SBA-15表面改性及其对介孔La0.8Sr0.2CoO3钙钛矿型催化剂结构和性能的影响
2017-11-01黄学辉商晓辉牛鹏举
黄学辉 商晓辉 牛鹏举
(武汉理工大学材料科学与工程学院,武汉 430070)
SBA-15表面改性及其对介孔La0.8Sr0.2CoO3钙钛矿型催化剂结构和性能的影响
黄学辉*商晓辉 牛鹏举
(武汉理工大学材料科学与工程学院,武汉 430070)
本文通过改进的水热法制备了孔径较大的有序介孔SBA-15,其孔径(Dp)为10.1 nm,并采用浓盐酸、三甲基氯硅烷(TMCS)和3-氨丙基三乙氧基硅烷(APTES)对SBA-15表面进行了羟基、甲基和氨基改性处理。以嫁接不同表面官能团的SBA-15为模板,合成了一系列菱方晶系钙钛矿型介孔La0.8Sr0.2CoO3氧化物,系统地研究了模板SBA-15的表面性能对La0.8Sr0.2CoO3氧化物结构和性能的影响。X射线衍射(XRD)图谱表明,外表面引入―CH3,内表面羟基化和引入―NH2的模板有利于合成结晶度较高的、物相单一的钙钛矿结构氧化物。小角XRD图谱、高分辨率透射电子显微镜(HRTEM)图像以及N2吸附测试结果表明,所制备的催化剂为介孔结构。CO氧化的催化活性测试表明,采用表面改性的模板合成的La0.8Sr0.2CoO3氧化物表现出较高的催化活性和稳定性,在140 °C时可以实现CO的完全转化,连续使用100 h后催化剂的催化活性几乎没有下降。X射线光电子能谱(XPS)、H2程序升温还原(H2-TPR)和O2程序升温脱附(O2-TPD)测试结果表明,催化剂表面丰富的吸附氧物种,高价态的Co离子,钙钛矿结构中Sr在表面富集,及优良的低温氧化还原性是催化活性提高的主要原因。
SBA-15;表面改性;模板法;介孔La0.8Sr0.2CoO3;低温CO氧化
1 Introduction
Catalytic oxidation of CO is always one of the research hotspots in the field of catalysis not only due to its extensive industrial applications, such as automobile exhaust emission control, chemical processing, CO2lasers, sensors, fuel cell technology, as well as air purification1,2, but also because of its relative simplicity as an ideal model reaction in heterogeneous catalysis3. Recently, many researchers devoted to developing novel and highly-efficient catalysts for CO oxidation1. Noble metal catalysts exhibit high activity for CO oxidation, but they are rare, expensive, less thermally stable and easy poisoning,which limits their applications2−5.
Perovskite complex oxides (ABO3) were found to be the most promising alternative to noble metal catalysts in a variety of catalytic processes due to their higher thermal stability and outstanding physicochemical properties4. Especially, La-Co-O perovskite oxides appear to be the most active among the LaBO3(B is transition atom from V to Ni) perovskite series for the CO complete oxidation6,7. In addition, aliovalent ion doping in A site can also influence the catalytic property of perovskite-type oxides by inducing structural modification. For example, the substitution of La3+ions by Sr2+ions in perovskite oxides can either increase the valence state of the B site ion or generate oxygen vacancies, leading to the improvement of oxidizing ability of catalysts7,8. Unfortunately, as a catalyst, the application of perovskite oxides obtained by traditional methods is limited owing to its low surface area and catalytic activity4,5.
To increase the surface area of perovskite-type catalysts,many investigators have developed new methods. For example,our group prepared porous La1−xSrxMn0.8Fe0.2O3(0≤x≤0.6)microspheres with a surface area of 55.73 m2·g−1through a low temperature molten salt approach9. Furthermore, some researchers dispersed perovskite catalysts on high surface area supports, e.g., mesoporous SiO210, SBA-15, MCM-4111,Ce1−xZrxO212, and mesoporous ZrO213to enhance the relative specific surface area. Recently, a template method has sparked extensiveattention due to its advantage in preparing perovskite oxides with high surface area5,14. For instance, threedimensionally ordered macroporous (3DOM) La0.6Sr0.4MnO315with the surface areas of 32−40 m2·g−1were fabricated using the well-arrayed polymethyl methacrylate (PMMA)-templating strategy. On the other hand, many mesoporous perovskite oxides such as mesoporousLaMnO316LaCoO317,18perovskite oxides with higher surface area have been synthesized via the nanaocasting technology by using mesoporous silica as the hard template.
However, there are few reports about the effect of surface properties of SBA-15 on mesoprous perovskite oxides via the template method. Abundant silanol groups on silica surface are essential for a high degree of impregnation19, for silanol groups are preserved on the silica surface in contrast to the silica removed surfactant by calcinations process14, the microwave-digested mesoporous silica has been selected to prepare mesoporous metal oxides. Amino-functionalized SBA-15 shows a high affinity for relatively heavier metal ions,such as Cu2+, Zn2+, Cr3+, Ni2+and Co2+20. 3D mesoporous Cr2O321and WO322have been successfully prepared using the amine-functionalized SBA-15 as the template. SBA-15 with the methyl groups on the external surface and amine on the internal surface is used as a template to prepare Ag nanorods with the length of tens to hundreds of nanometers, the methyl groups on the external surface suppress the growth of metal particles outside the mesochannels23. Amination of the surface silanols of the silica results in the modification of acid-base property of the silica surface, which is beneficial to accommodate large amount of acidic species22.
Herein, the effect of surface properties of SBA-15 on mesoprous perovskite oxides prepared via a nanocasting route was investigated. Firstly, we prepared mesoprous SBA-15 with larger pore diameter than that achieved previously,subsequently the external surface of SBA-15 was functionalized by methyl groups, and the internal surface was modified by more hydroxyl or amino groups. The catalytic activity of mesoporous La0.8Sr0.2CoO3was evaluated by CO oxidation. In addition, a possible explanation for the effect of surface properties on La0.8Sr0.2CoO3perovskite oxides was proposed.
2 Experimental
2.1 Materials
Pluronic P123 (relative molecular weight is 5800,EO20PO70EO20) and pluronic F127were purchased from Sigma-Aldrich. Lanthanum nitrate hexahydrate (≥ 99%),APTES (≥ 99%), TMCS (≥ 99%) were obtained from Aladdin.Ethyl silicate (AR), HCl (concentration 36%−38%), NaOH (≥96%), citric acid monohydrate (≥ 99.5%), cobalt nitrate hexahydrate (≥ 98.5%), strontium nitrate (≥ 99.5%), ethanol (≥99.7%) were obtained from Sinopharm Chemical Reagent Co.Deionized water was used in the whole synthesis.
2.2 Preparation and modification of silica templates
Mesoporous silica SBA-15 was prepared following the modified version of Ref.24. The main difference was P123 and F127 together as a structure-directing agent. Typically,surfactant F127 and P123 with the molar ratio of 0.0065 were dissolved in HCl solution. Several hours later, ethyl silicate(TEOS) was added into the homogeneous solution with continuous stirring at 35 °C for additional 24 h. The molar ratio for TEOS : P123 : HCl: H2O in the mixture was 1 : 0.017 : 6 :167. Then the solution was transferred to a Teflon-sealed autoclave and aged statically at 110 °C for 36 h. The resulting white precipitate was collected by filtration, then washing and drying, the dry powder was marked as SBA-15-s. SBA-15 was acquired by direct calcination of SBA-15-s at 350 °C for 6 h to remove the surfactant. Otherwise, SBA-15-s was treated by TMCS before calcination, and the obtained product was denoted as CH3-SBA-15, which means that the external surface of SBA-15 was modified by methyl groups. The methyl modification process was carried out according to Ref. 23.CH3-SBA-15-OH referred to ―CH3groups on the outer surface and hydroxylation treatment on the inner surface to produce more ―OH groups. Typically, CH3-SBA-15 evenly dispersed in concentrated HCl, and the mixture was stirred at RT for 8 h, and then filtered, washed with distilled water until it was neutral, dried in air and the powder was labeled as CH3-SBA-15-OH. CH3-SBA-15-OH/NH2means that the surface of CH3-SBA-15-OH was further amino functionalized,and the amino group modification process was the same as Ref.23. Prior to modification by 3-aminopropyltriethoxysilane(APTES), the CH3-SBA-15-OH was dried under vacuum at 100 °C for 12 h to remove residual water.
2.3 Synthesis of meseporous La0.8Sr0.2CoO3perovskite
The synthesis procedure of mesoporous La0.8Sr0.2CoO3is as follows: stoichiometric amounts of lanthanum nitrate hexahydrate (La(NO3)3·6H2O), cobalt nitrate hexahydrate(Co(NO3)2·6H2O), strontium nitrate (Sr(NO3)2) were dissolved in aqueous ethanol solvent. Citric acid monohydrate, as the chelating agent, was dissolved into the nitrate solution, and the molar ratio of the citric acid to total metal ions (La3+, Co2+and Sr2+) was 1:1. Then the as-prepared mesoporous silica was added into the complex solution. The solution was heated under stirring constantly until it became viscous, dried in an oven.Subsequently, the dry solid composite was heated in air at a rate of 5 °C·min−1from room temperature to 300 °C and further 650 °C, and the powder was sintered at the two temperatures for 2 h, respectively. Afterwards the obtained perovskite oxides were poured into 2 mol·L−1NaOH solution and stirred at 80 °C for 6 h to remove silica template. After being washed and dried,the template-free oxides were acquired. The synthesized samples were referred to as LSC-X (X=1, 2, 3), corresponding to the different templates SBA-15, CH3-SBA-15-OH and CH3-SBA-15-OH/NH2, respectively.
2.4 Characterization
The crystal structure and composition were determined by powder X-ray diffraction (XRD) using a D/max-RB type X-ray diffractometer (Bruker AXS, Germany) with Cu Kαradiation source (λ=0.15418 nm). The crystallite size was calculated with the Scherrer equation. The morphologies as well as the corresponding selected area electron diffraction (SAED)patterns of the samples were obtained by a JEM-2100F (JEOL,Japan) high-resolution transmission electron microscope(HRTEM) at an acceleration voltage of 200 kV. Fourier transform infrared (FTIR) spectra were recorded on a Nicolet 6700 spectrometer (Thermo, USA) in a wavenumber range of 4000 to 400 cm–1and the samples were prepared with KBr.Hydrogen temperature programmed reduction (H2-TPR)experiments were carried out on a Micromeritics AutoChem II 2920 apparatus (Micromeritics, USA) with a thermal conductivity detector (TCD) under a flow rate of 50 mL·min−1(5% H2/Ar). Approximately 50 mg of sample was pretreated with Ar at 100 °C for 2 h, prior to running the H2-TPR experiments, and the temperature was raised from room temperature to 850 °C at a rate of 10 °C·min−1. O2temperature programmed desorption (O2-TPD) experiments were carried out using BELCAT-B-293 (BEL, Japan) analyzer. Before each experiment, 100 mg of sample was pretreated in O2(20 mL·min−1) at 500 °C for 1 h, the temperature was raised at the rate of 10 °C·min−1, then cooled down to room temperature under the same gas composition. Subsequently, O2was switched to He, and desorption profile recorded from room temperature to 950 °C at a heating rate of 10 °C·min−1.Nitrogen adsorption/desorption measurements were performed at −196 °C using an ASAP 2020 M analyzer (Micromeritics,USA) utilizing the BET model for the calculation of specific surface areas. The pore size distribution was calculated from the adsorption isotherm curves using the Barrett-Joyner-Halenda (BJH) method. Before measurement,the samples were degassed in vacuum at 120 °C for 5 h. X-ray photoelectron spectroscopic (XPS) measurements were performed on an ESCALAB 250 Xi system (Thermo, China)equipped with Al Kαradiation source (hν= 1486.6 eV) at room temperature and under a vacuum of 10−7Pa. The error of binding energy was ±0.2 eV and the binding energy was calibrated referencing to C 1s peak (Eb= 284.6 eV).
2.5 Catalytic performance
The catalytic activity in oxidation of CO was examined with a continuous-flow fixed-bed quartz reactor (inner diameter is 8 mm) at a temperature range from room temperature to 180 °C.The temperature was controlled using a temperature controller connected to a thermocouple inserted in the reactor. The catalysts (approximately 100 mg) were dispersed on quartz wool and loaded into reactor; the mass ration of catalyst to quartz wool is 1:5.2% (volume fraction) CO, 3% O2and Ar as balance gas were fed through a mass flow controller at a total flow rate of 200 mL·min−1, and the space velocity (GHSV) was 20000 h−1. The outlet gas composition was determined by gas chromatograph (GC-7890 II, China) equipped with FID and TCD double detector.
3 Results and discussion
3.1 Characterization of the silica templates
The small-angle XRD spectrum in Fig.1 exhibits three characteristic diffraction peaks of two-dimensional hexagonal structure corresponding to (100), (110) and (200)diffractions22,23,25. It is evident that the long-range order mesostructure is retained after hydroxylation and modification with methyl or amino, but the mesopore order of CH3-SBA-15-OH/NH2decreases for the peak intensity decreases26. On the contrary, the peak intensity for CH3-SBA-15-OH increases,indicating that the order and stability of mesoporous SBA-15 increase after hydroxylation, which is in favor of dipping of precursor. Also we could see that the main diffraction peaks shift slightly to higher angle compared to the unmodified mesoporous silica template, which is owing to the shrinkage of the aperture during surface modification, indicating that the functional amino groups and silanols have been grafted into the SBA-15 pores22,27.
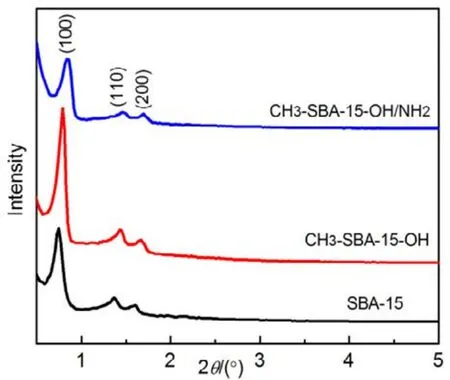
Fig.1 Small-angle XRD patterns of silica template.
Fig.2 shows the TEM images of all the templates. We can clearly see the parallel channels with two-dimensional hexagonal structure for each sample, suggesting that the long-range order mesostructure is reserved in CH3-SBA-15-OH and CH3-SBA-15-OH/NH2during the functionalization reaction. The results are in good agreement with the XRD analysis. Furthermore, the pore size of SBA-15 is about 10 nm as analyzed by Digital Micrographdemo software, which is consistent with the nitrogen adsorption results.
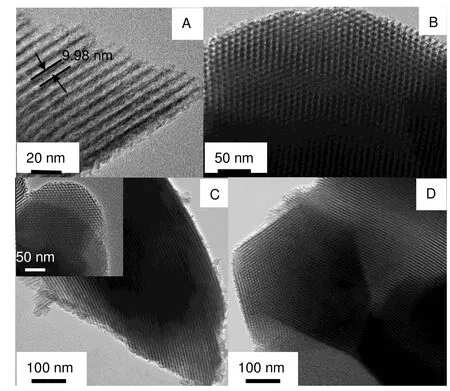
Fig.2 TEM images of SBA-15 (A, B), CH3-SBA-15-OH (C) and CH3-SBA-15-OH/NH2 (D).
According to the IUPAC classification, the N2adsorption/desorption isotherms of SBA-15, CH3-SBA-15-OH and CH3-SBA-15-OH/NH2display a typical type IV isotherm with a hysteresis loop of H1(Fig.3), indicating that they possess highly ordered mesoporous structure. Apparently,CH3-SBA-15-OH/NH2has a relatively low adsorption volume.In addition, all the samples show a narrow pore size distribution, and the textural parameters, such as the BET surface area, the pore size and the cumulative adsorption pore volume for the synthesized samples, are summarized in Table 1.Unmodified SBA-15 has larger pore size (10.1 nm) and higher surface area (802 m2·g−1). The BET surface area, pore size and the pore volume of CH3-SBA-15-OH/NH2, CH3-SBA-15-OH are smaller than those of SBA-15, further indicating that ―NH2and ―OH are anchored onto the inner surface of SBA-1527. The results are corresponding to the XRD analysis.
The successful modification of the hydroxyl, methyl group or amine group can be further demonstrated by FT-IR analysis as shown in Fig.4. For each sample, the absorption bands at 1080, 470 and 804 cm−1are assigned to asymmetric stretching,bending mode center and symmetric stretching vibrations of Si―O―Si, respectively, which confirms the existence of an SBA-15 framework23,25,27. The bands at 1630 and 3432 cm−1are ascribed to the bending vibration and stretching modes of ―OH group23,25,26, respectively, and weak peaks associated with Si-OH groups stretching vibration in the range of 940−960 cm−1are also present. Compared with SBA-15, the peak intensity of CH3-SBA-15-OH increases at 960, 1630 and 3413 cm−1, suggesting that there are much more silanols generated on the surface of silica. In contrast, the intensity of ―OH group peaks decreases in CH3-SBA-15-OH/NH2, resulting from the reaction between Si―OH and APTES23. On the modified SiO2samples, the absorption bands appearing in the range of 2850−3000, 1490 and 1380cm−1are corresponding to the C―H stretching and bending vibrations23,25,27,28. The N―H bending vibration around 687 cm−125−27and the presence of N―H stretching vibration at 1570 cm−1further confirmed the incorporation of amino groups in CH3-SBA-15-OH/NH227.
3.2 Characterizations of mesoporous LSC-X perovskite
Wide-angle XRD pattern of the samples is shown in Fig.5A.All samples possess rhombohedral perovskite crystal structure similar to LaCoO3(JCPDS PDF# 48-0123 R3c) and the diffraction peaks could be well indexed. One weak diffraction signal at 2θ= 36.8° attributable to the (311) reflection of Co3O4(JCPDS PDF# 45-1003 Fd3m) has been detected in the LSC-1 perovskite oxide, indicating the formation of cubic phase Co3O4in the perovskite when using unmodified SBA-15 as a template. The average sizes were estimated by Sherrer’s equation D(hkl) = 0.89λ/(βcosθ)29for XRD peak at 2θ = 33°,which are in the range of 16−18 nm for La0.8Sr0.2CoO3patterned by hard template SBA-15 with different surface properties, respectively. It can be clearly observed that diffraction peak of Co3O4becomes weaker and even disappear in LSC-2 and LSC-3, respectively. On the contrary, diffraction peaks of main phase perovskite become much sharper, which implies the improved crystallinity of perovskite oxides17,demonstrating that surface modified SBA-15 contributes to synthesizing well crystallized pure perovskite phase by the co-nanocasting template method, which may be due to the heat released by decomposition of APTES and TMCS during calcination, leading to a better crystallization of perovskite phase.
The small-angle XRD pattern of the synthesized LSC-X samples is illustrated in Fig.5B, one weak characteristic diffraction peak corresponding to (l00) crystal plane can be recorded for the sample LSC-2 and LSC-3, suggesting that some mesostructure has been obained18. During impregnation of CH3-SBA-15-OH and CH3-SBA-15-OH/NH2, the hydrophobic external surface, the strong chelating ability of amine to metal ions citrate complex or the abundant silanol groups on the internal surface would favor the encapsulation of nitrate precursors into the mesochannels, so there are more precursors adsorbed into the channels of templates.
Fig.6(A−C) shows the TEM images of the samples LSC-X,respectively. It can be seen that a certain mesoporous structure of the replica LSC-X has been obtained, but the channel structure is not very clear, and big particles are also observed,suggesting that there are still some particles grown on the outer surface of SBA-15. This kind of mesopore can provide good mass transfer to the reactant molecules and easy accessibility to the active sites in the catalytic process, and finally improve the catalytic efficiency17. The prepared mesoporous La0.8Sr0.2CoO3has polycrystalline character according to the electron diffraction rings in the selected area electron diffraction(SAED) pattern, as shown in the inset of Fig.6A. For HRTEM image of the LSC-3 (insert in Fig.6C), the lattice fringes corresponding to the (110) plane of orthorhombic La0.8Sr0.2CoO3perovskite are clearly visible, suggesting that perovskite oxides are well crystallized. The EDS pattern of LSC-3 (Fig.6D) shows that the atomic ratio of La : Sr : Co is close to 0.8 : 0.2 : 1. Additionally, very weak Si signal can be detected, and there was about 4% (mass fraction) Si remained after etching with NaOH, which may be due to the strong interaction between silica and rare earth elements, as was also found by others e.g. Ref. 16−18.
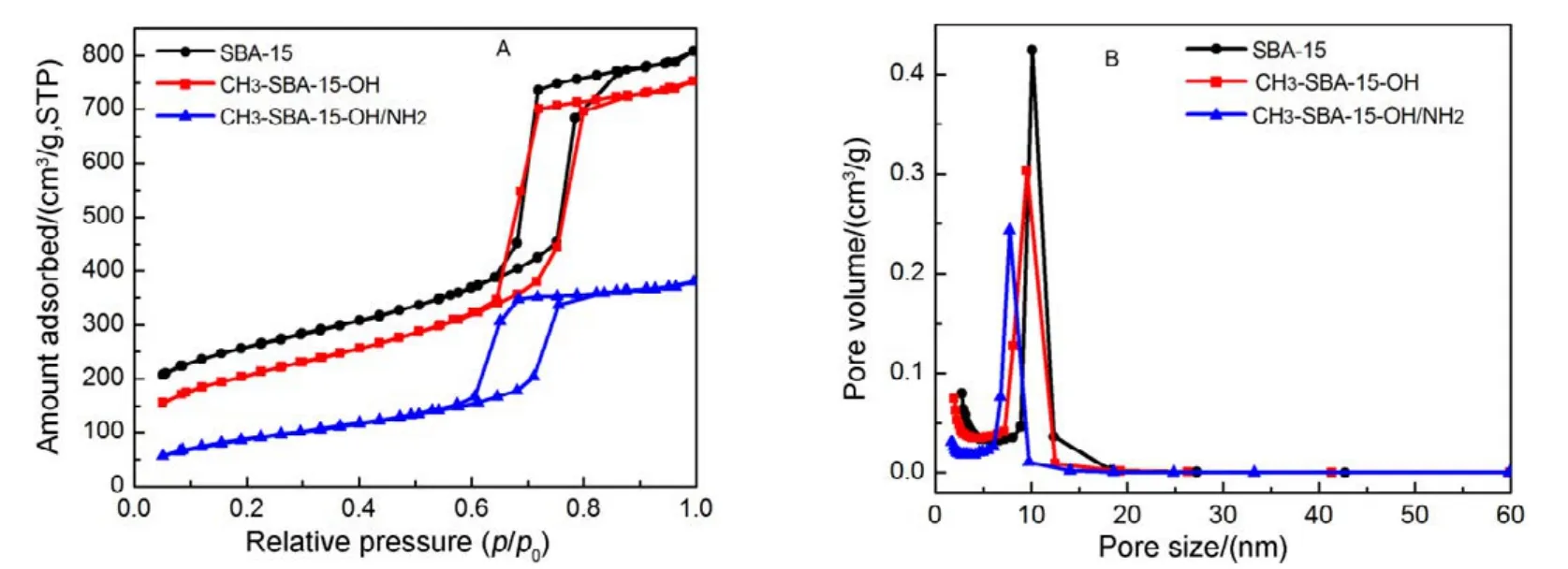
Fig.3 N2 adsorption-desorption isotherms (A) and pore size distribution curves (B) of silica template.
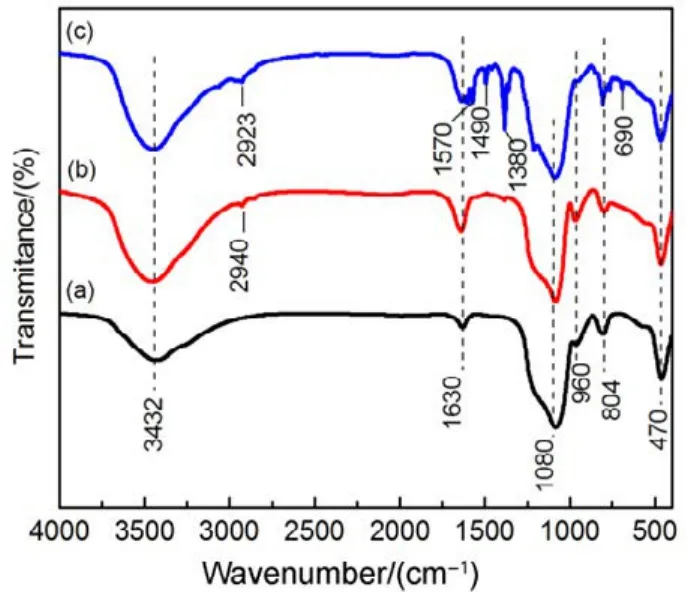
Fig.4 FT-IR spectra of silica template.
Nitrogen adsorption–desorption isotherms and the corresponding BJH pore size distribution curves of the mesoporous La0.8Sr0.2CoO3are shown in Fig.7. All samples correspond to type-IV isotherm with hysteresis loops appeared in the relative pressure range of 0.5−1.0, which is the feature of mesoporous materials synthesized by the hard template methods17,18, The BET surface area, pore volume and pore size distribution of mesoporous LSC-X are listed in Table 1. The as-obtained mesoporous LSC-1 oxide exhibits the highest surface area (148 m2·g−1). The modification of silica template leads to a decline of surface area and pore size, therefore, the surface area of corresponding perovskite oxides become lower.On the other hand, the surface area of LSC-3 is nearly 1/3 of that of corresponding template, confirming that template CH3-SBA-15-OH/NH2can improve the filling efficiency of the precursor to a certain extent. In addition, all samples have narrow pore diameter distribution with the peak located at around 7.2 nm (shown in Table 1) according to the BJH analysis.
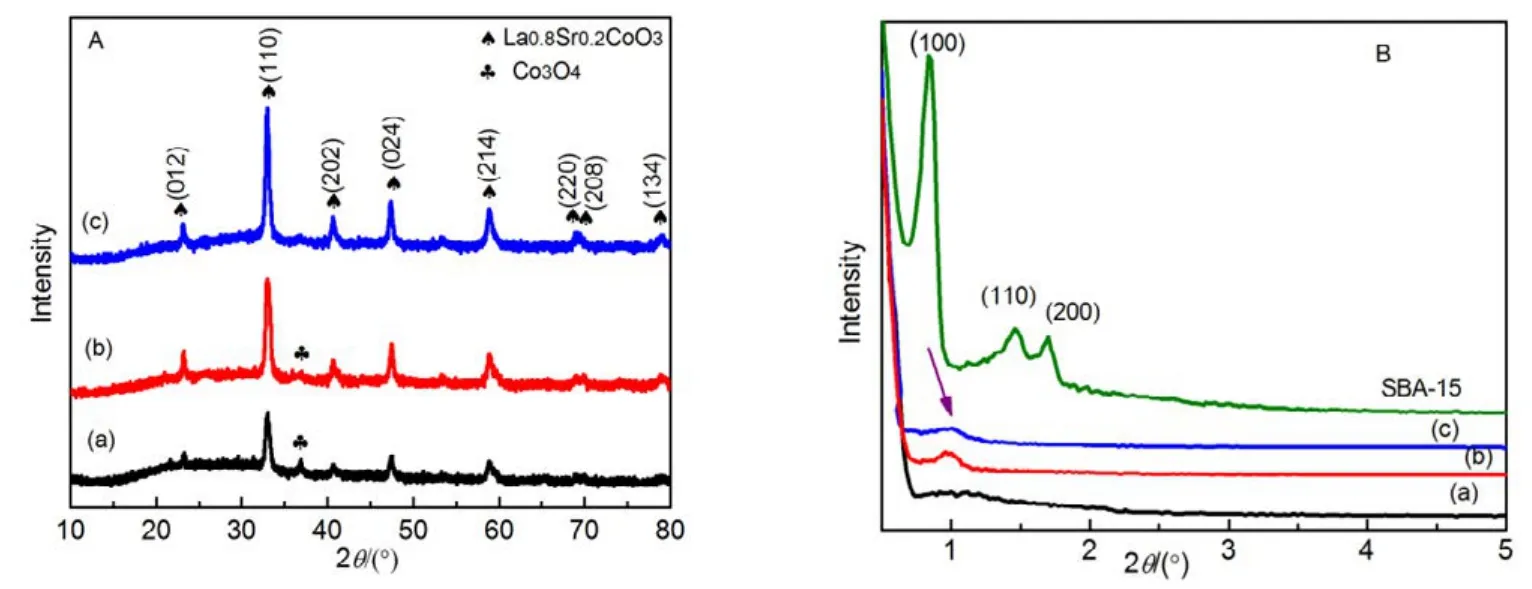
Fig.5 Wide-angle XRD patterns (A) and small-angle XRD patterns (B) of LSC-X.
The surface element compositions, metal oxidation states,and adsorbed oxygen species of the samples are characterized by XPS. Fig.8A shows the 2p spectra of cobalt in LSC-X perovskite. There is no shake-up satellite at 786−788 eV,suggesting the absence of Co2+in the outmost layers30,31. The Co 2p3/2spectra of all LSC-X perovskite oxides show peaks around 780.5 eV (as listed in Table 2), which is higher than 779.8 eV assigned to Co3+in perovskite oxides30,34, indicating that Co4+ions exist together with Co3+ions31,32,35,41. To maintain the charge balance in the samples during the substitution of Sr2+for La3+ions in the A sites, there are some Co4+ions formed, which contributes to the higher catalytic activity7,33. In addition, it should be noted that the binding energy of Co 2p3/2in LSC-X increases with the sequence of LSC-1 < LSC-2 <LSC-3, indicating that the proportion of Co4+ions increases with the modification of templates31. From Fig.8A, one can clearly observe that the asymmetrical Co 2p3/2peak could be decomposed into two components at EB≈ 779.8 and 781.2, attributed to the surface Co3+and Co4+species35,respectively. After quantitatively analyzing the Co 2p3/2XPS spectra of the samples, as shown in Table 2, it can be seen that the Co4+/Co3+molar ratio increases obviously from LSC-1 to LSC-3, and the LSC-3 possessed the highest surface Co4+/Co3+molar ratio (1.99). Based on the XPS results (not shown),residual Si2+ions were detected on the surface of LSC. It can be concluded that the interaction between SiO2and metal ions became stronger as the amino groups and silanol groups modified the surface of SBA-15, so there would be more Si2+entered into the lattice. According to the electroneutrality principle, more Co4+would be generated in LSC-3. At the same time, the Co 2p3/2peaks of catalysts become stronger with the modification of the template, which further proves the existence of the high valence Co ions29. From Table 2, it can be clearly seen that Co enrichment is obtained on the surface of La0.8Sr0.2CoO3samples, which is pronounced in LSC-3 and believed to conduce to good catalytic activity.
As shown in Fig.8B, the asymmetrical O 1s spectrum of each sample could be decomposed into four components at EB≈529.2, 530.3, 531.3 and 532.2 eV, assignable to the surface lattice oxygen O2−, adsorbed oxygen (e.g. O−2, O22−or O−),hydroxyl groups or carbonate (CO) species, and molecular water adsorbed on the surface respectively17,33,34. Both O−and O22−are strongly electrophilic reactants that are very active for oxidation, especially at low temperature17,29,33. The content(33.4% and 37.2% respectively) of adsorbed oxygen species in LSC-2 and LSC-3 are much higher than that (21%) in LSC-1.In addition, the Oads/Olatratio of LSC-X increases from 2.4 to 3.7 (Table 2). The existence of more Co4+ions increases the oxygen vacancies of oxide materials and surface oxygen vacancies is beneficial to the adsorption, activation and mobility of oxygen36, which will accelerate the formation of more adsorbed oxygen species. In addition, surface active oxygen species promote the enhancement in catalytic performance for low temperature oxidation4,39.
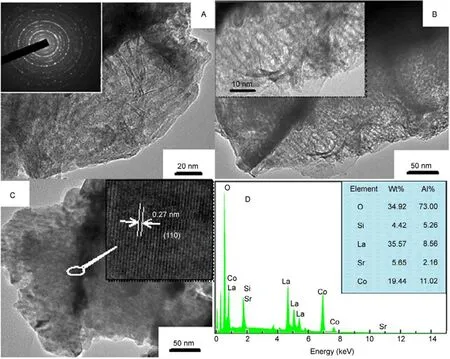
Fig.6 TEM images of LSC-1 (A), LSC-2 (B), LSC-3 (C), EDS pattern of LSC-3 (D).
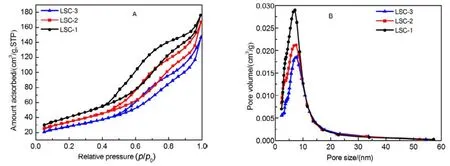
Fig.7 N2 adsorption-desorption isotherms (A) and pore size distributi on curves (B) of LSC-X.
As shown in Fig.8C, the Sr 3d peak can be fitted by two sets of Sr 3d5/2and 3d3/2doublets, the contributions at lower EB(132.0 eV for Sr 3d5/2and 134.0 eV for Sr 3d3/2) can be assigned to the Sr cations in the near-surface region of the perovskite lattice (Srlat). The peaks observed at higher EB(133.3 eV for Sr 3d5/2and 135.5 eV for Sr 3d3/2) can be related to the surface states of strontium (Srsur), which could be correlated to the presence of SrO or Sr-OH surface secondary phases37. One can see that the Srlatcontributions become larger in the LSC-2 and LSC-3 samples, as supported by the fact that the ratio of the Srlatto the Srsurfor the LSC-3 samples (1.52) is much larger than that for the LSC-1 catalyst (0.87). As the presence of increased oxygen vacancy content will drive Sr in the perovskite structure toward the surface37. Sr enrichment in the perovskite structure in the near-surface region can induce the increased content of lattice defect or high-valence cobalt ions,which can improve the catalytic activity. In contrast, the presence of secondary surface phases at high coverage can depress the oxygen surface exchange rate37,38. The increased amount of Sr ions in the perovskite lattice may play a critical role in enhanced catalytic activity.
H2-TPR tests were performed to investigate the reducibility of the samples, and the results are illustrated in Fig.9A. For the LSC samples, there are two reduction peaks, indicating thatreduction proceeds according to the two consecutive steps as follows40:
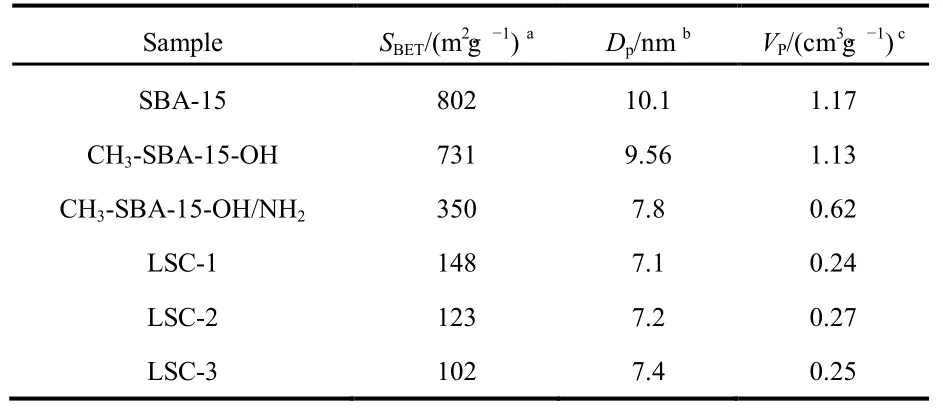
Table 1 N2 adsorption-desorption measurement result of SBA-15-X and LSC-X.
2LaCoO3+H2→2LaCoO2.5+H2O (1)
2LaCoO2.5+H2→La2O3+2Co0+2H2O (2)
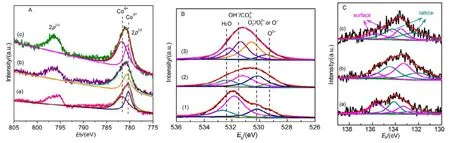
Fig.8 Co 2p (A),O 1s (B) and Sr 3d (C) XPS spectra of LSC-X.
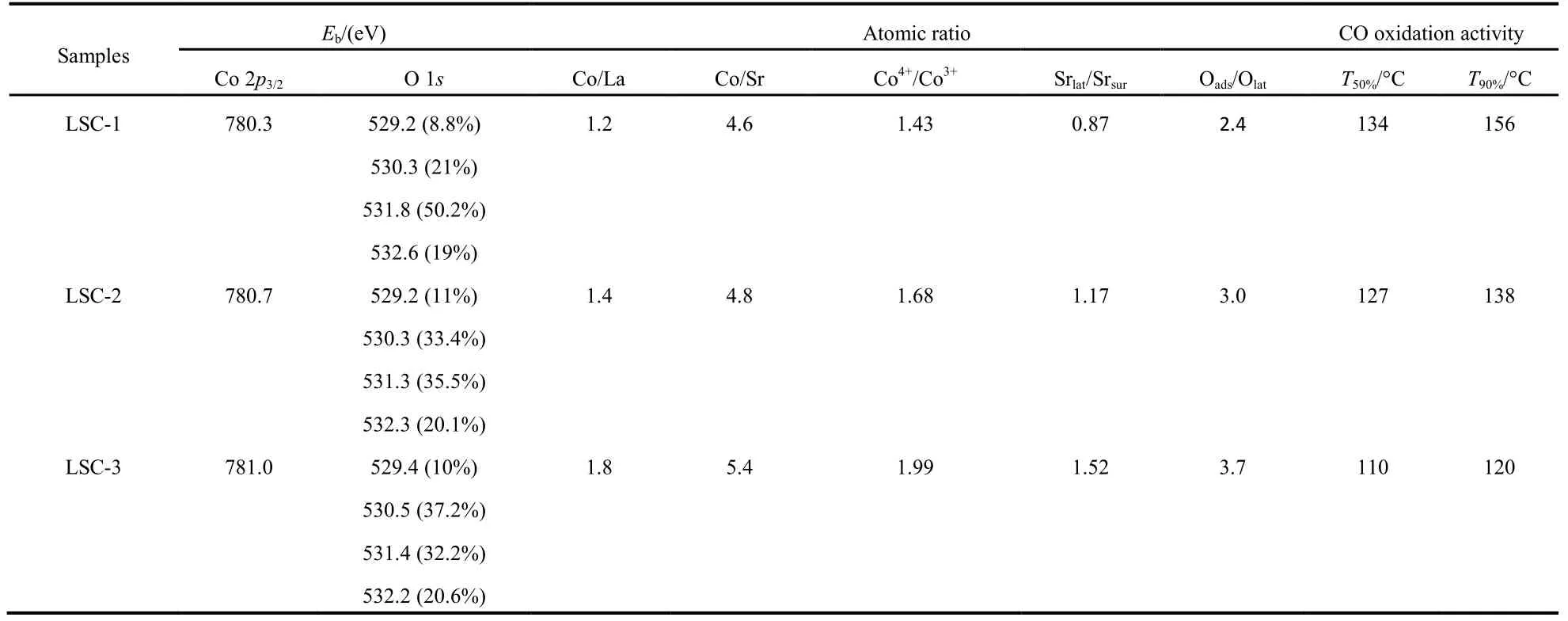
Table 2 XPS results and CO catalytic activity of mesoporous LSC-X.
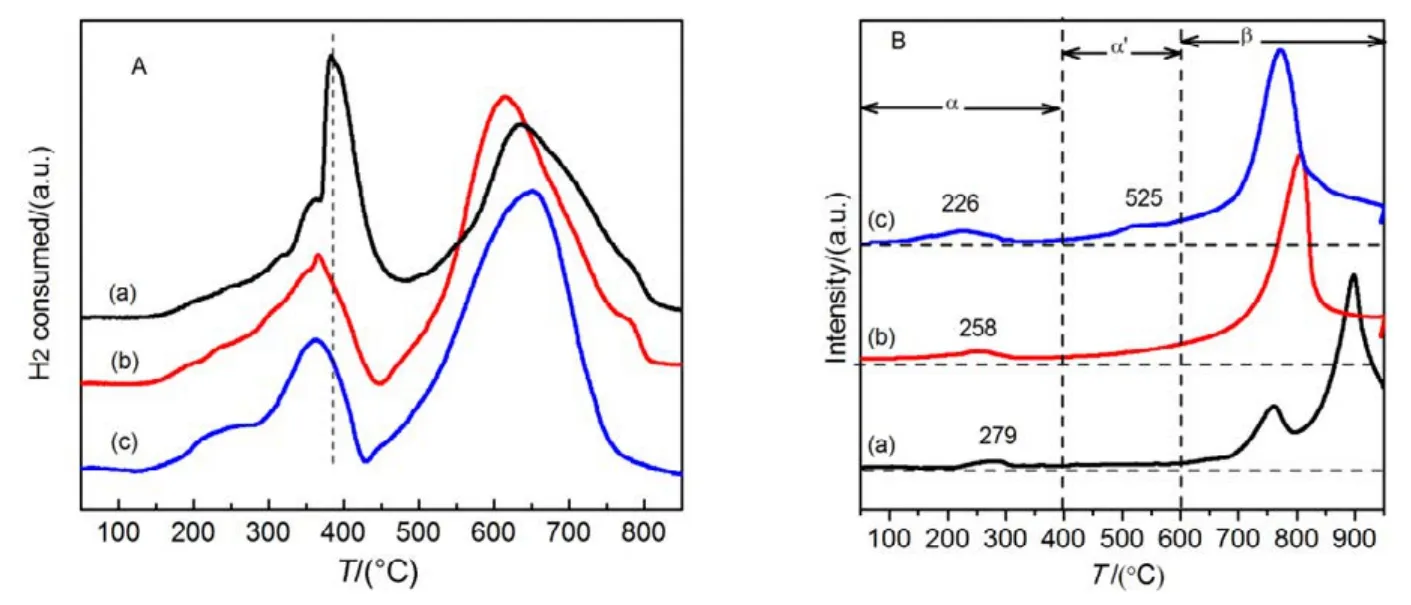
Fig.9 H2-TPR and O2-TPD profiles of LSC-X.
The TPR peak around 370 °Cis assigned to the reduction of Co3+to Co2+in the form of LaCoO2.5, and the peak around 650 °C is due to the reduction of Co2+to Co0, respectively39,40.It is noted that the first reduction peak of mesoporous La0.8Sr0.2CoO3prepared by modified SBA-15 shifts to lower temperature, which may be associated with the higher content of adsorbed oxygen species5. Moreover, a small shoulder is observed around 245 °C for LSC-3, the phenomenon may be due to more high-valence cobalt ions appearing on the surface of LSC-3, leading to a relatively low reduction temperature17,which were testified by XPS results. It is evident that the LSC-3 sample possesses the lowest onset reduction temperature(358 °C) and a shoulder seam, suggesting that the sample is the most reducible5.
By using the curve-fitting approach, H2consumptions amounts of each reduction peak of the samples were calculated,and the results were summarized in Table 3. The H2consumptions in the low-temperature region (<450 °C) are 1.8,1.9 and 2.1 mmol·g−1, while those in the high-temperature region (>450 °C) are 3.5, 4.0 and 4.1 mmol·g−1for the LSC-1,LSC-2 and LSC-3 samples, respectively. It is apparent that the H2consumptions of the LSC-2 and LSC-3 are much larger than that of the LSC-1, demonstrating that the surface modified SBA-15 templates lead to an enhancement in reducibility.
O2-TPD experiments were performed in order to investigate the surface and bulk oxygen species, as well as the mobility of oxygen for the as prepared catalysts. As illustrated in Fig.9B,the LSC-3 reveals three distinct O2-desorption peaks. As reported in reference41, oxygen desorption below 400 °C (α-O)can be ascribed to chemisorbed oxygen species that are related to the oxygen vacancy in the surface or subsurface. Moreover,the peak at 400−600 °C (αʹ-O) is attributed to surface lattice oxygen species as generated from lattice defects, and the peak above 600 °C (β-O) corresponds to those oxygen species liberated mostly from the bulk of crystal structure, which is often as an indicator of the mobility of the oxygen in the bulk42.The desorption temperature of α-O shifts towards lower temperature in the LSC-2 and LSC-3, and the amounts of weakly adsorbed oxygen species (α-O) increase in the following order: LSC-1 < LSC-2 < LSC-3 (Table 3),which are consistent with the XPS analysis, as shown in Table 2, the molar ratio of chemisorbed oxygen (Oads)/lattice oxygen (Olat)for LSC-3 is 3.7, but that for LSC-1 is only 2.4. Furthermore, it is evident that LSC-3 possesses the highest αʹ-O desorption value, indicating that there are more defects in the LSC-3 than the others. The high oxygen desorption ability and oxygen mobility of LSC-3 are proposed due to its much higher lattice defects and oxygen vacancy, which is beneficial to the promotion of catalyst performance.
Before evaluating the catalytic activity of mesoporous LSC-X, a blank experiment only loading quartz wool in the reactor was carried out, under the conditions of 2% CO and 3%O2in volume fraction, Ar as balanced air, and GHSV = 20000 h−1, no conversion of CO below 350 °C, and only 0.3%conversion rate were detected until 500 °C, indicating that no significant homogeneous reactions took place under the adopted reaction conditions5,36. Fig.10A shows the catalytic property of mesoporous La0.8Sr0.2CoO3oxides for CO oxidation as a function of reaction temperature, and all samples exhibit excellent catalytic activity for CO oxidation at a temperature range from 120 to 180 °C. The catalytic activity increases with an order of LSC-3 > LSC-2 > LSC-1, which is in the opposite order of surface area, implying that surface area is not the determining factor for catalytic activity of catalysts. In order to better evaluate the activities of these samples, the temperatures(T50%and T90%, respectively) required for 50% and 90% CO conversion are employed, as summarized in Table 2. In terms of the sample LSC-3, the complete CO conversion achieved at 140 °C, which is 50 °C lower than that in Ref. 18. Furthermore,T90%of LSC-2 and LSC-3 (138 and 120 °C, respectively) was much lower than that (252.3 °C) in our previous research43. It is obvious that the prepared mesoporous LSC-X samples possess much higher catalytic activity for CO oxidation. Moreover,T90%of LSC-3 is 36 °C lower than that of LSC-1, indicating the surface properties of the template have some effect on the catalytic activity of mesoporous La0.8Sr0.2CoO3via a nanocasting route.
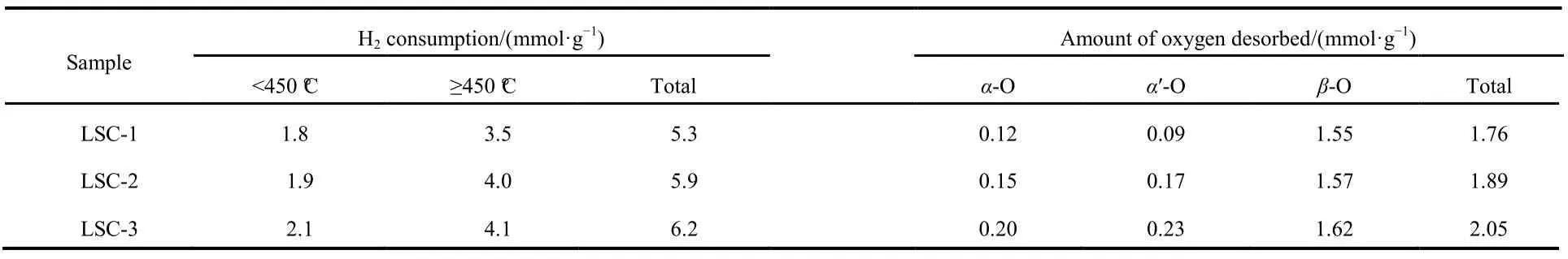
Table 3 The data obtained from H2-TPR and O2-TPD analysis.
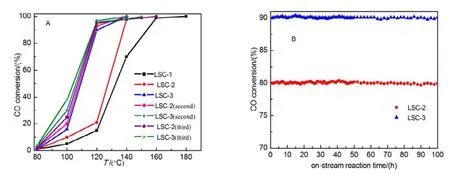
Fig.10 Catalytic activities in CO oxidation of the fresh and used catalysts (A) and CO conversion as a function of on-stream reaction time over the mesoporous LSC-2 and LSC-3 (B).
Specifically, the higher catalytic activation can be interpreted in accordance with the CO oxidation mechanism on perovskite oxides. It is reported that CO oxidation over perovskite proceeds by way of a superficial process, CO adsorbs on the perovskite oxides and reacts with surface oxygen species. The CO oxidation process on perovskite can be described as follows6:
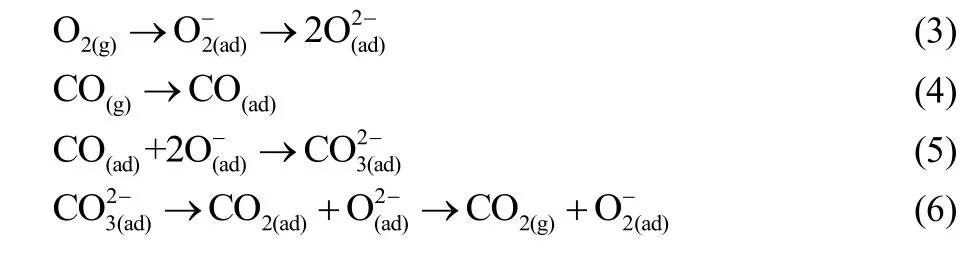
It can be concluded that the surface oxygen species concentration and desorption rate of CO2play a critical role for CO oxidation reaction on perovskite catalysts. Meanwhile,Eq.(6) should be the rate-determining step6, and the desorption process of CO2is associated with surface cations reducibility30.Obviously, the higher CO oxidation activity of LSC-3at low temperature can be explained by better reducibility of Co ions and higher content of adsorbed oxide species on the surface,which was consistent with the XPS, H2-TPR and O2-TPD results.
In addition, the second and third catalytic cycle were performed for mesoporous LSC-2 and LSC-3 perovskite oxides after one complete catalytic test and the results are shown in Fig.10A. Interestingly, the conversion curves are much better than those of the corresponding fresh catalysts, since the low-temperature conversion rate is found to be increased. In the second run, T50%of mesoporous LSC-2 and LSC-3 samples are 108 and 105 °C respectively, which are lower than those (127 and 110 °C respectively) in fresh catalysts, the similar phenomenon was also found in our previous research shown in reference 9. One can observed that the low-temperature conversion rate in the third cycle is higher than before, but complete conversion temperature (T100%) becomes higher.Overall, the low-temperature catalytic activity is improved. The long-time stability experiments were conducted over LSC-2 and LSC-3 oxides for CO oxidation reaction to evaluate the catalytic stability. As shown in Fig.10B, it is conspicuous that there are no significant drops in the activity within 100 h of on-stream reaction, suggesting that the mesoporous LSC-2 and LSC-3 samples are catalytically durable.
4 Conclusions
In summary, mesoporous La0.8Sr0.2CoO3perovskite oxides with good crystallinity are successfully fabricated by a nanocasting route from the modified SBA-15. The catalysts prepared by modified SBA-15 exhibit better structure and higher catalytic activity for CO oxidation at low-temperature.In particular, the CO complete conversion can achieve at 140 °C, which is much lower than that in previous studies. The higher adsorbed species concentration, better low-temperature reducibility, the existence of Co4+and good crystallinity, as well as mesoporous structure are responsible for the enhanced catalytic activity of thus-prepared perovskite catalysts. The surface properties of mesoporous silica have some effect on the structure and physicochemical properties of metal oxides, and modified mesoporous silica is expected to be applicable to synthesize other metal oxide nanomaterials with high surface area.
(1) Qi, J.; Chen, J.; Li, G. D.; Li, S. X.; Gao, Y.; Tang, Z. Y.EnergyEnviron. Sci. 2012, 5, 8937.doi: 10.1039/C2EE22600F
(2) Li, X. Z.; Ni, C. Y.; Lu, X. W.; Zuo, S. X.; Liu, W. J.; Yao, C. Catal.Sci. Technol. 2016, 6, 545. doi: 10.1039/C5CY00909J
(3) Liang, Q.; Zhao, Z.; Liu, J.; Wei, Y. C.; Jiang, G. Y.; Duan, A. J.Acta Phys. -Chim. Sin. 2014, 30, 129. [梁 倩, 赵 震, 刘 坚, 韦岳长,姜桂元, 段爱军. 物理化学学报, 2014, 30, 129.]doi: 10.3866/PKU.WHXB201311201.
(4) Suh, M. J.; Park, Y. K.; Ihm, S. K. Catal. Today 2016, 265, 210.doi: 10.1016/j.cattod.2015.08.057
(5) Li, X. W.; Dai, H. X.; Deng, J. G.; Liu, Y. X.; Zhao, Z. X.; Wang, Y.;Yang, H. G.; Au, C. T. Appl. Catal. A-Gen. 2013, 458, 11.doi: 10.1016/j.apcata.2013.03.022
(6) Pena, M. A.; Fierro, J. L. G. Chem. Rev. 2001, 101, 1981.doi:10.1021/cr980129f
(7) Einaga, H.; Nasu, Y.; Oda, M.; Saito, H. Chem. Eng. J. 2016, 283, 97.doi: 10.1016/j.cej.2015.07.051
(8) Seyfi, B.; Baghalha, M.; Kazemian, H. Chem. Eng. J. 2009, 148, 306.doi: 10.1016/j.cej.2008.08.041
(9) Huang, X. H.; Niu, P. J.; Shang, X. H. Chin. J. Catal. 2016, 37, 1431.doi: 10.1016/S1872-2067(16)62502-0.
(10) Ding, Q.; Xian, H.; Tan, Y. S.; Tsubaki, N.; Li, X. G. Catal. Sci.Technol. 2013, 3, 1493. doi: 10.1039/C3CY00105A
(11) Wang, N.; Yu, X. P.; Wang, Y.; Chu, W.; Liu, M. Catal. Today 2013,212, 98. doi: 10.1016/j.cattod.2012.07.022
(12) Alifanti, M.; Florea, M.; Pârvulescu, V. I. Appl. Catal. B-Environ.2007, 70, 400. doi: 10.1016/j.apcatb.2005.10.037
(13) Kustov, A. L.; Tkachenko, O. P.; Kustov, L. M.; Romanovsky, B. V.Environ. Int. 2011, 37, 1053. doi: 10.1016/j.envint.2011.05.002
(14) Tian, B. B.; Liu, X. Y.; Yang, H. F.; Xie, S. H.; Yu, C. Z.; Tu, B.;Zhao, D. Y. Adv. Mater. 2003, 15, 1370.doi: 10.1002/adma.200305211
(15) Arandiyan, H.; Dai, H. X.; Deng, J. G.; Liu, Y. X.; Bai, B.Y.; Wang,Y.; Li, X. W.; Xie, S. H.; Li, J. H. J. Catal. 2013, 307, 327.doi: 10.1016/j.jcat.2013.07.013
(16) Nair, M. M.; Kleitz, F.; Kaliaguine, S. Chin. J. Catal. 2016,37, 32.doi: 10.1016/S1872-2067(15)60909-3.
(17) Wang, Y. J.; Ren, J. W.; Wang, Y. Q.; Zhang, F. Y.; Liu, X. H.; Guo, Y.;Lu, G. Z. J. Phys. Chem. C. 2008, 112, 15293.doi:10.1021/jp8048394
(18) Wang, Y. X.; Cui, X. Z.; Li, Y. S.; Shu, Z.; Chen, H. G.; Shi, J. L.Micropor. Mesopor. Mat. 2013, 176, 8.doi: 10.1016/j.micromeso.2013.03.033
(19) Valdés-Solís, T.; Marbán, G.; Fuertes, A. B. Chem. Mater. 2005, 17,1919. doi:10.1021/cm0477321
(20) Liu, A. M.; Hidajat, K.; Kawi, S.; Zhao, D.Y. Chem Commun 2000,13, 1145. doi: 10.1039/B002661L
(21) Zhu, K. K.; Yue, B.; Zhou, W. Z.; He, H. Chem. Commun. 2003, 1, 98.doi: 10.1039/B210065G
(22) Yue, B.; Tang, H. L.; Kong, Z. P.; Zhu, K. K.; Dickinson, C. Chem.Phys. Lett. 2005, 407, 83. doi: 10.1016/j.cplett.2005.03.066
(23) Huang, X. B.; Yang, M.; Wang, G.; Zhang, X. X.; Micropor. Mesopor.Mat. 2011, 144, 171. doi: 10.1016/j.micromeso.2011.04.012
(24) Zhao, D. Y.; Feng, J. L.; Huo, Q. S.; Melosh, N.; Fredrickson, G. H.;Chmelka, B. F.; Stucky, G. D. Science 1998, 279, 548.doi: 10.1126/science.279.5350.548
(25) Zhu, Y. H.; Li, H.; Zheng, Q.; Xu, J. Q.; Li, X. X. Langmuir 2012, 28,7843. doi:10.1021/la300560j
(26) Chen, F.; Liu, S. W.; Yu, J. G. Phys. Chem. Chem. Phys. 2016, 18,18161.doi: 10.1039/C6CP03037H
(27) Nomura, A.; Jones, C. W. Ind. Eng. Chem. Res. 2015, 54, 263.doi:10.1021/ie504165d
(28) Nomura, A.; Jones, C. W. ACS. Appl. Mater. Inter. 2013, 5, 5569.doi:10.1021/am400810s
(29) Xu, J. F.; Liu, J.; Zhao, Z.; Xu, C. M.; Zheng, J. X.; Duan, A. J.; Jiang,G. Y. J. Catal. 2011, 282, 1. doi: 10.1016/j.jcat.2011.03.024
(30) Glisenti, A.; Pacella, M.; Guiotto, M.; Natile, M. M.; Canu, P. Appl.Catal. B-Environ. 2016, 180, 94. doi: 10.1016/j.apcatb.2015.06.017
(31) Rousseau, S.; Loridant, S.; Delichere, P.; Boreave, A.; Deloume, J. P.;Vernoux, P. Appl. Catal. B-Environ. 2009, 88, 438. doi:10.1016/j.apcatb.2008.10.022
(32) Wang, L.; Fang, S. Q.; Feng, N. J.; Wan, H.; Guan, G. F.; Chem. Eng.J. 2016, 293, 68. doi: 10.1016/j.cej.2016.02.038
(33) Merino, N. A.; Barbero, B.P.; Eloy, P.; Cadus, L. E.; Appl. Surf. Sci.2006, 253, 1489. doi: 10.1016/j.apsusc.2006.02.035
(34) Ding, J. C.; Li, H. Y.; Cai, Z. X.; Wang, X. X.; Guo, X. Sensor Actuat.B-Chem. 2016, 222, 517. doi: 10.1016/j.snb.2015.08.099
(35) Ding, J. J.; Zhou, Y. N.; Sun, Q.Yu, X. Q.; Yang, X. Q.; Fu, Z. W.Electrochimica Acta 2013, 87, 388.doi:10.1016/j.electacta.2012.09.058
(36) Li, X. Y.; Zhang, Z. P.; Ma, J. T.; Zhu, Z. Z.; Meng, Y. M. Acta Phys.-Chim. Sin. 1996, 12, 502. [李绪渊, 张自萍, 马建泰, 朱宗祯, 孟益民. 物理化学学报, 1996, 12, 502.]doi: 10.3866/PKU.WHXB19960605
(37) Crumlin, E. J.; Mutoro, E.; Liu, Z.;Grass M. E.; Biegalski M. D.; e Yueh-Lin Lee, Morgan, D.; Christen, H. M.; Bluhm, H.; Yang Shao-Horn. Energ. Environ. Sci. 2012, 5, 6081.doi: 10.1039/c2ee03397f
(38) Jung, W. C.; Tuller, H. L.; Energ. Environ. Sci. 2012, 5, 5370.doi: 10.1039/c1ee02762j
(39) Li, X. W.; Dai, H. X.; Deng, J. G.; Liu, Y. X.; Xie, S. H.; Zhao, Z. X.;Wang. Y.; Guo, G. S.; Arandiyan, H. Chem. Eng. J. 2013, 228, 965.doi: 10.1016/j.cej.2013.05.070
(40) Deng, H. B.; Lin, L.; Liu, S. J. Energ. Fuel 2010, 24, 4797.doi: 10.1021/ef100768e
(41) Zhang, J. Y.; Tan, D. D.; Meng, Q. J.; Weng, X. L.; Wu, Z. B. Appl.Catal. B-Environ. 2015, 172, 18.doi: 10.1016/j.apcatb.2015.02.006
(42) Levasseur, B.; Kaliaguine, S. Appl. Catal. B-Environ. 2009, 88, 305.doi: 10.1016/j.apcatb.2008.11.007
(43) Liao, H. Preparation, Modification and Performance of Mesoporous Perovskite-type La-Co-O. M. S. Dissertation, Wuhan University of Technology, Wuhan, 2015. [廖 晖. 介孔La-Co-O钙钛矿型催化剂的制备、改性及性能研究 [D]. 武汉: 武汉理工大学, 2015.]
Surface Modification of SBA-15 and Its Effect on the Structure and Properties of Mesoporous La0.8Sr0.2CoO3
HUANG Xue-Hui*SHANG Xiao-Hui NIU Peng-Ju
(School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, P. R. China)
Mesoporous SBA-15 with mesopore diameter up to 10.1 nm was prepared by a hydrothermal method, and was further functionalized to obtain different surface properties. Thus-prepared SBA-15 was employed as a template to synthesize rhombohedrally crystallized mesoporous La0.8Sr0.2CoO3perovskite via a nanocasting method. The surface properties of the SBA-15 were adjusted by treatment with concentrated hydrochloric acid, trimethylchlorosilane (TMCS), and 3-aminopropyltriethoxysilane(APTES). A series of characterization techniques verified that all the synthesized templates possessed ordered two-dimensional hexagonal mesoporous structure, and the surface was successfully modified with methyl and amino groups. The mesoporous perovskite structure was formed in the samples and the surface properties of SBA-15 significantly influenced the structure and properties of La0.8Sr0.2CoO3perovskite oxides. Wide-angle X-ray diffraction patterns suggested that the modified silica templates were conducive to the formation of pure perovskite frameworks with good crystallinity. The catalysts alsopossessed mesoporous structure, as confirmed by small-angle XRD patterns, high-resolution transmission electron microscopy images, and nitrogen adsorption analysis. Moreover, the La0.8Sr0.2CoO3materials synthesized using surface-functionalized templates exhibited relatively higher catalytic activity and stability in CO oxidation. Complete CO conversion could be achieved at 140 °C using the thus-prepared La0.8Sr0.2CoO3materials, and no significant loss in catalytic activity was observed after 100 h of on-stream reaction experiments. X-ray photoelectron spectroscopy, H2temperature-programmed reduction, and O2temperature-programmed desorption experiments revealed that the existence of Co4+,Sr enrichment in the perovskite structure, and high content of adsorbed oxygen species play a critical role in the enhanced catalytic activity of the catalysts. We also proposed the possible reasons for the effect of surface properties of the silica templates on the structure and properties of the La0.8Sr0.2CoO3nanomaterials.
SBA-15; Surface modification; Template; Mesoporous La0.8Sr0.2CoO3;Low-temperature CO oxidation
December 22, 2016; Revised: March 21, 2017; Published online: April 10, 2017.
O643;TQ138.1+2
10.3866/PKU.WHXB201704103 www.whxb.pku.edu.cn
*Corresponding author. Email: huangxh@whut.edu.cn; Tel: +86-27-87642570.
© Editorial office of Acta Physico-Chimica Sinica
