腹腔镜保脾胰体尾整块切除手术治疗胰腺癌的技术细节及预后
2017-10-20孙志鹏朱昱冰阿民布和李天雄张能维
孙志鹏 朱昱冰 阿民布和 樊 庆 李天雄 张能维
(首都医科大学附属北京世纪坛医院肿瘤外科,北京大学第九临床医学院, 北京 100038)
·临床研究·
腹腔镜保脾胰体尾整块切除手术治疗胰腺癌的技术细节及预后
孙志鹏△朱昱冰△阿民布和 樊 庆 李天雄 张能维*
(首都医科大学附属北京世纪坛医院肿瘤外科,北京大学第九临床医学院, 北京 100038)
目的阐述腹腔镜保脾胰体尾整块切除手术的技术细节,并对其治疗胰腺癌的短期、长期肿瘤学预后及合并症情况进行评价。方法采用回顾性病例对照分析的方法,研究对象为北京世纪坛医院2007年1月1日至2010年1月1日间术前诊断为I期、II期胰体尾癌,且无脾门淋巴结转移的23例病人,其中17例接受腹腔镜保脾胰体尾整块切除手术,同期6例行脾切除的腹腔镜胰体尾整块切除手术,对两组的手术时间、术中出血等术中情况,近期、远期肿瘤学预后及合并症情况进行汇总分析,并与开腹手术的同期文献报道的相关指标进行比较。结果6例腹腔镜保脾胰体尾整块切除手术的平均手术时间为(203±54)min。平均出血量为(208±106)mL。1例由于严重的腹腔粘连中转开腹。短期合并症率为47%(n=8),胰瘘的发生率为41%(n=7)。无围术期死亡病例。肿瘤平均直径为(32±12)mm,平均淋巴结获得数量为(19.8±9.3)个。切缘均为阴性。病人的1、3、5年总生存率分别为64.7%、52.9%、41.2%,无复发生存率分别为58.8%、47.1%、35.3%。这些指标与同时期行脾切除的腹腔镜胰体尾手术及同期开腹胰体尾手术报道相比,差异无统计学意义。结论腹腔镜保脾胰体尾整块切除手术治疗术前诊断为I期、II期,且无脾门淋巴结转移的胰体尾癌,具有可接受的近期、远期肿瘤学预后及手术合并症发生率。
腹腔镜保脾胰体尾整块切除手术;胰腺癌;整块切除技术;根治性顺势胰体尾脾切除术
在过去的20年里,腹腔镜胰体尾手术经历了从最初的尝试应用的个案报道,到逐渐地用在诊断性腹腔镜探查、标本活检、胰体尾切除、胰十二指肠切除的过程[1]。由于腹腔镜胰体尾切除的难度不大,在良性及低度恶性的疾病中,该手术方式得到了广泛应用[2]。
2003年,Strasberg等[3]首先提出了根治性顺行胰体尾脾整块切除的概念(radical antegrade modular pancreatosplenectomy, RAMPS)。其后Mitchem等[4]对后方离断平面进行了研究,将其分为离断部分Gerota筋膜的前RAMPS平面和完全剥离Gerota筋膜的后RAMPS平面。并得出后RAMPS切除平面可获得更好的R0切除率。获得更好的远期生存率。然而以欧美流派为代表的RAMPS手术虽然对于后方清扫平面给予了明确界定,但对于胰体尾癌的淋巴结清扫及神经清扫范围的描述仍十分模糊[5]。
日本的胰腺癌诊疗指南[6-7]一直有对胰腺癌淋巴结的明确分组,第5版及第6版有对神经丛切除范围的明确描述。尽管这样的手术规范仍缺乏充分的循证医学证据支持,但这是少有的明确淋巴结分组及神经清扫范围的指南之一。因此根据日本胰腺癌诊疗指南第5版(2003年)[8]及RAMPS手术后方离断平面,笔者设计了腹腔镜胰腺癌整块切除手术。标准保脾胰体尾整块切除技术的目前术式并未确定。因此笔者的研究目的是阐述笔者所理解的保脾的腹腔镜胰体尾整块切除(laparoscopic distal pancreatectomy,LDP)手术的技术细节,并参考汇总既往的胰体尾肿瘤手术的近期、远期疗效的研究[4,9-12],对该手术技术的近期、远期肿瘤学预后予以评估。
1 资料与方法
1.1病例资料
从2007年1月1日至2010年1月1日,共计60名病人进行了胰腺癌手术,其中23例病人进行了腹腔镜手术。17例为腹腔镜下保脾胰体尾整块切除手术,6例为腹腔镜下胰体尾+脾切除手术(包含1例中转开腹)。2002年,大量的临床研究[12-15]显示:腹腔镜手术可用于胰腺癌治疗。对于良性及术前根据美国癌症联合会(American Joint Committee on Cancer,AJCC)临床分期为I期、Ⅱ期,术前影像学考虑无脾门淋巴结转移的胰体尾癌,均将腹腔镜手术作为治疗方案之一。关于术后合并症的分析,采取Clavian-Dindo的分类方法[14]。胰瘘的定义及分级标准采用国际胰瘘研究组织(International Study Group of Pancreatic fistular, ISGPF)的定义及分级标准[15]。
1.2腹腔镜胰体尾整块切除手术的技术细节
整块切除的概念:1)整块切除Gerota筋膜、Toldt筋膜、胰体尾;从左侧自胰尾部开始翻起肾前筋膜至肠系膜上动静脉。2)切除第一站,第二站淋巴结;清扫8a、8p、10、11p、11 d、18和7、9、14p、14 d、15两组淋巴结(表1)。3)腹腔神经丛清扫:清扫腹腔神经丛第一部、第二部左半周(表2)。在无脾门及胃网膜左血管及淋巴结侵犯的情况下,保留脾脏。手术技术细节详见图1、图2,手术技术路线见图3。
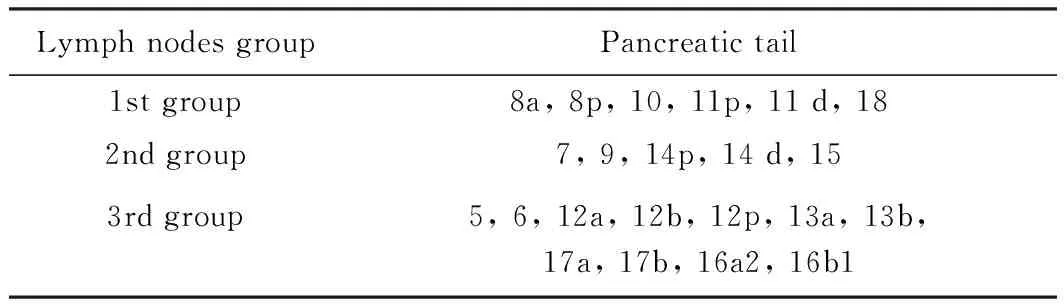
表1 胰腺淋巴结的分组(日本胰腺癌诊疗指南第5版,2003年)[8]Tab.1 Pancreatic lymph nodes group in General Rulesfor the Study of Pancreatic Cancer (5th edition, 2003)[8]
1.3统计学方法
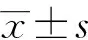
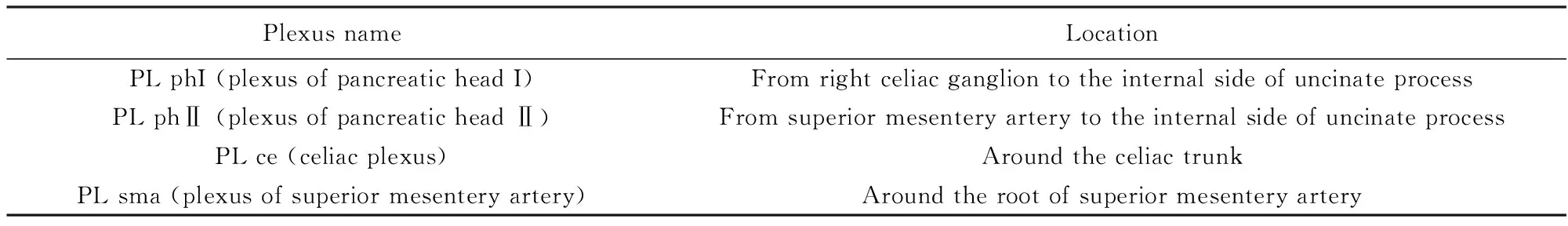
表2 胰腺相关神经丛编号及名称(日本胰腺癌诊疗指南第5版,2003年)[8]Tab.2 Pancreatic nerve plexus group in General Rules for the Study of Pancreatic Cancer (5th edition, 2003)[8]
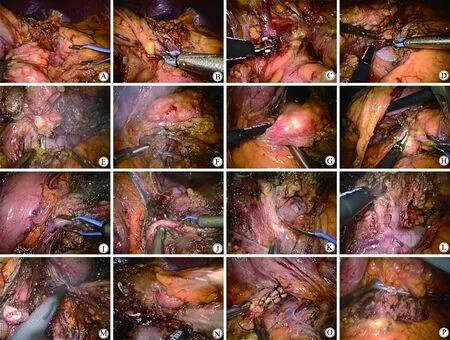
图1 保脾胰体尾整块切除技术Fig.1 The technique of standard en-bloc spleen-preserving LDP
A: Dissect the gastro-colic ligament, disconnect the transverse mesocolon from the Gerota fascia.B: At the level above the upper surface of the pancreas isolate LGEV and the communicating veins from the distal pancreas. Preserve the LGEV.C: Dissect between the communicating vessels to the distal pancreas.D: At the tail of the pancreas, dissect Gerota fascia from the lower border of left renal vein upward to the left kidney.E: Continue to dissect Gerota fascia to the left border of the SMA. Dissect the lymph nodes and celiac ganglion group Ⅱ around SMA preserving 5 mm ganglion at the right side.F: Continue to dissect upward to the splenic vein. Expose the splenic vein and IMV, ligate and cutoff the IMV.G: Penetrate the pancreas from the posterior surface at the root of splenic vessels.H: Dissect the pancreas at the root of splenic vessels with Harmonic scalpel.I: Isolate the splenic vein at the root, and dissect it.J: Isolate the splenic artery at the root, and dissect it.K: Dissect the lymph nodes and celiac ganglion group I around the celiac trunk preserving 5 mm ganglion at the right side.L: Dissect the retroperitoneum adipose tissue at the upper border of the pancreas. Expose the left adrenal vessles and adrenal gland.M: Preserve the adrenal gland if it hasn’t been invaded. Dissect the retroperitoneum adipose tissue to the origin.N: Ligate and dissect the spleen vessles at the tail of the pancreas.O: Inverting suture the stump of the pancreas.P: Leave a drainage tube in the surgical site;LDP:laparoscopic distal panceatectomy;LGEV:left gastro-epiploic vein;SMA:superior mesentery artery;IMV:inferior mesenteric vein.
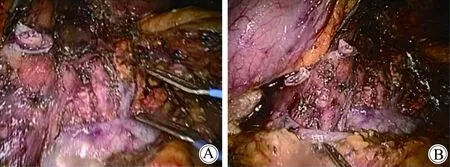
图2 取出标本后的腹膜后组织结构Fig.2 The posterior peritoneal structure afterdislodge the specimen
A: The stump of spleen artery, left renal vein, left adrenal vessels, and superior mesentery artery were shown in the picture after surgery.B: The stump of spleen vein was shown in the picture.
2 结果
2.1病人的一般特征
在23例胰腺癌病人中,12例为男性,11例为女性。平均年龄(65±11.4)岁。平均体质量指数(body mass index, BMI)为(25.9±4.4)kg/m2。美国麻醉医师协会(American Society of Anesthesiologists, ASA)评分均值:2.2。无病人行新辅助化疗。
2.2短期预后评估
病人手术情况详见表3。17例病人行保脾的LDP手术,6例病人行腹腔镜切脾的LDP手术。总体平均手术时间(203 ± 54) min。平均出血量(208 ± 106)mL。1例病例由于腹腔内严重粘连而中转开腹。在手术时间上,切脾的LDP与保脾的LDP差异无统计学意义[(198±59) minvs(223 ± 29) min,P=0.45]。出血量差异亦无统计学意义[(184 ± 65)mLvs(275 ± 109)mL,P=0.49)]。
腹腔镜胰体尾切除术总体的合并症率为47.8%(n=11),胰瘘的发生率为39.1%(n=9)。保脾(n=8)与切脾(n=3)两组之间合并症的发生率差异无统计学意义(48%vs50%,P=0.90)。所有的病人均在30 d内出院。4名病人发生了Ⅲa级别的合并症。2例病人接受了超声引导下腹腔积液穿刺引流。2例病人接受了经内镜逆行性胰胆管造影术(endoscopic retrograde cholangiopancreatography, ERCP)检查以确定胰瘘。3例病人诊断为脾梗死。12例病人经增强CT检查脾血运正常。2例病人术后未行增强CT检查。中转开腹病人为腹腔镜胰体尾脾整块切除组病人。因术中粘连较重,中转开腹,术后病理为Ⅲ期(T3N1M0)。
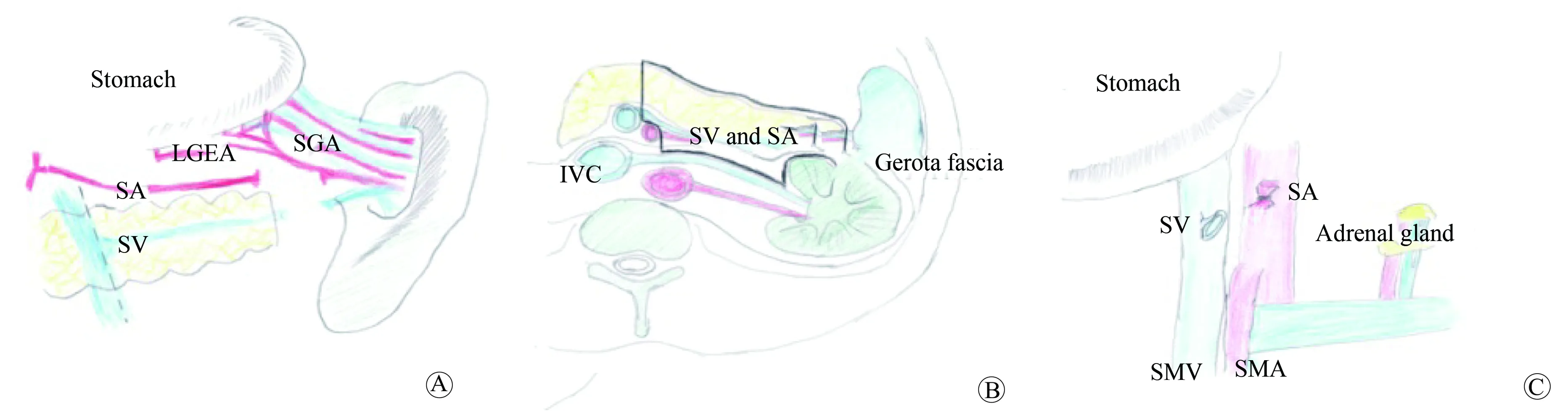
图3 整块切除手术技术的示意图Fig.3 The diagram of en-bloc resection range
A: After dissecting the spleen artery and vein, the blood flow of spleen compensated by left gastro-epiploic vessels and short gastric vessels.B: Gerota fascia, spleen artery and vein and the distal pancreas were removed with en-bloc technique.C: The posterior peritoneal structures were shown after the specimen removed.SA: spleen artery;SV: spleen vein;LGEA: left gastro-epiploic artery;SGA: short gastric artery;IVC: inferior vena cava;SMA: superior mesentery artery;SMV: superior mesentery artery.
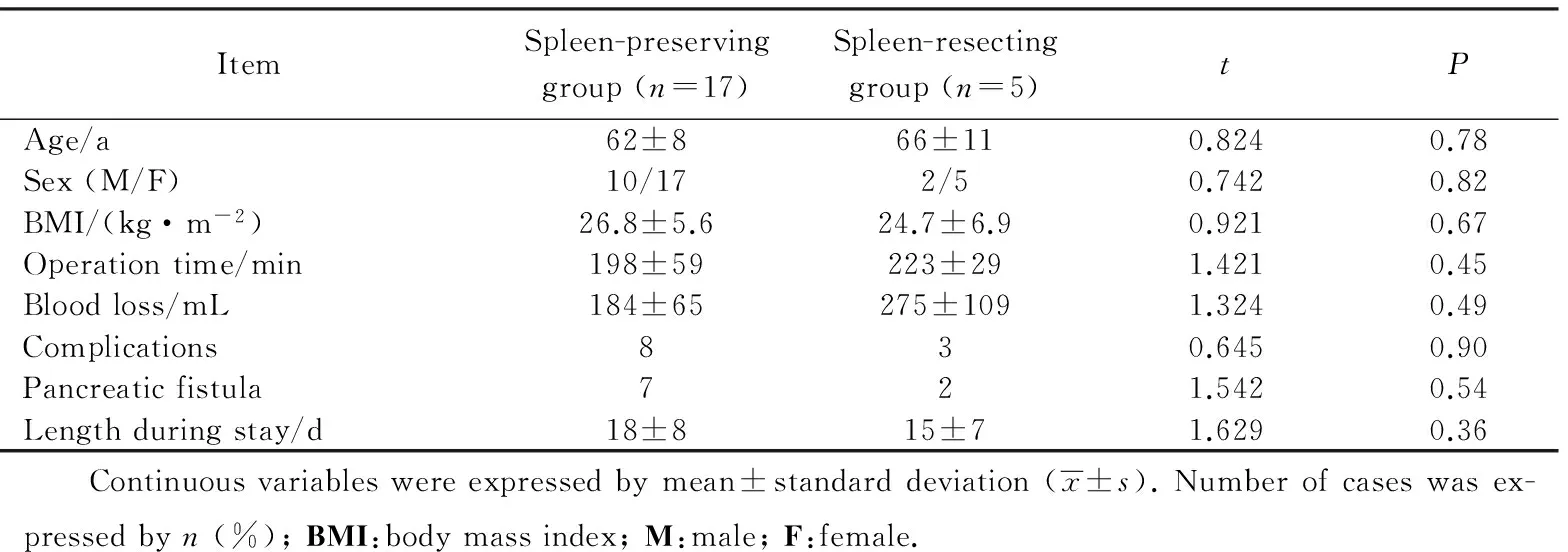
表3 保脾与切脾手术组的病人特征比较 Tab.3 Patient character of the spleen preserving and resecting group
所有脾梗死的病人均接受了保守观察,并获得了自行治愈。病人的平均住院时间(17±8) d。在住院时间上,保脾与切脾组差异无统计学意义[(18±8)dvs(15±7)d,P=0.36]。肿瘤的平均直径为(32±12)mm。平均的淋巴结获得数量为(19.8±9.3)个。14例病人的淋巴结转移阳性个数≥1 个。病理结果为18例导管腺癌,4例导管内乳头状癌,1例黏液腺癌。所有的手术切缘均为阴性。病理分期为:2例 IA期,5例 IB期,2例 ⅡA期,13例 ⅡB期, 1例 Ⅲ期(表4)。从保脾组和切脾组病理结果分层对比来看。两组无论大小、病理类型、切缘、分期情况,差异均无统计学意义。
2.3长期预后评估
在5年的观察期内,平均的生存时间是19个月。1、3、5年的生存率分别是64.7%、52.9%和41.2%。6例病人出现了术后复发。1例病人同时出现原位复发和肝转移。2例病人为腹膜后复发。平均的复发时间是14个月。1、3、5年的无复发生存率分别是58.8%、47.1%、35.3%(图4)。
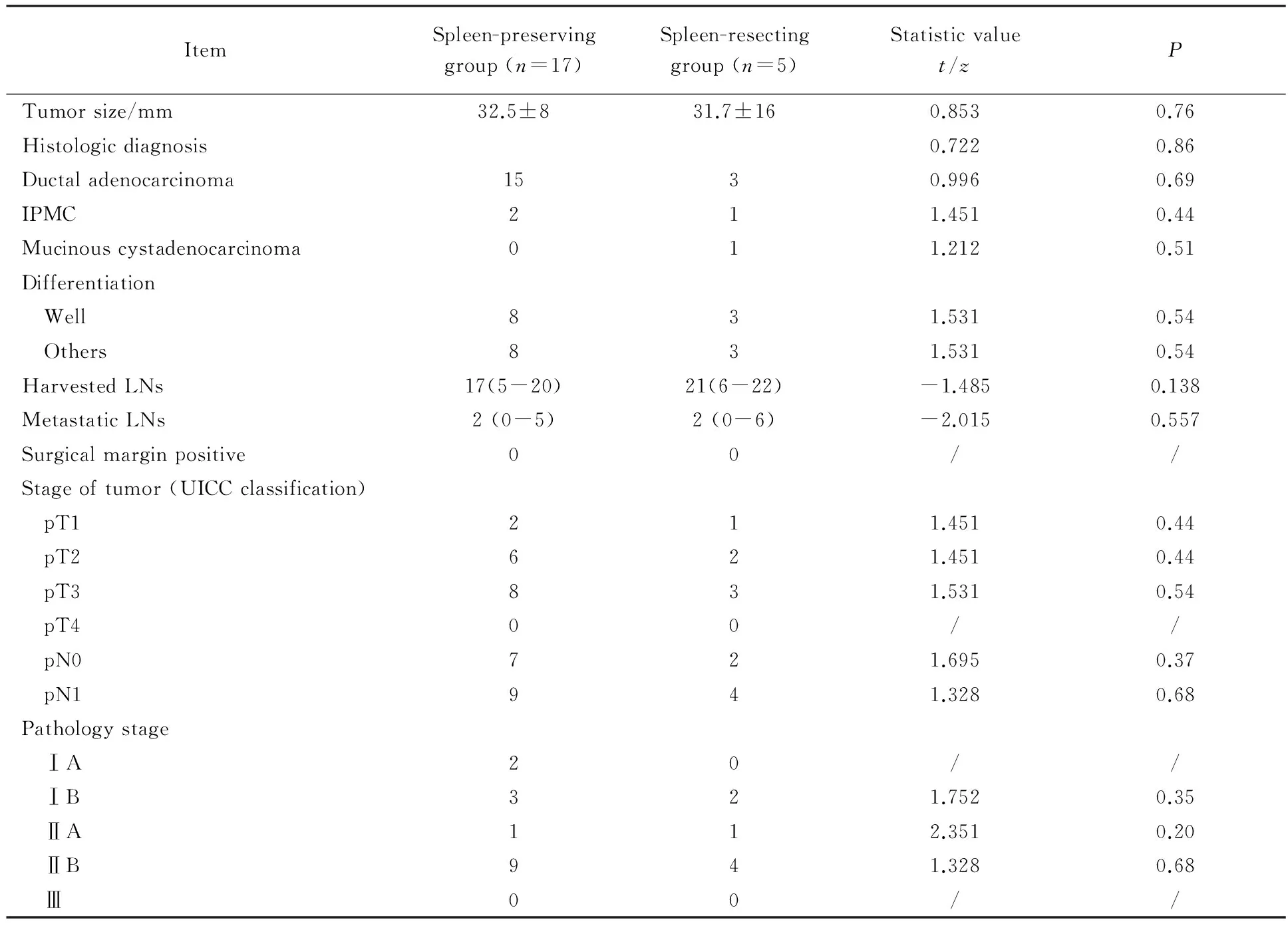
表4 保脾与切脾手术组的病理分期结果 (国际抗癌联盟第六版,2006年)[16]Tab.4 Pathology outcomes (Union for International Cancer Control Classification, 6th edition)[16]
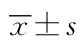
3 讨论
在最近的研究报道[14-15,17]中显示,关于腹腔镜胰体尾整块切除手术治疗胰腺癌的长期预后是比较满意的(5年的总生存率约为33%)。笔者的临床数据来源于单中心。腹腔镜胰体尾整块切除概念,依赖于胰腺肿瘤的R0切除,N2淋巴结清扫及左半周神经丛清扫。以RAMPS手术为整块切除范围,结合日本胰腺癌诊疗指南的淋巴结及神经清扫范围。
笔者的研究与最近的研究[4,9-12]表明(表5):腹腔镜胰体尾切除淋巴结获取的数量与开腹胰体尾切除手术相似(19.8vs15.5个)。笔者最满意的是100%的R0切除率。切除的肿瘤直径也与开腹胰体尾脾切除术(open distal pancreatectomy, ODP)相似。腹腔镜手术与开腹手术相比,出血量明显减少(208 mLvs747 mL)。其主要原因是腹腔镜的放大作用与气腹作用下腹压的增大。
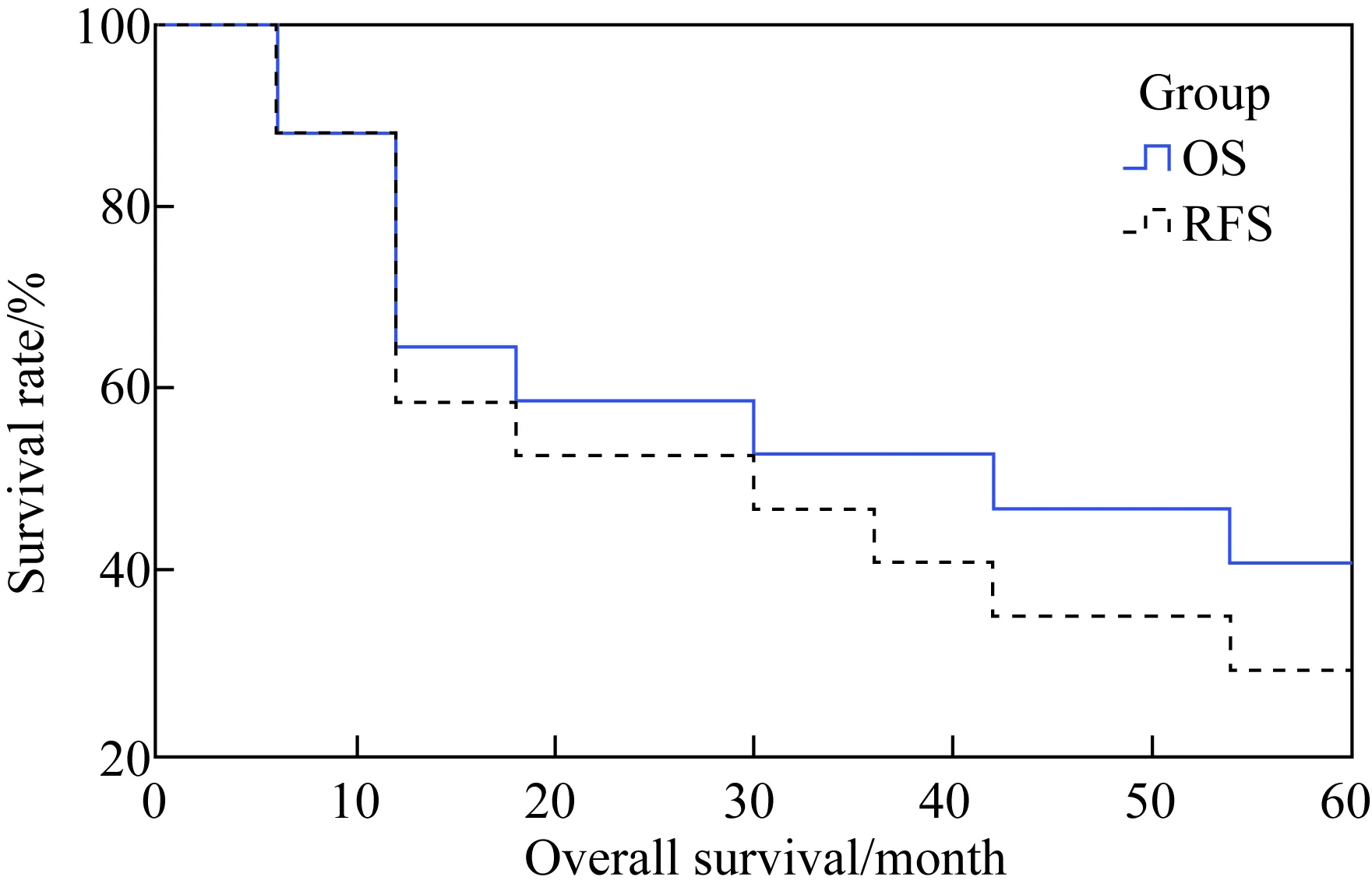
图4 17例腹腔镜胰体尾整块切除手术的生存曲线Fig.4 The overall survival (OS) and recurrencefree survival (RFS) curve
The observation period was 5 years after surgery.The survival rate of 1, 3, 5 years after surgery was 64.7%, 52.9% and 41.2% respectively. The recurrence free survival rate of 1, 3, 5 years after surgery was 58.8%, 47.1% and 35.3% respectively.
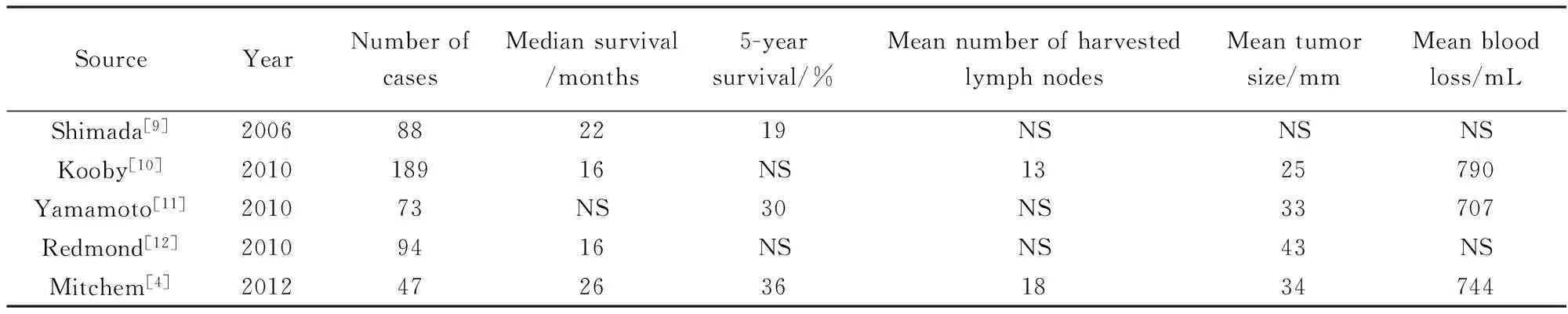
表5 既往研究报告中所报道的开腹胰体尾切除手术预后Tab.5 The prognosis reported by previous studies about open distal pancreatectomy
NS:not stated.
腹腔镜胰体尾整块切除技术强调整块切除Gerota筋膜、Toldt筋膜、脾血管及胰体尾,来确保阴性切缘。相对而言,标准的ODP并不需要常规切除Gerota筋膜和Toldt筋膜。对于是否要切除脾脏是一个有争议的话题。一些研究[18]表明这种手术发生脾梗死的概率为11%到29%。直到目前仍未有任何指南或专家共识指出行胰体尾切除手术必须要切除脾脏。
胰体尾癌有时会伴有淋巴结转移,胰头神经丛、腹腔干神经丛、肠系膜上动脉神经丛侵犯[19-20]。但是全部切除神经会有严重的腹泻和呕吐。因此笔者保留上述神经丛神经的右半周,切除左半周。这也符合日本胰腺癌诊疗指南的要求,与部分学者的临床实践[21]相同。
在本研究中,腹腔镜胰体尾整块切除的预后与之前的报道[1-2,10,15]相似,在这些报道中病人平均的生存时间13到26个月不等。5年生存率19%~36%不等。截至目前,依据笔者所检索到的文献。这是第一份关于腹腔镜胰体尾整块切除手术长期预后的报告。本研究的局限性在于:首先这是一项回顾性病例对照研究。并且标本的病例类型也只有3种,对照组仅有6例病例。前瞻性随机对照实验和大样本量的研究,可以避免相关统计偏移,长期的肿瘤学预后还有待于进一步评估。但由于胰体尾肿瘤的发病率较低,这样的实验也很难于开展。
总之,腹腔镜保脾胰体尾切除手术是一项安全、有效的手术技术。近期和远期预后均可以接受,可以作为胰体尾肿瘤的治疗方案。
[1] Merkow J, Paniccia A, Edil B H. Laparoscopic pancreaticoduodenectomy: a descriptive and comparative review[J]. Chin J Cancer Res, 2015, 27(4): 368-375.
[2] Postlewait L M, Kooby D A. Laparoscopic distal pancreatectomy for adenocarcinoma: safe and reasonable?[J]. J Gastrointest Oncol, 2015, 6(4): 406-417.
[3] Strasberg S M, Drebin J A, Linehan D. Radical antegrade modular pancreatosplenectomy[J]. Surgery, 2003, 133(5): 521-527.
[4] Mitchem J B, Hamilton N, Gao F, et al. Long-term results of resection of adenocarcinoma of the body and tail of the pancreas using radical antegrade modular pancreatosplenectomy procedure[J]. J Am Coll Surg, 2012, 214(1): 46-52.
[5] 杨尹默. 胰十二指肠切除术中淋巴结清扫范围争议与共识[J]. 中国实用外科杂志, 2016, 36(8): 843-846.
[6] 杨尹默. AJCC第八版及日本胰腺学会第七版胰腺癌TNM分期的更新要点及内容评介[J]. 中华外科杂志, 2017, 55(1): 20-23.
[7] Kishi Y, Shimada K, Hata S, et al. Definition of T3/4 and regional lymph nodes in gallbladder cancer: which is more valid, the UICC or the Japanese staging system?[J]. Ann Surg Oncol, 2012, 19(11): 3567-3573.
[8] Kondo S. Japanese Pancreas Society Staging Systems for Pancreatic Cancer[M]. New York: Springer, 2003.
[9] Shimada K, Sakamoto Y, Sano T, et al. Prognostic factors after distal pancreatectomy with extended lymphadenectomy for invasive pancreatic adenocarcinoma of the body and tail[J]. Surgery, 2006, 139(3): 288-295.
[10] Kooby D A, Gillespie T, Bentrem D, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches[J]. Ann Surg, 2008, 248(3): 438-446.
[11] Yamamoto J, Saiura A, Koga R, et al. Improved survival of left-sided pancreas cancer after surgery[J]. Jpn J Clin Oncol, 2010, 40(6): 530-536.
[12] Redmond K J, Wolfgang C L, Sugar E A, et al. Adjuvant chemoradiation therapy for adenocarcinoma of the distal pancreas[J]. Ann Surg Oncol, 2010, 17(12): 3112-3119.
[13] Sugiura T, Okamura Y, Ito T, et al. Surgical Indications of distal pancreatectomy with celiac axis resection for pancreatic body/tail cancer[J]. World J Surg, 2017, 41(1): 258-266.
[14] 施思, 项金峰, 徐近, 等. 2016版国际胰腺外科研究组术后胰瘘定义和分级系统更新内容介绍和解析[J]. 中国实用外科杂志, 2017, 37(2): 149-152.
[15] De Rooij T, Sitarz R, Busch O R, et al. Technical aspects of laparoscopic distal pancreatectomy for benign and malignant disease: review of the literature[J]. Gastroenterol Res Pract, 2015, 2015: 472906.
[16] Edge S B. AJCC Cancer Staging Manual, 6th edn[M]. New York: Springer, 2005.
[17] Okada K, Kawai M, Tani M, et al. Surgical strategy for patients with pancreatic body/tail carcinoma: who should undergo distal pancreatectomy with en-bloc celiac axis resection?[J]. Surgery, 2013, 153(3): 365-372.
[18] Kim S H, Kang C M, Satoi S, et al. Proposal for splenectomy-omitting radical distal pancreatectomy in well-selected left-sided pancreatic cancer: multicenter survey study[J]. J Hepatobiliary Pancreat Sci, 2013, 20(3): 375-381.
[19] 陈汝福, 周泉波. 神经周围浸润在胰腺癌病人预后中的意义[J]. 中华肝脏外科手术学电子杂志, 2014, (2): 4-6.
[20] 迟学成. 腹部巨大肿瘤临床特点与治疗分析[J]. 中华肿瘤防治杂志,2015,22 (10):806-808.
[21] Kawabata A, Hamanaka Y, Suzuki T. Potentiality of dissection of the lymph nodes with preservation of the nerve plexus around the superior mesenteric artery[J]. Hepatogastroenterology, 1998, 45(19): 236-241.
Detailoftheen-bloctechniqueandprognosisofspleen-preservinglaparoscopicdistalpancreatectomyforpancreaticcancer
Sun Zhipeng△, Zhu Yubing△, Aminbuhe, Fan Qing, Li Tianxiong, Zhang Nengwei*
(DepartmentofOncologySurgery,BeijingShijitanHospital,CapitalMedicalUniversity,PekingUniversityNinthSchoolofClinicalMedicine,Beijing100038,China)
ObjectiveThe aim of our study was to illustrate the detail of the spleen-preserving en-bloc technique as while as the short-term, long-term outcomes.MethodsDescribe the detail of the en-bloc technique with pictures. Evaluate the prognosis of successive 23 cases who underwent the laparoscopic distal pancreatectomy (LDP) surgery. There were 17 cases that underwent spleen-preserving LDP while 6 cases underwent spleen-resecting LDP.ResultsThe average surgery time was (203 ± 54)minutes, the average blood loss volume was (208 ± 106)mL. One case was transferred to open surgery because of severe adhesion. The complication rate was 47% (n=8) in short-term after surgery. Pancreatic fistula rate was 41% (n=7). No lethal case occurred. The average diameter of the tumor was (32 ± 12)mm. Average number of the lymph nodes obtained was (19.8 ± 9.3). All the cutting edges were negative. Survival rate of the patient after 1, 3, 5 years were 64.7%, 52.9% and 41.2%. These records showed no statistical significance compared with spleen-resecting LDP and open distal pancreatectomy (ODP) surgeries.ConclusionThe en-bloc spleen-preserving LDP can be performed by experienced surgeons. This surgery has good short-term and long-term outcome.
spleen-preserving laparoscopic distal pancreatectomy; pancreatic cancer; en-bloc technique; radical antegrade modular pancreaticosplenectomy
北京市医院管理局扬帆重点课题基金资助(ZYLX201512)。This study was supported by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support(ZYLX201512).
*Corresponding author, E-mail:zhangnw1@sohu.com
时间:2017-10-14 16∶29
http://kns.cnki.net/kcms/detail/11.3662.R.20171014.1629.054.html
10.3969/j.issn.1006-7795.2017.05.019]
R735.9
2017-02-28)
编辑 陈瑞芳
△共同第一作者
