利用表面模板调控低维有机纳米材料的拓扑结构
2017-11-01范其瑭朱俊发
范其瑭 朱俊发
(中国科学技术大学,国家同步辐射实验室和苏州纳米科学与技术协同创新中心,合肥 230029)
利用表面模板调控低维有机纳米材料的拓扑结构
范其瑭 朱俊发*
(中国科学技术大学,国家同步辐射实验室和苏州纳米科学与技术协同创新中心,合肥 230029)
“自下而上”的纳米结构制备法是一种在原子或分子水平上构筑纳米结构的方法;相比于“自上而下”的蚀刻技术,其可实现更小尺寸的半导体器件的制备。因此,这种“自下而上”制备法将是纳米技术未来的重要发展方向。然而,其中一个存在已久的关键问题是如何实现对所构筑的纳米拓扑结构进行精确控制。此文综述了近期关于如何调控由表面乌尔曼反应合成的纳米材料拓扑结构的研究。其中包括以下三个方面:首先,利用纳米结构与衬底晶格参数之间的匹配关系控制金属有机链的形状;其次,利用超栅格或者超分子模板对长链进行有效剪裁;最后,利用纳米结构与衬底之间对称性的匹配来调控环状和链状产物的形成。另外,我们对寻求普适性模板以调控更广泛类型的纳米材料的拓扑结构进行了展望。
结构调控;表面模板;乌尔曼反应;表面合成;金属有机;聚合物;大环分子
1 Introduction
The last decade has seen the prosperity of “top-down”techniques like lithography applied in semiconductor manufacturing industry. However, this technique will soon reach its limit due to quantum size effect, thus the future of nanotechnology should lie in the “bottom up” approach1. One of the ultimate goal of the “bottom up” nanotechnology is to build the so-called “molecular chip”, that is, all the components in the chip are based on single or several molecules, which includes molecular wires, transistors, capacitors and etc2,3. In order to allow a considerable carrier transportation within the chip, covalent connections should be established between these components4. The organic chemistry has shed light on the realization of this goal due to diverse organic reactions that can be employed for covalent linking of versatile organic building blocks, including Ullmann, glaser, or dehydrogenative couplings5. Up to date, with specially designed and functionalized precursors, macromolecular structures with topological diversity and complex functions have been achieved directly on metal surfaces in ultrahigh vacuum (UHV) conditions. This includes graphene6−9or graphyne10−13derivatives, covalent/organometallic linear polymers14−24or cyclic rings25,26.
Typically, the controlling of the dimensions of the formed macromolecular nanostructure can be achieved by using precursors with different number of the reactive groups (i.e.,halogen, alkynyl). For instance, the two-fold and multi-fold(three-fold or higher) halogen substituted haloarenes typically undergo Ullmann coupling into one-dimensional (1D)19,25,27and two-dimensional (2D)9,20,21,28−31polyphenylenes, respectively. In particular, the widths of the 1D chains can be regulated directly by the sizes of the precursors. This is evidenced by the synthesis of 1D graphene nanorribons with different widths from haloarenes with different sizes6,7,32. Additionally, the edge topology of the graphene nanoribbons can be steered into armchaired or zigzag shape by means of geometry design of the precursors8. So far, the lengths of 1D chains obtained viaon-surface reactions mostly exhibit Γ-distribution due to the diffusion-controlled reaction process of precursors7,33,34. This raises an unresolved issue, i.e., how to control the chain length,which is of huge significance in the fabrication of molecular conducting wire with defined length.

Another interesting category of nanostructures is the cyclic polymers or macrocycles. The electronic properties of cyclic macromolecules are distinguished from their linear counterparts due to their endless topology, which may find new demands in the applications of molecular electronics35,36.Typically, the two-fold substituted precursor should react into 1D chains. Alternatively, the encounter of the two ends of a chain results in the formation of rings. However, the latter has seldom been observed due to the typical dominance of chain formation in ring-chain competition. Therefore, great challenge still remains in how to steer the ring-chain competition toward the direction of ring formation. To settle the above mentioned issues, we present several successful approaches in our recent studies for the topology regulation of 0D rings and 1D chains,including the ring size, chain length, and ring-chain competition. This is typically achieved by steering the reaction pathway of the precursors via multiple surface templates, as illustrated in Scheme 1. For instance, the lattice and symmetry matchings between the adsorbates and the templates have substantial influence on the toplogy of the formed nanostructures (Scheme 1(a, c)). The lengths of the chains can be unified and tuned by striped template, as shown in Scheme 1a. Additionally, the striped template can facilitate the formation of rings with tunable sizes due to the steric effect exerted therein (Scheme 1b). These initial success shed light on the developing of more general strategies for topology regulation of other macromolecular nanostructures, such as the size and shape of 2D networks as presented in Scheme 1d.
2 Regulation of chain topologies
Most of the organic reactions occurred on surfaces havedifferent pathways comparing to those in solution, especially when the surface plays a role as a template. In our recent works,the two-fold brominated 4,4ʹ-dibromo-m-terphenyl (DMTP)molecules were employed for the fabrication of chains with different topologies on surfaces with different symmetries, i.e.,Cu(111) and Cu(110). The deposition of submonolayer of DMTP molecules onto the six-fold symmetric Cu(111) at 300 K leads to the formation of exclusive zigzag organometallic chains with six orientations (marked with green arrows) as shown in Fig.1a26. This indicates that the DMTP molecules has twelve optimal adsorption configurations on the Cu(111)surface. The detailed structure of the zigzag organometallic chains, which are composed of m-terphenyl (MTP) units connected by C―Cu―C bonds with all-trans configuration, is revealed by the molecularly resolved STM image overlaid with molecular model in Fig.1b. In addition, a periodicity of 2.65 nm along the chain has been measured.

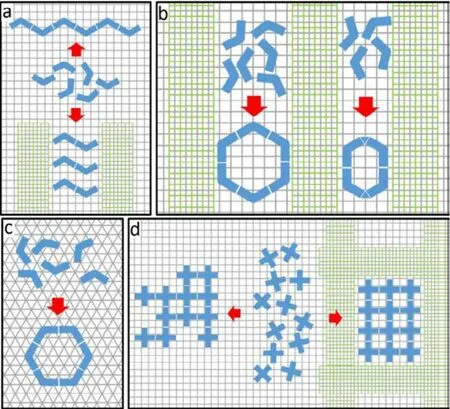
Scheme 1 Illustrations for the topology regulations of on-surface nanostructures with different surface templates.
However, the outcome is different on a two-fold symmetric Cu(110) surface. As shown in Fig.1c, organometallic chains with two different topologies (pointed with green and blue arrows) have been observed after deposition of submonolayer of DMTP onto Cu(110) held at 300 K27. The detailed structures of these two types of chain islands are revealed by their magnified views, as shown in Fig.1d and e. One type is the zigzag organometallic chain, which is similar to that formed on Cu(111) as illustrated by the overlaid molecular model in Fig.1d. However, the periodicity (2.79 nm) along the chain is larger than the chains formed on Cu(111) (2.65 nm). This is most possibly due to the different lattice matching of the zigzag chains with different surfaces. Moreover, only two orientations were observed for the zigzag organometallic chains formed on Cu(110), indicating four optimal adsorption configurations of the MTP unit. Again, this should result from the symmetry matching between the zigzag organometallic chains and the substrates. The other type is the trapezoidal-waved organometallic chains consists of m-terphenyl (MTP) units connected by C―Cu―C bonds with alternative cis/trans configuration, as shown in Fig.1e. The formation of trapezoidal-waved chain is the result of incorporation of MTP units with four optimal adsorption configurations into a single chain.
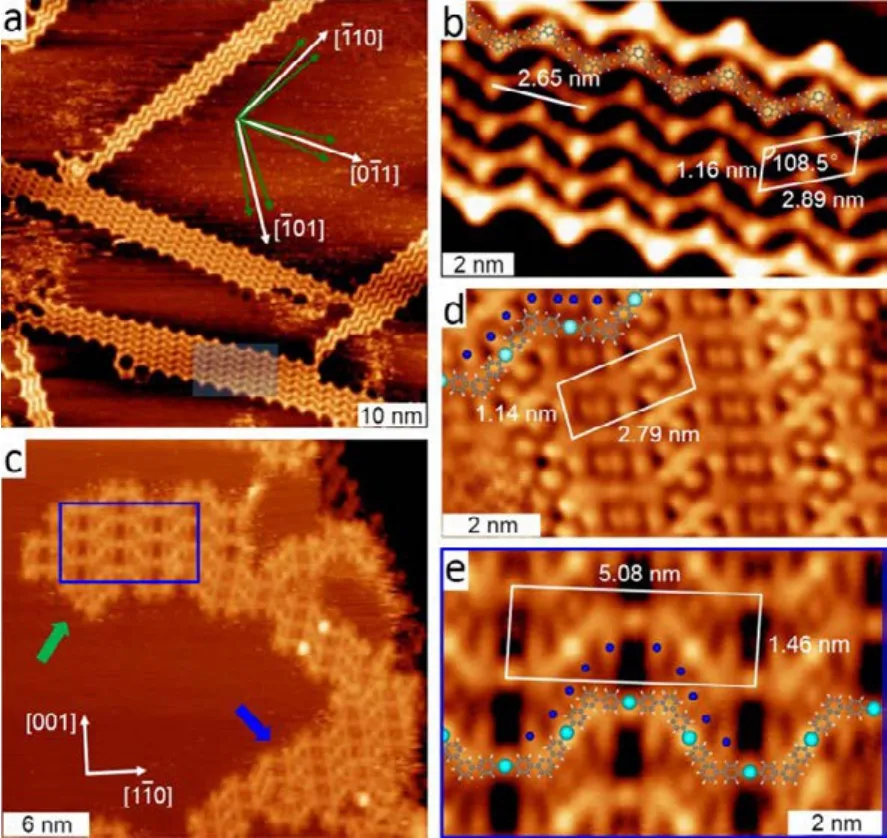
Fig.1 (a) Constant-current STM image taken after deposition of 4,4′-dibromo-m-terphenyl (DMTP) onto Cu(111) at 300 K at a coverage of 0.2 ML; tunneling parameters: U = −3.6 V, I = 0.02 nA.(b) Higher-resolution image of the shaded area in panel (a) with overlaid molecular model and unit cell, U = −3.6 V, I = 0.01 nA.(c) STM image obtained at 300 K after 0.48 ML DMTP molecules were deposited onto Cu(110) at 300 K. U = −2.9 V, I = 0.02 nA. (d)High resolution STM image of a section of the same all-trans zigzag structure as marked by blue arrow in (b), U = −2.0 V, I = 0.02 nA.(e) Magnified view of the blue framed region in panel (b) showing the cis/trans chains, U = −2.9 V, I = 0.03 nA.
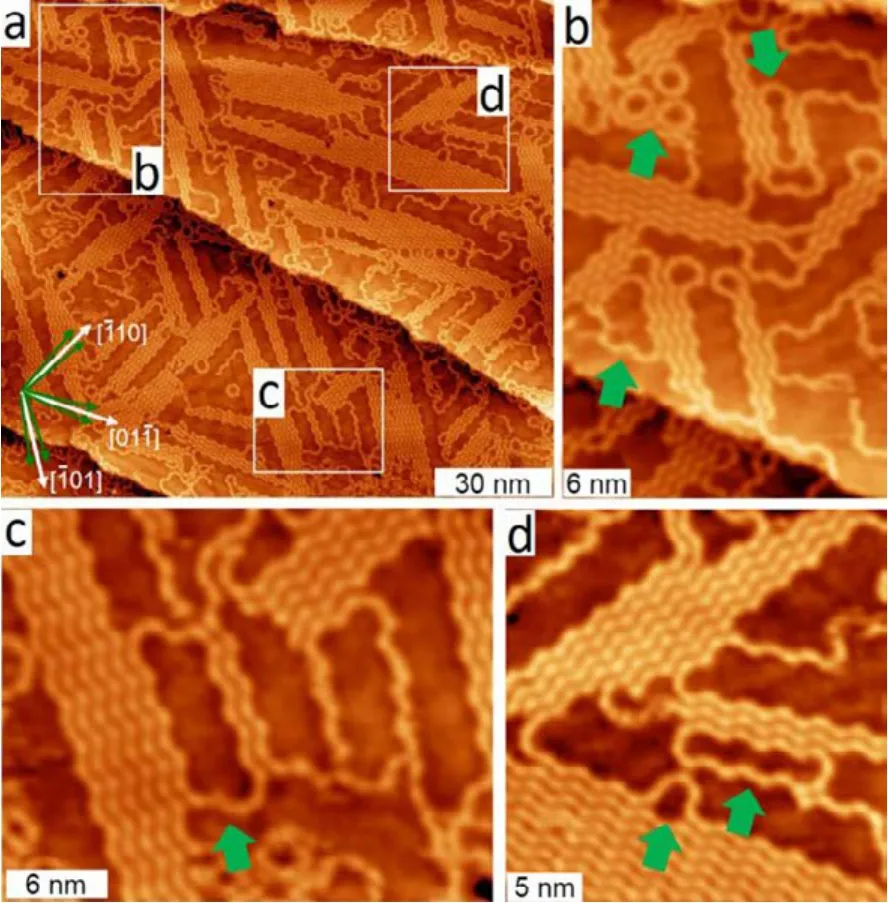
Fig.2 STM images of the organometallic oligomers and polymers obtained after deposition of 0.55 ML DMTP onto Cu(111) at 300 K; U = −2.75 V, I = 0.08 nA.
Similarly, the incorporation of MTP units on Cu(111) with six different optimal adsorption configurations enables the formation of “back-folding” organometallic chain or macrocycles as discussed below. Fig.2a shows the STM image after deposition of 0.55 monolayer (ML) DMTP onto Cu(111)at 300 K37. The encounter of zigzag organometallic chain domains at higher coverage leads to the formation of back-folding organometallic chains or cyclic organometallic oligomers around the domain boundaries (marked by white frames in Fig.2). As shown in Fig.2b, a cluster of three adjacent hexagons (MTP-Cu)6has been observed in the upper left region,the formation of which can be reckoned as the encounter and connection of three (MTP-Cu)2oligomeric chains oriented along three directions with mutual angles of 120°. In such a case, the variation of lengths of the three oligomeric chains can lead to the formation of organometallic cycles with different shapes and sizes. This includes the macrocycles (MTP-Cu)14and (MTP-Cu)16on the right-hand and the lower part of Fig.2b,respectively. Also, larger macrocycles (MTP-Cu)22and(MTP-Cu)18were observed as shown in Fig.2c and 2d (pointed with green arrows). Therefore, the formation of chains with different number of orientations and topologies on Cu(111) and Cu(110) strongly reflects the template role of the substrate during Ullmann reaction of DMTP monomers. Similar template effect has been observed for stepped surfaces which provide striped terraces to align the growth of polymer chains along the step edges10,38. Besides the surface corrugation, the nature of the substrate material also has significant influence on the formed nanostructures via Ullmann reaction. This is evidenced by the formation of 2D porous graphene network from cyclohexa-m-phenylene (CHP) monomer with different order and compactness on Cu(111), Ag(111) and Au(111) surfaces9.
3 Regulation of chain length
Except from the regulation of the chain shapes mentioned above, the width of the chains was typically steered by employment of precursors with different sizes. For instance, the two-fold brominated linear 4,4ʹ-dibromo-p-terphenyl monomer forms 1D polyphenylene chain with a width of three carbon atoms19. Additionally, the use of a wider two-fold brominated 1,4,5,8-tetrabromonaphthalene precursor leads to the formation of graphene nanoribbon with a width of five carbon atoms (i.e.,5-armchaired graphene nanoribbon (5-AGNR))32. Further increasing the width of the precursor, i.e., the employment of 10,10ʹ-dibromo-9,9ʹ-bianthryl, results in the formation of even wider 7-AGNR.7Nevertheless, the studies related to the regulation of the lengths of chains have seldom been reported.Typically, the 1D chains formed via on-surface synthesis exhibit a Γ-distribution as a result of the diffusion controlled assembly process, such as the formation of graphene nanoribbons on the Au(788) stepped surface34.
This case also ocurrs for organometallic chains acquired after deposition of DMTP onto both Cu(110) and Cu(111) surface.Fig.3a shows the islands of the zigzag organometallic chains obtained by deposition of 0.77 ML DMTP on Cu(111) at 440 K37. Although the size of the islands has been enlarged by applying a higher substrate temperature during deposition, the shapes of the islands remain irregular. This indicates that the lengths of the chains still follow certain distribution determined by their assembly process, as revealed by the magnified view of the island in Fig.3b. Similarly, increasing the tempreature of Cu(110) to 383 K during the deposition of DMTP leads to the formation of exclusive zigzag organometallic chains rather than the mixture of zigzag and trapezoidal-waved chains, as shown in Fig.3(c, d)39. This is most possibly due to that the zigzag chains are thermodynamically more stable than the trapezoidal-waved chains. Because the former has a higher packing density which would maxmize the mutual attractions between chains. Note that this interpretaion also explains the elimination of organometallic cycles on Cu(111) (Fig.3a) at 440 K, which is also the less stable products due to their lower packing density comparing to the chains. The domains of zigzag organometallic chains formed on the Cu(110) surface also exhibit irregular shapes, which depend mostly on the shapes of the step edges.
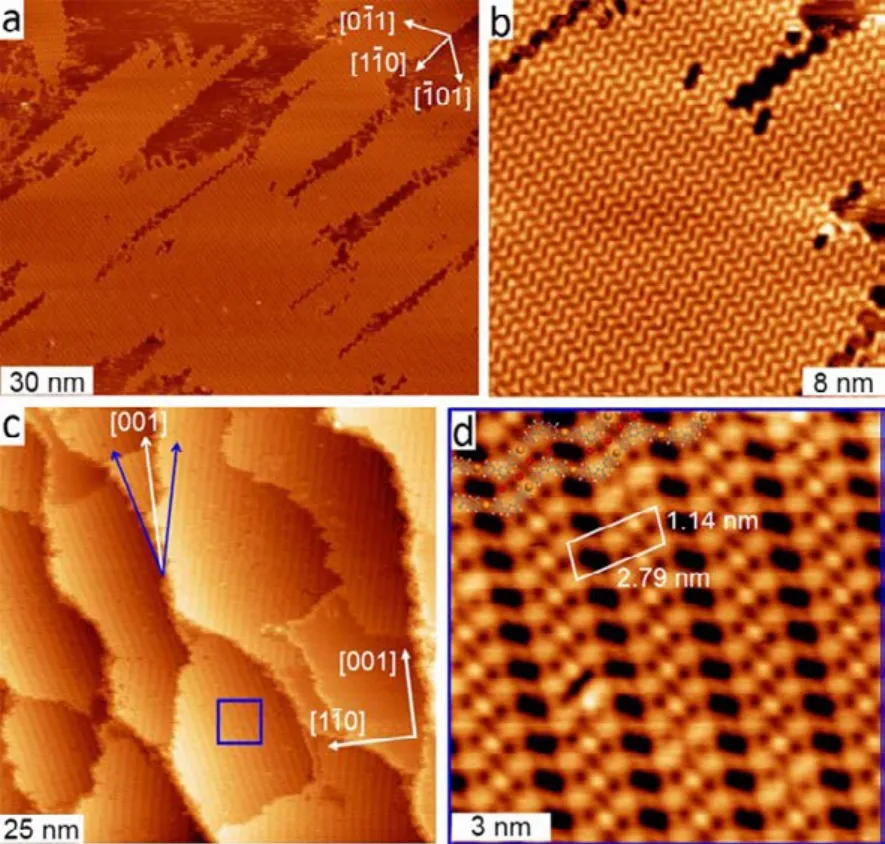
Fig.3 (a) STM image of large islands of the organometallic polymer obtained by deposition of 0.77 ML DMTP onto Cu(111)held at 440 K; U = −3.6 V, I = 0.01 nA. (b) Magnified image of the central area in (a), U = −3.6 V, I = 0.02 nA. (c) Overview STM image taken after deposition of 0.77 ML DMTP onto Cu(110) held at 383 K. (d) Zoom-in of the blue-framed region in panel (c)overlaid with the molecular model.
In order to tailor the lengths of these zigzag organometallic chains, special template was employed to regulate the Ullmann reaction of DMTP monomers. The template is a supergrating surface, i.e., a Cu(110)-(2 × 1)O superstructure39. The supergrating template was prepared by an appropriate exposure of Cu(110)surface to O2atmosphere at an elevated temperature. This procedure results in the formation of a submonolayer of Cu―O chains on the Cu(110) surface. Therefore, the supergrating consists of alternatively arranged Cu―O chain stripes and bare Cu stripes, both of which orient along the [001] direction. In addition, the widths of the bare Cu stripes can be adjusted by controlling the oxidation extent of Cu(110): increasing the exposure of O2at the same substrate temperature makes the bare Cu stripes narrower, whereas keeping the exposure constant but increasing the substrate temperature would result in broader Cu stripes and larger distances between them.
Fig.4a shows the overview STM image taken after deposition of 0.66 ML DMTP onto the Cu(110)-(2 × 1)O surface composed of Cu stripes (with widths from 14 to 18 nm)held at 383 K39. Domains of well-ordered short zigzag organometallic chains were observed on the bare Cu stripes.They are separated by Cu―O stripes, which are imaged as darker stripes. Fig.4b shows the magnified STM image of a zigzag organometallic chain domain with a width of 16.6 nm.The lengths of the chains in this domain can be calculated to be 18.2 nm according to their constant angle (24°) relative to the[001] direction. As can be seen, the long zigzag organometallic chains are tailored into short oligomeric chains with molecular accuracy. It should be noted that the successful tailoring of the zigzag organometallic chains with Cu(110)-(2 × 1)O striped surface requires the condition that the presence of the Cu―O chains does not affect the registry of the zigzag organometallic chains with the Cu(110) surface lattice. This is evidenced by the observations that the two mirror domains of the zigzag organometallic chains formed on the Cu(110)-(2 × 1)O striped surface have the same orientations as the domains formed on the clean Cu(110) surface (marked with blue arrows in Fig.4a).
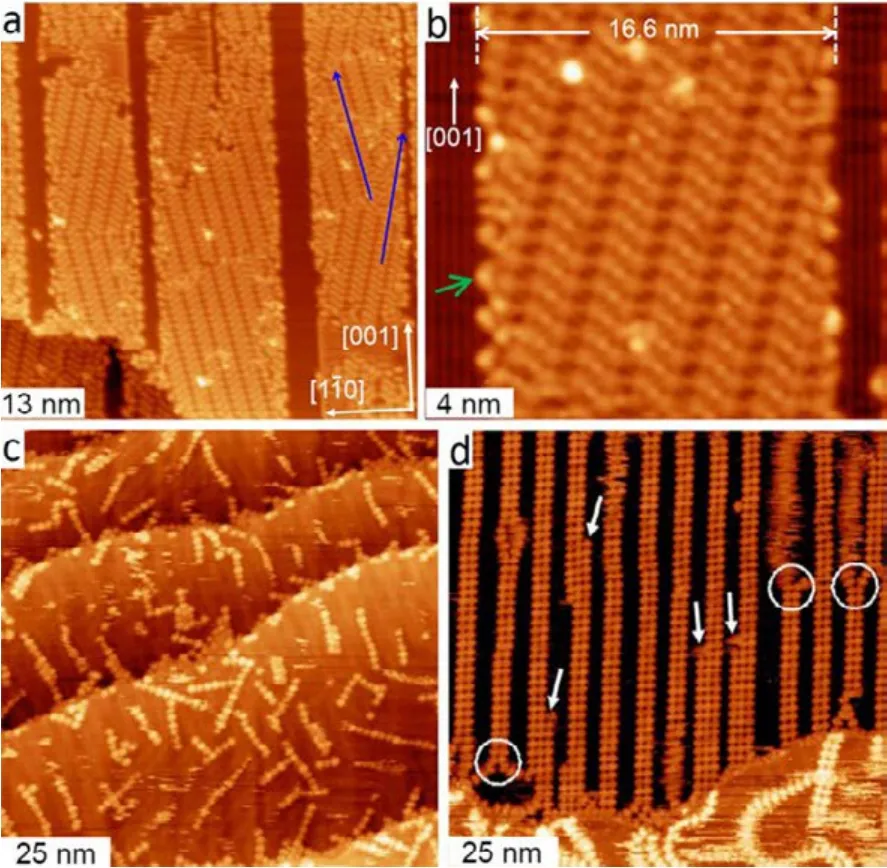
Fig.4 (a) STM image taken after deposition of 0.66 ML DMTP onto the Cu(110)-(2 × 1)O striped surface (Θo = 0.15 ML) held at 383 K. (b) Magnified view of a region from the sample in panel (a).(c) STM image showing macromolecular structures formed from 5,15-bis(4′-bromophenyl)-10,20-bis(4′-phenyl)porphyrin on Au(111) (data acquired at 200 K). (d) STM image of the sample prepared in two steps, where in each step,5,15-bis(4′-bromophenyl)-10,20-bis(4′-pyridyl)porphyrin molecules deposited on the substrate at room temperature,annealed at 180 °C, and cooled down to room temperature.
Another alternative approach for the regulation of chain length is steering the chain growth with supramolecular template rather than the substrate. It utilizes noncovalent binding supramolecular motifs to steer the formation of covalently bounded nanostructures, such as the metal―ligand binding, hydrogen bonding, and even weaker π−π interactions.This approach has been typically employed in solution synthesis and results in the acquirement of molecules with diverse topologies and properties that are otherwise difficult to achieve40. Lin and co-authors have successfully transplanted this method to the metal surface, who employed a linear coordination template to control the on-surface polymerization41.As illustrated in Fig.4c, the polymerization of 5,15-bis(4ʹ-bromophenyl)-10,20-bis(4ʹ-phenyl)porphyrin (DBDPP) on Au(111)surface under the assistance of copper leads to the formation of porphyrin chains with varied lengths, which again obey the Γ-distribution.
However, the deposition of 5,15-bis(4ʹ-bromophenyl)-10,20-bis(4ʹ-pyridyl)porphyrin (DBDPyP) molecules with copper onto Au(111) at 180 °C results in the formation of the double-row (DR) dimeric chains with a high yield of > 60%, as shown in Fig.4d. The DR chains consist of covalently linked porphyrin dimers which are then linked by pyridyl (Py)―Cu―pyridyl coordination bonds. The different outcomes stem from the modification of DBDPP molecule by replacing the two orthogonal phenyls with two pyridyl groups. This strongly indicates that the formation of long Py―Cu―Py bonded coordination chains steers the C―C coupling reaction process.The reaction process can be described as follows: The growth of DR chains starts with the preliminary formation of covalently bounded porphyrin dimers. Then, in the intermediate state, the anchoring of two additional precursors were achieved by the formation of two Py―Cu―Py coordination bonds. In the final step, the anchored two precursors undergo C―C Ullmann coupling forming the longer DR chain. Note that one requisite for the step-by-step growth of DR chains from the porphyrin dimer seeds is that they are stable during the annealing duration. This is guaranteed by the fact that the decomposition of DR chains requires breaking two metal―ligand bonds simultaneously, which is energetically very unfavorable. Therefore, Linʹs work presents an effective approach for steering the lengths of covalent porphyrin chains by controlling the assembly kinetics via the coordination chain template with Py―Cu―Py bonds.
4 Controlling of ring-chain competition
One of the other significant aspects of the topology of macromolecule is their continuity, i.e., the cyclic topology.Typically, the chemical and physical properties of macromolecular/polymer chain depend strongly on their terminal functional groups42. In view of this, the properties of a chain should alter significantly if they undergo terminal encounter and covalent linkage into a cycle with terminal-free topology. This has been evidenced by numerous studies, in which the comparison of the properties of chains and their cyclic homologues reveals a huge difference between them. For instance, the cyclic polymers bear a higher density, lower intrinsic viscosity, and higher thermostability35,36,43−48. However, challenges still exist in controlling the formation of macrocycles and the characterization of their properties with powerful techniques. In this part, we will briefly review the studies concerning the steering of the ring-chain competition toward the direction of ring formation with special templates.
As discussed above, the deposition of DMTP onto clean Cu(110) surface at 383 K leads to the formation of exclusive zigzag organometallic chains. In order to steer the formation of organometallic rings, we have employed again the partially oxidized Cu(110)-(2 × 1)O surface as the template39.Nevertheless, the widths of the Cu stripes are in the range of 2.6 to 3.6 nm. As shown in Fig.5a, the deposition of 0.22 ML DMTP onto this template with narrow Cu stripes held at 383 K leads to the formation of organometallic tetragons (MTP-Cu)4(marked with green and white arrows) and hexagons(MTP-Cu)6(marked with yellow arrow). Noteworthy, these species are typically not the single products obtained on Cu stripes with a particular width (other byproducts can be observed). However, the total yield of tetramers and hexamers on this surface reaches a high level comparing to that on the clean Cu(110) surface. Fig.5b shows the high-resolution STM image of a section of the cyclic organometallic tetragons(MTP-Cu)4, which consists of two cis-(MTP-Cu)2dimers linked by two bent C―Cu―C bonds as illustrated by the adsorption model in Fig.5c. The formation of extraordinary bent rather than straight bridges (marked with yellow ovals in Fig.5c) is mainly due to the strong spatial confinement effect imposed by Cu―O chains, which is also proposed as the main stimulus for the formation of cyclic species.
Furthermore, the rotated but identical cyclic organometallic(MTP-Cu)4tetragons are formed on the slightly wider Cu stripes with a width of 3.1 nm. These tetragons bear an orientation perpendicular to that formed on Cu stripes with width of 2.6 nm, as shown in Fig.5d. This change in the orientation is proposed to be driven mainly by a maximum utilization of the width of the wider Cu stripe (3.1 nm);otherwise additional MTP units would fill the remaining blank area of the wider Cu stripe. An additional increase of the width of Cu stripe to 3.6 nm results in the formation of the cyclic organometallic hexagons (MTP-Cu)6, as shown in Fig.5f. The corresponding adsorption model of these hexagons is displayed in Fig.5g, which can be regarded as formed by insertion of two additional MTP units (marked with white rectangles) into the left and right cis-(MTP-Cu)2dimers in the tetragon in Fig.5b.Therefore, by the employment of Cu(110)-(2 × 1)O surface with narrow Cu stripes (2.6−3.6 nm) as template, the organometallic ring-chain competition has been successfully steered towards the direction of ring formation. In addition, the sizes of the macrocyles can be tuned by the widths of Cu stripes.
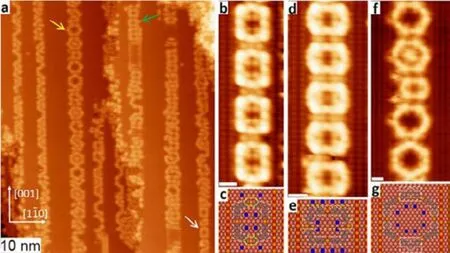
Fig.5 (a) Overview STM image taken after deposition of 0.22 ML DMTP onto Cu(110)-(2 × 1)O with a coverage of oxygen of 0.35 ML held at 383 K. The panels (b), (d) and (f) show the magnified viewof organometallic species formed on the sample in panel (a).The corresponding molecular models are shown in (c), (e) and (g).(b, c) Tetramer (MTP-Cu)4. (d, e) Rotated tetramer (MTP-Cu)4.(f, g) Hexamer (MTP-Cu)6. Grey spheres represent carbon atoms;white, hydrogen; blue, bromine; red, oxygen; coppery, copper atom. Tunneling parameters: (a) U = 0.6 V, I = 0.07 nA;(b, d) U = 0.8 V, I = 0.10 nA; (f) U = 0.5 V, I = 0.07 nA.
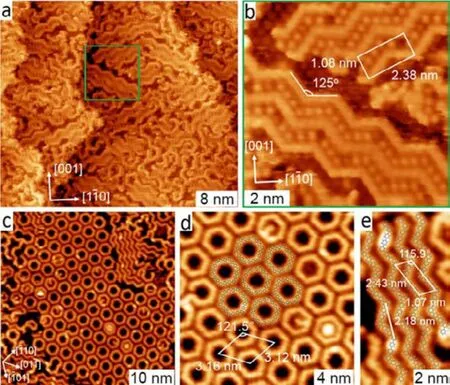
Fig.6 (a) Overview STM image taken after deposition of 0.84 ML DMTP onto Cu(110) at 383 K followed by annealing at 458 K,U = −0.7 V, I = 0.04 nA. (b) Zoom-in STM image of the green framed region in panel (b), U = −1.5 V, I = 0.06 nA. (c) Overview STM image obtained at 300 K after deposition of DMTP onto Cu(111) held at 550 K. (d) Magnified view of the hyperbenzene island overlaid with molecular models and a unit cell. U = −3.6 V,I = 0.01 nA. (e) Magnified view of a small section with oligophenylene chains.
Besides the organometallic case, competition of the formation of covalently ring-chain species has also been controlled by using effective template. As shown in Fig.6a,deposition of 0.84 ML DMTP onto Cu(110) at 383 K followed by annealing at 458 K leads to the exclusive formation of domains of zigzag oligophenylene chains27. No cyclic species are observed on Cu(110). The high selectivity for chain formation is due mainly to the commensurability and the symmetry matching between the formed zigzag oligophenylene chains and the two-fold symmetric Cu(110) substrate. Fig.5b shows the magnified view of the zigzag oligophenylene chains,the unit cell of which is 1.08 × 2.38 nm. Notably, the newly formed zigzag oligophenylene chains are stretched along the chain direction. Thus, the angle of the bend (between two adjacent straight oligophenylene moiety) of the zigzag chains is calculated to be 125°. This stretching is most likely driven by adsorbate−substrate interactions and results in a better commensurability with the surface lattice.
However, the employment of the six-fold symmetric Cu(111) surface as the substrate during deposition of DMTP leads to the formation of oligophenylene rings with a high yield26. As shown in Fig.6c, ordered arrays of hexagonal rings as the main products together with chain-like and other structures as minor products are obtained after deposition of DMTP onto Cu(111) at 550 K. With the size and profile consideration, the hexagonal rings are verified as hyperbenzene consisting of six MTP units connected by C―C bonds, as illustrated by the overlaid molecular model in Fig.6d.Comparing to the formation of exclusive two-fold symmetric zigzag oligophenylene chains on Cu(110), it is the six-fold symmetry of the Cu(111) substrate leads to the high selectivity of the reaction pathway with regard to the formation of hyperbenzene. Apart from the hyperbenzene arrays, zigzag oligophenylene chains (Fig.6e) are obtained as byproducts.Notably, their periodicity (2.18 nm) along the chain is considerably smaller than that of the oligophenylene chains(2.38 nm) formed on Cu(110) surface. This is due mainly to the different commensurabilities achieved between zigzag oligophenylene chains and the two different substrates.Therefore, the matching of the symmetries and lattice parameters between the covalent nanostructure and the substrate have huge impact on their growth kinetics, and thus can be employed to regulate their topologies.
5 Conclusion and outlook
In conclusion, in this paper we have reviewed recent works concerning the topology regulation of the nanostructures formed via on-surface reactions. First, the regulation of the shapes of organometallic chains is achieved by different lattice matching. As a result of the commensurability, the deposition of DMTP onto six-fold symmetric Cu(111) leads to the formation of exclusive zigzag organometallic chains. However,a mixture of zigzag and trapezoidal-waved organometallic chains forms on the two-fold symmetric Cu(110) under similar conditions. Second, the lengths of organometallic chains can be regulated by the super-grating or metal-directed supramolecular templates. By employing a Cu(110)-(2 × 1)O supergrating, the long zigzag organometallic polymeric chains are tailored into short oligomeric chains with uniform lengths, which can also be tuned via controlling of the width of grating. Additionally,the covalently bounded porphyrin chains are regulated into dimers with high yield by the formation of Py―Cu―Py coordination bonded chain template. Thirdly, the competition between the formation of covalent ring and chain from DMTP can be controlled by employing templates with different symmetries. DMTP forms exclusive zigzag oligophenylene chains on the two-fold symmetric Cu(110) surface, however,forms cyclic hyperbenzenes with high yield on the six-fold symmetric Cu(111) surface.
Although the topology of a certain category of organometallic or covalent chains can be controlled by several approaches presented in this review, huge challenges still remain in the application of these approaches to a wider range of 1D chains and 2D covalent networks. For instance, the currently used Cu(110)-(2 × 1)O supergrating template deconstructs when annealed with DMTP to 420 K, and thus cannot be used to regulate the topology of covalently bounded nanostructures. To settle the potential similar issues, searching for diverse 1D and 2D supergrating surfaces that are more robust under the reaction of precursor monomers is demanded. One possible example is the 1D supergrating formed by assembly of silicon nanowire on Ag(110),49although this may in return raise other issues concerning the interaction between the organic precursors and the supergratings. However, with the assistance of powerful surface science techniques, the further exploration of more general methods to steer the topologies of a broader range of nanostructures on surfaces is just a matter of time.
(1) Barth, J. V.; Costantini, G.; Kern, K. Nature 2005, 437, 671.doi: 10.1038/nature04166
(3) Champness, N. R. Nat. Nanotechnol. 2007, 2, 671.doi: 10.1038/nnano.2007.355
(4) Perepichka, D. F.; Rosei, F. Science 2009, 323, 216.doi: 10.1126/science.1165429
(5) Fan, Q.; Gottfried, J. M.; Zhu, J. Acc. Chem. Res. 2015, 48, 2484.doi: 10.1021/acs.accounts.5b00168
(6) Cai, J.; Pignedoli, C. A.; Talirz, L.; Ruffieux, P.; Sode, H.; Liang, L.;Meunier, V.; Berger, R.; Li, R.; Feng, X.; Müllen, K.; Fasel, R. Nat.Nanotechnol. 2014, 9, 896. doi: 10.1038/nnano.2014.184
(7) Cai, J.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, S.;Muoth, M.; Seitsonen, A. P.; Saleh, M.; Feng, X.; Muellen, K.; Fasel,R. Nature 2010, 466, 470. doi: 10.1038/nature09211
(8) Ruffieux, P.; Wang, S.; Yang, B.; Sánchez-Sánchez, C.; Liu, J.;Dienel, T.; Talirz, L.; Shinde, P.; Pignedoli, C. A.; Passerone, D.;Dumslaff, T.; Feng, X.; Müllen, K.; Fasel, R. Nature 2016, 531, 489.doi: 10.1038/nature17151
(9) Bieri, M.; Nguyen, M. T.; Groning, O.; Cai, J. M.; Treier, M.;Ait-Mansour, K.; Ruffieux, P.; Pignedoli, C. A.; Passerone, D.;Kastler, M.; Müllen, K.; Fasel, R. J. Am. Chem. Soc. 2010, 132,16669. doi: 10.1021/ja107947z
(10) Cirera, B.; Zhang, Y. Q.; Bjӧrk, J.; Klyatskaya, S.; Chen, Z.; Ruben,M.; Barth, J. V.; Klappenberger, F. Nano Lett. 2014, 14, 1891.doi: 10.1021/nl4046747
(11) Gao, H. Y.; Wagner, H.; Zhong, D.; Franke, J. H.; Studer, A.; Fuchs,H. Angew. Chem. Int. Ed. 2013, 52, 4024. doi:10.1002/anie.201208597
(12) Liu, J.; Chen, Q.; Xiao, L.; Shang, J.; Zhou, X.; Zhang, Y.; Wang, Y.;Shao, X.; Li, J.; Chen, W.; Xu, G. Q.; Tang, H.; Zhao, D.; Wu, K.ACS Nano 2015, 9, 6305. doi: 10.1021/acsnano.5b01803
(13) Zhang, Y. Q.; Kepcija, N.; Kleinschrodt, M.; Diller, K.; Fischer, S.;Papageorgiou, A. C.; Allegretti, F.; Bjӧrk, J.; Klyatskaya, S.;Klappenberger, F.; Ruben, M.; Barth, J. V. Nat. Commun. 2012, 3,1286. doi: 10.1038/ncomms2291
(14) Di Giovannantonio, M. E. G., M.; Lipton-Duffin, J.; Meunier, V.;Cardenas, L.; Fagot Revurat, Y.; Cossaro, A.; Verdini, A.; Perepichka,D. F.; Rosei, F.; Contini, G. ACS Nano 2013, 7, 8190.doi: 10.1021/nn4035684
(15) Sun, Q.; Zhang, C.; Kong, H.; Tan, Q.; Xu, W. Chem. Commun. 2014,50, 11825. doi: 10.1039/c4cc05482b
(16) Park, J.; Kim, K. Y.; Chung, K. H.; Yoon, J. K.; Kim, H.; Han, S.;Kahng, S. J. J. Phys. Chem. C 2011, 115, 14834.doi: 10.1021/jp203129f
(17) Basagni, A.; Sedona, F.; Pignedoli, C. A.; Cattelan, M.; Nicolas, L.;Casarin, M.; Sambi, M. J. Am. Chem. Soc. 2015, 137, 1802.doi: 10.1021/ja510292b
(18) Sun, Q.; Zhang, C.; Li, Z.; Kong, H.; Tan, Q.; Hu, A.; Xu, W. J. Am.Chem. Soc. 2013, 135, 8448. doi: 10.1021/ja404039t
(19) Wang, W. H.; Shi, X. Q.; Wang, S. Y.; Van Hove, M. A.; Lin, N. J. Am.Chem. Soc. 2011, 133, 13264. doi: 10.1021/Ja204956b
(20) Lafferentz, L.; Eberhardt, V.; Dri, C.; Africh, C.; Comelli, G.; Esch, F.;Hecht, S.; Grill, L. Nat. Chem. 2012, 4, 215.doi: 10.1038/nchem.1242
(21) Grill, L.; Dyer, M.; Lafferentz, L.; Persson, M.; Peters, M. V.; Hecht,S. Nat. Nanotechnol. 2007, 2, 687. doi: 10.1038/nnano.2007.346
(22) Chung, K. H.; Koo, B. G.; Kim, H.; Yoon, J. K.; Kim, J. H.; Kwon, Y.K.; Kahng, S. J. Phys. Chem. Chem. Phys. 2012, 14, 7304.doi: 10.1039/c2cp23295b
(23) Lipton-Duffin, J. A.; Miwa, J. A.; Kondratenko, M.; Cicoira, F.;Sumpter, B. G.; Meunier, V.; Perepichka, D. F.; Rosei, F. Proc. Natl.Acad. Sci. U. S. A. 2010, 107, 11200. doi: 10.1073/pnas.1000726107
(24) Zhong, D.; Franke, J. H.; Podiyanachari, S. K.; Blomker, T.; Zhang,H.; Kehr, G.; Erker, G.; Fuchs, H.; Chi, L. Science 2011, 334, 213.doi: 10.1126/science.1211836
(25) Lipton-Duffin, J. A.; Ivasenko, O.; Perepichka, D. F.; Rosei, F. Small 2009, 5, 592. doi: 10.1002/smll.200801943
(26) Fan, Q.; Wang, C.; Han, Y.; Zhu, J.; Hieringer, W.; Kuttner, J.; Hilt,G.; Gottfried, J. M. Angew. Chem. Int. Ed. 2013, 52, 4668.doi: 10.1002/anie.201300610
(27) Dai, J.; Fan, Q.; Wang, T.; Kuttner, J.; Hilt, G.; Gottfried, J. M.; Zhu,J. Phys. Chem. Chem. Phys. 2016, 18, 20627.doi: 10.1039/C6CP03551E
(28) Gutzler, R.; Walch, H.; Eder, G.; Kloft, S.; Heckl, W. M.; Lackinger,M. Chem. Commun. 2009, 29, 4456. doi: 10.1039/b906836h
(29) Eichhorn, J.; Strunskus, T.; Rastgoo-Lahrood, A.; Samanta, D.;Schmittel, M.; Lackinger, M. Chem. Commun. 2014, 50, 7680.doi: 10.1039/c4cc02757d
(30) Fan, Q.; Wang, C.; Liu, L.; Han, Y.; Zhao, J.; Zhu, J.; Kuttner, J.; Hilt,G.; Gottfried, J. M. J. Phys. Chem. C 2014, 118, 13018.doi: 10.1021/jp5037475
(31) Fan, Q.; Wang, T.; Liu, L.; Zhao, J.; Zhu, J.; Gottfried, J. M. J. Chem.Phys. 2015, 142, 101906. doi: 10.1063/1.4906214
(32) Zhang, H.; Lin, H.; Sun, K.; Chen, L.; Zagranyarski, Y.; Aghdassi, N.;Duhm, S.; Li, Q.; Zhong, D.; Li, Y.; Müllen, K.; Fuchs, H.; Chi, L.J. Am. Chem. Soc. 2015, 137, 4022. doi: 10.1021/ja511995r
(33) Han, P.; Akagi, K.; Federici Canova, F.; Mutoh, H.; Shiraki, S.; Iwaya,K.; Weiss, P. S.; Asao, N.; Hitosugi, T. ACS Nano 2014, 8, 9181.doi: 10.1021/nn5028642
(34) Linden, S.; Zhong, D.; Timmer, A.; Aghdassi, N.; Franke, J. H.;Zhang, H.; Feng, X.; Müllen, K.; Fuchs, H.; Chi, L.; Zacharias, H.Phys. Rev. Lett. 2012, 108, 216801.doi: 10.1103/PhysRevLett.108.216801
(35) Laurent, B. A.; Grayson, S. M. Chem. Soc. Rev. 2009, 38, 2202.doi: 10.1039/b809916m
(36) Endo, K., Endo, K. Adv. Polym. Sci. 2008, 217, 121.doi: 10.1007/12_2008_157
(37) Fan, Q.; Wang, C.; Han, Y.; Zhu, J.; Kuttner, J.; Hilt, G.; Gottfried, J.M. ACS Nano 2014, 8, 709. doi: 10.1021/nn405370s
(38) Saywell, A.; Schwarz, J.; Hecht, S.; Grill, L. Angew. Chem. Int. Ed.2012, 51, 5096. dio: 10.1002/anie.201200543
(39) Fan, Q.; Dai, J.; Wang, T.; Kuttner, J.; Hilt, G.; Gottfried, J. M.; Zhu,J. ACS Nano 2016, 10, 3747. dio: 10.1021/acsnano.6b00366
(40) Anderson, S.; Anderson, H. L. Templated Organic Synthesis;Wiley-VCH: Weinheim, Germany, 2000.doi: 10.1002/9783527613526.ch01
(41) Lin, T.; Shang, X. S.; Adisoejoso, J.; Liu, P. N.; Lin, N. J. Am. Chem.Soc. 2013, 135, 3576. doi: 10.1021/ja311890n
(42) Jia, Z.; Monteiro, M. J. J. Polym. Sci. A-Polym. Chem. 2012, 50, 2085.doi: 10.1002/pola.25999
(43) Zhang, K.; Tew, G. N. React. Funct. Polym. 2014, 80, 40.doi: 10.1016/j.reactfunctpolym.2014.01.012
(44) Yamamoto, T. Polym. J. 2013, 45, 711. doi: 10.1038/pj.2012.213
(45) Tezuka, Y. Polym. J. 2012, 44, 1159. doi: 10.1038/pj.2012.92
(46) Yamamoto, T.; Tezuka, Y. Polym. Chem. 2011, 2, 1930.doi: 10.1039/c1py00088h
(47) Hoskins, J. N.; Grayson, S. M. Poly. Chem. 2011, 2, 289.doi: 10.1039/c0py00102c
(48) Kricheldorf, H. R. J. Polym. Sci. A -Polym. Chem. 2010, 48, 251.doi: 10.1002/pola.23755
(49) Sahaf, H.; Masson, L.; Léandri, C.; Aufray, B.; Le Lay, G.; Ronci, F.Appl. Phys. Lett. 2007, 90, 263110. doi: 10.1063/1.2752125
Controlling the Topology of Low-Dimensional Organic Nanostructures with Surface Templates
FAN Qi-Tang ZHU Jun-Fa*
(National Synchrotron Radiation Laboratory and Collaborative Innovation Center of Suzhou Nano Science and Technology, University of Science and Technology of China, Hefei 230029, P. R. China)
The future of nanotechnology lies in the “bottom up” approach, which aims at building nanostructures at an atomic or molecular level so as to minimize the sizes of chips and other nano-devices. However, one of the long-term unresolved issues for “bottom up” nanotechnology is the precise control of the topologies of fabricated nanostructures. In this contibution, we review recent studies with regard to the control of the topologies of nanostructures formed via the on-surface Ullmann reaction of haloarenes. This includes three aspects: control of the shape of the organometallic chain via lattice matching between the adsorbate nanostructure and the substrate; tailoring the chains by employing super-gratings or supramolecular templates; and steering the covalent ring-chain competition in the reaction of precursors towards ring formation through adsorbate-substrate symmetry matching. Further,we present future directions for the development of more general templates for the regulation of the topologies of a broader range of nanostructures.
Topology regulation; Surface template; Ullmann reaction; On-surface synthesis;Organometallic; Polymer; Macrocycle molecule
January 12, 2017; Revised: March 10, 2017; Published online: April 7, 2017.
his Bachelor of Science in Physics from Nanchang University in 2011 and Ph.D of Engineering in Nuclear Science and Technology from University of Science and Technology of China (USTC) in 2016. He is currently working as a postdoctoral researcher in Philipps-Universität Marburg under the support of Humboldt Research Fellowship. His research interests focus on surface supramolecular assembly and on-surface synthesis of macromolecules.
ZHU Jun-Fa, received his Ph.D. in Physical Chemistry from USTC in 1999.After several years working in the Institute of Experimental Physics, Johannes-Kepler-Universität Linz, Austria, Lehrstuhl für Physikalische Chemie II, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany and Department of Chemistry, University of Washington, USA, he returned to USTC in December, 2006, and became a professor at National Synchrotron Radiation Laboratory, USTC under the support of “Hundred Talent Program” of Chinese Academy of Sciences. His research interests mainly focus on in-situ studies of surface chemistry and catalysis, surface/interface structures and properties of functional materials, and surface coordination chemistry.
O649
10.1038/nchem.1276
[Feature Article]
10.3866/PKU.WHXB201704074 www.whxb.pku.edu.cn
*Corresponding author. Email: jfzhu@ustc.edu.cn; Tel: +86-551-63602064.
The project was supported by the National Natural Science Foundation of China (21473178) and National Key Basic Research Program of China (973)(2013CB834605).
国家自然科学基金项目(21473178)和国家重点基础研究发展规划项目(973) (2013CB834605)资助
© Editorial office of Acta Physico-Chimica Sinica
FAN Qi-Tang,
