C,Singh N K,Singh R P.J.Indian Chem.Soc.,1986,63:466
[23]Trivedi M,Chandra M,Pandey D S,et al.J.Organomet.Chem.,2004,689:879-882
[24]Singh S K,Chandra M,Pandey D S.J.Organomet.Chem.,2004,689:2073-2079
[25]Gopinathan S,Pardhy S A,Gopinathan C,et al.Inorg.Chim.Acta,1986,111:133-138
[26]Cui S X,Zhao Y L,Zhang J P,et al.Polyhedron,2009,28:980-986
[27]Cui S X,Zhao Y L,Zhang J P,et al.Synth.Met.,2009,159:2191-2193
[28]Hao Z M,Zhang X M.Dalton Trans.,2011,40:2092-2098
[29]Zeng Y F,Hu X,Liu F C,et al.Chem.Soc.Rev.,2009,38:469-480
[30]Kang L C,Chen X,Wang X S,et al.Dalton Trans.,2011,40:5200-5209
[31]Naiya S,Biswas C,Drew M G B,et al.Inorg.Chem.,2010,49:6616-6627
[32]Tandon S S,Bunge S D,Motry D,et al.Inorg.Chem.,2009,48:4873-4881
[33]Mukherjee S,Gole B,Chakrabarty R,et al.Inorg.Chem.,2009,48:11325-11334
[34]Tian C B,Li Z H,Lin J D,et al.Eur.J.Inorg.Chem.,2010:427-437
[35]Zhang L F,Yu M M,Ni Z H,et al.J.Mol.Struct.,2011,1006:629-634
[36]Gao Q,Xie Y B,Thorstad M,et al.CrystEngComm,2011,13:6787-6793
[37]Weng D F,Wang Z M,Gao S.Chem.Soc.Rev.,2011,40:3157-3181
[38]Wang S,Zuo J L,Gao S,et al.J.Am.Chem.Soc.,2004,126:8900-8901
[39]Sheldrick G M.SHELXS-97 and SHELXL-97,University of Göttingen,Germany,1997.
[40]Tandon S S,Bunge S D,Rakosi R,et al.Dalton Trans.,2009:6536-6551
[41]Alley K G,Bircher R,Waldmann O,et al.Inorg.Chem.,2006,45:8950-8957
[42]Adhikary C,Koner S.Coord.Chem.Rev.,2010,254:2933-2958
[43]Demeshko S,Leibeling G,Maringgele W,et al.Inorg.Chem.,2005,44:519-528
[44]Nagaraja C M,Kumar N,Maji T K,et al.Eur.J.Inorg.Chem.,2011:2057-2063
[45]Karasawa S,Koga N.Inorg.Chem.,2011,50:2055-2057
[46]Li B,Zhang J P,Yong X,et al.Dalton Trans.,2011,40:4459-4464
[47]Vallejo J,Castro I,Ruiz-García R,et al.J.Am.Chem.Soc.,2012,134:15704-15707
[48]Han Y,Xu H,Liu Y,et al.Chem.Eur.J.,2012,18:13954-13958
[49]Kahn O.Molecular Magnetism.New York:Wiley-VCH,1993.
[50]Zeng Y F,Zhao J P,Hu B W,et al.Chem.Eur.J.,2007,13:9924-9930
