Advanced diffusion-weighted magnetic resonance imaging in the evaluation of white matter axons in patients with idiopathic normal pressure hydrocephalus
2018-01-05MasaakiHori,KouheiKamiya,RyusukeIrie
Advanced diffusion-weighted magnetic resonance imaging in the evaluation of white matter axons in patients with idiopathic normal pressure hydrocephalus
With the increasing application of regenerative medicinein vivo,non-invasive and accurate methods for estimating the white matter axons in neuronal tissue have become increasingly important.As a non-invasive method for patients, magnetic resonance (MR)imaging has demonstrated potential as a promising tool for evaluating axonsin vivo. In particular, diffusion-weighted MR imaging(dMRI) and its applications, such as white matter tractography and the metrics derived from dMRI data (i.e., mean diffusivity and fractional anisotropy (FA)), have been used for the semi-quantitative measurement of normal and diseased axons of neuronal tissues,particularly in neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and idiopathic normal pressure hydrocephalus (iNPH), because the diagnosis and monitoring of the status of neurodegenerative diseases are usually estimated based on clinical symptoms, gene abnormalities and imaging findings.Moreover, recent advances in MR imaging, such as more complicated and dedicated dMRI analyses, are emerging and can be compared with conventional dMRI. Unlike the previous conventional dMRI, recent advances in dMRI are based on the theory of the non-Gaussian assumption of water molecule diffusivity and/or compartment model analysis because the Gaussian assumption of water molecule diffusion in the tissue made by conventional dMRI theory has drawbacks and limitations that restrict the more accurate estimation of the microstructures of actual neuronal tissues. In actual neuronal tissues, Gaussian distributions of water molecules are rarely observed because tissue microstructures, such as cell membranes, organelles and myelin sheaths, can act as barriers to water molecules. Therefore, emerging and advanced dMRI analyses have the potential to provide new and additional information regarding the actual neuronal tissue microstructures compared with conventional dMRI.
iNPH is among the several types of neurodegenerative diseases. iNPH is a disease of unknown etiology, and its major triad of symptoms consists of gait disturbance, dementia and bladder incontinence. A characteristic feature of iNPH is that its symptoms can be relieved by a neurosurgical operation that drains excess cerebrospinal fluid (CSF), which is not the case for most neurodegenerative disorders. An improvement of symptoms is observed in patients with iNPH following shunt surgery, but the mechanism and origin of the symptoms of iNPH still require clarification.For example, the decreased excess CSF is not the only reason for improvement because some patients’ symptoms improve without a marked change in the dilated ventricles after shunt surgery. In a more complicated scenario, microstructural changes in the neuronal tissue may be the cause of the symptoms. Applying advanced dMRI for iNPH patients is expected to provide additional and substantive information.
Diffusion kurtosis imaging (DKI) is an advanced dMRI technique that accesses the non-Gaussianity of water molecule displacement as a dimensionless metric, such as the mean kurtosis (MK). In iNPH patients, both the FA and MK are correlated with cognitive impairment, especially in the frontal lobe white matter; however, the MK may be superior for evaluating the changes in the subcortical white matter (Kamiya et al., 2016). Moreover, because it measures directionally speci fic kurtosis, DKI was found to potentially offer a more comprehensive and sensitive detection of tissue microstructural changes in a rodent maturation study (Cheung et al., 2009). Another dMRI analysis used the recently introduced MR technique of neurite orientation dispersion and density imaging (NODDI) to examine brain tissue using a three-compartment model that consisted of the intra-cellular component of restricted water, which indicates structures such as axons; the hindered extra-cellular water, which indicates structures such as glia; and the free (Gaussian-distributed)water of the CSF (Zhang et al., 2012). This technique can effectively evaluate the neurite abnormalities in the corticospinal tract (CST)in the brain, which are closely related to the symptom of gait disturbance in iNPH patients (Irie et al., 2017).
Q-space imaging (QSI) has also been introduced as an advanced dMRI technique for research and clinical purposes. This dMRI technique does not assume a predefined shape of the probability density function (PDF) for the diffusion displacement of the water molecules. In other words, whether the distribution of water molecules is Gaussian is not problematic for QSI measurements. Brie fly,in QSI, the PDF of molecular water diffusion displacement is obtained by means of a Fourier transform of the measured diffusion echo attenuation of the dMRI data, and the full-width at half-maximum (FWHM) of the displacement PDF correlates with the localized space between barriers, including the cell membrane and/or myelin sheaths, in neuronal tissuesin vivo. Therefore, QSI has the potential to provide microstructural information about neuronal tissues, including the cell compartment size and axon diameter.However, the precise assessment of axonal architecture using the QSI technique usually requires high gradient amplitudes in the MR imaging system, which are not safe for humans, and long scanning times (~several hours), which are not realistic forin vivohuman studies or clinical use. As an alternative method for analyzing QSI data, a two-component low-qfit model has been proposed. This model fits the measured echo attenuation at only the lowq-values to extract the root mean square displacement of the water molecule diffusion. High-performance MR imaging systems with high gradient amplitudes are not needed for this approach. Moreover, good correlations between the metrics derived from two-component low-qfit QSI data and pathologic data related to axon diameters in excised mouse spinal cords have been reported in the literature (Ong and Wehrli, 2010). However, these authors scanned the excised spinal cords of pigs for 5 hours, which is not suitable for clinical applications. Using this approach with optimized imaging parameters to achieve a reasonable scanning time in a clinical MR scanner,one study that utilized QSI analysis with the two-component low-qfit model found that iNPH patients exhibited a signi ficant increase in the intra-axonal volume fraction in the CST at the paraventricular level, but no significant difference was observed in the axon diameter compared with the normal controls (Kamiya et al., 2014).Another study using the same technique reported that the extracellular space of the posterior limb of the internal capsule (PLIC) was signi ficantly higher than that in the same patients after lumboperitoneal shunt surgery, and no signi ficant differences were observed in the PLIC axon diameters among the normal controls or in the patients before and after surgery (Hori et al., 2016) (Figure 1).
These are very interesting and important findings regardingin vivoaxons and their surrounding structures. In patients with iNPH, past investigations suggest that compression and stretching of the white matter tracts of the CST may be the cause of reliable gait disturbance (Nakanishi et al., 2013). After neurosurgical procedures, such as lumboperitoneal shunt surgery, the symptoms soon improve in some patients with iNPH. Therefore, it was expected that irreversible structural damage was not directly observed in the axons and that microstructural changes, including narrowing of the peri-axonal space (extracellular space), accounted for the symptom. The results of the above two example studies support this hypothesis. Additionally, the extracellular space in patients with iNPH before treatment is wider than that in normal controls and significantly narrower than that in patients after shunt surgery (Hori et al., 2016). These findings may indicate that the cause of iNPH is partially explained by an inefficiency of the extracellular out flow pathway of the CSF in the extracellular space that includes the venous side of the glymphatic system, which might be developed as a parallel pathway for CSF resorption and exit from the ventricles in patients with iNPH (Bradley, 2015).
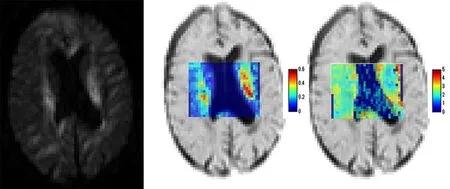
Figure 1 Example images of QSI analysis using the two-component low-q fit model.A 65-year-old man with iNPH. Examples of the raw diffusion image (b =3,000 s/mm2) for q-space imaging, intra-axonal volume fraction maps and axon diameter maps (μm) on inverted T2–weighted images.
There are still several limitations to the application of advanced dMRI to the investigation of white matter axonsin vivo. First,dMRI techniques generally require more time for data acquisition compared with conventional diffusion-weighted imaging (DWI).Additionally, much more time will be needed for images with higher spatial resolutions. In clinical settings, routine DWI only requires 1 minute or less for data acquisition, whereas advanced dMRI requires more than twice as much time. However, MR scanning times can shortened depending on the MR imaging technique; for example, the parallel imaging and simultaneous multi-slice techniques have been used over the past several years. Therefore, from a technical perspective, the long acquisition time of advanced dMRI will be solved by progress in the software and hardware of MR imaging systems in the near future. As a second limitation, although the diffusion metrics have potential as imaging biomarkers for investigating axonsin vivo, reproducibility is a concern because the MR imaging signal produces a relative value. There are several factors that could be problematic for image reproducibility, such as differences between MR scanner venders, models, imaging parameters and software (including sequence designs), in all types of advanced and conventional dMRI. For example, theb-value dependences on diffusion tensor imaging (DTI) quantitation and the sensitivity of detecting neural tissue changes have been reported (Hui et al.,2010). Therefore, caution must be taken when interpreting dMRI metrics, and con firmation of the reliability and standardization of imaging techniques for advanced dMRI must be established for imaging biomarkers of axonsin vivo.
Despite the numerous limitations mentioned above, advanced dMRI, especially QSI, has the potential to non-invasively provide novel information about axonsin vivoand will be a necessary tool for research and clinical use. The microstructural changes of axons and peri-axonal tissues can be directly measured as quantitative values with advanced dMRI techniques;e.g., axon diameters can be measuredin vivo. These advances indicate that the monitoring of axons in patients is possible and will provide a powerful support tool for managing patients with degenerative diseases and for examining degenerative and regenerative processes.
This work was supported by JSPS KAKENHI Grant Number JP16H06280, Grant-in-Aid for Scientific Research on InnovativeAreas-Resource and technical support platforms for promoting research ‘Advanced Bioimaging Support’ and the ImPACT Program of Council for Science, Technology and Innovation (Cabinet Office,Government of Japan).
Masaaki Hori*, Kouhei Kamiya, Ryusuke Irie
Department of Radiology, Juntendo University School of Medicine,Tokyo, Japan (Hori M, Kamiya K, Irie R)
Department of Radiology, The University of Tokyo, Tokyo, Japan(Kamiya K, Irie R)
*Correspondence to:Masaaki Hori, mahori@juntendo.ac.jp.
orcid:0000-0002-1791-8032 (Masaaki Hori)
How to cite this article:Hori M, Kamiya K, Irie R (2017) Advanced diffusion-weighted magnetic resonance imaging in the evaluation of white matter axons in patients with idiopathic normal pressure hydrocephalus.Neural Regen Res 12(12):1974-1975.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewer:Takashi Noguchi, Boston Medical Center, USA.
Bradley WG (2015) CSF flow in the brain in the context of normal pressure hydrocephalus. AJNR Am J Neuroradiol 36:831-838.
Cheung MM, Hui ES, Chan KC, Helpern JA, Qi L, Wu EX (2009) Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. Neuroimage 45:386-392.
Hori M, Kamiya K, Nakanishi A, Fukunaga I, Miyajima M, Nakajima M, Suzuki M, Suzuki Y, Irie R, Kamagata K, Arai H, Aoki S (2016) Prospective estimation of mean axon diameter and extra-axonal space of the posterior limb of the internal capsule in patients with idiopathic normal pressure hydrocephalus before and after a lumboperitoneal shunt by using q-space diffusion MRI. Eur Radiol 26:2992-2998.
Hui ES, Cheung MM, Chan KC, Wu EX (2010) B-value dependence of DTI quantitation and sensitivity in detecting neural tissue changes. Neuroimage 49:2366-2374.
Irie R, Tsuruta K, Hori M, Suzuki M, Kamagata K, Nakanishi A, Kamiya K, Nakajima M, Miyajima M, Arai H, Aoki S (2017) Neurite orientation dispersion and density imaging for evaluation of corticospinal tract in idiopathic normal pressure hydrocephalus. Jpn J Radiol 35:25-30.
Kamiya K, Hori M, Miyajima M, Nakajima M, Suzuki Y, Kamagata K, Suzuki M, Arai H, Ohtomo K, Aoki S (2014) Axon diameter and intra-axonal volume fraction of the corticospinal tract in idiopathic normal pressure hydrocephalus measured by q-space imaging. PLoS One 9:e103842.
Kamiya K, Kamagata K, Miyajima M, Nakajima M, Hori M, Tsuruta K, Mori H, Kunimatsu A, Arai H, Aoki S, Ohtomo K (2016) Diffusional kurtosis imaging in idiopathic normal pressure hydrocephalus: correlation with severity of cognitive impairment. Magn Reson Med Sci 15:316-323.
Nakanishi A, Fukunaga I, Hori M, Masutani Y, Takaaki H, Miyajima M,Aoki S (2013) Microstructural changes of the corticospinal tract in idiopathic normal pressure hydrocephalus: a comparison of diffusion tensor and diffusional kurtosis imaging. Neuroradiology 55:971-976.
Ong HH, Wehrli FW (2010) Quantifying axon diameter and intra-cellular volume fraction in excised mouse spinal cord with q-space imaging. Neuroimage 51:1360-1366.
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61:1000-1016.
2017-10-16
10.4103/1673-5374.221149
杂志排行
中国神经再生研究(英文版)的其它文章
- Roles of neural stem cells in the repair of peripheral nerve injury
- Conductive polymer scaffolds to improve neural recovery
- The contribution of oligodendrocytes and oligodendrocyte progenitor cells to central nervous system repair in multiple sclerosis: perspectives for remyelination therapeutic strategies
- MicroRNAs in Parkinson’s disease and emerging therapeutic targets
- Surgical reconstruction of spinal cord circuit provides functional return in humans
- Environmental cues determine the fate of astrocytes after spinal cord injury
