污染水体中河蚬的生物毒性响应研究进展
2017-10-13郭晓宇李茹枫冯成洪韩志华
郭晓宇,李茹枫,冯成洪,,*,韩志华
1. 北京师范大学环境学院水环境模拟国家重点实验室,北京 1008752. 北京师范大学环境学院水沙科学教育部重点实验室,北京 1008753. 环境保护部南京环境科学研究所,南京 210042
污染水体中河蚬的生物毒性响应研究进展
郭晓宇1,李茹枫2,冯成洪1,2,*,韩志华3
1. 北京师范大学环境学院水环境模拟国家重点实验室,北京 1008752. 北京师范大学环境学院水沙科学教育部重点实验室,北京 1008753. 环境保护部南京环境科学研究所,南京 210042
河蚬作为广泛分布于世界各国的典型底栖生物,由于其活动性低、滤食性等特征被广泛用作指示生物研究多种水体污染物的生物有效性。但迄今为止,尚没有系统论述污染水体中河蚬生物毒性响应的研究进展。为此,本文从污染物种类、测试指标、试验参数等角度探讨了过去30多年间河蚬在氨、重金属、有机污染物生物富集及生物毒性效应等方面的研究过程及主要成果。以往研究主要以河蚬生物体内累积、形态学及行为学观察、生化指标、代谢组学、基因完整性等指标表征污染水体的生物毒性效应,并随着分子生物学的发展已逐步由多指标全面表征代替单一指标测试。此外,现有研究多偏重于重金属和持久性有机污染物,对氨、新型污染物及纳米材料的河蚬生物毒性效应探讨尚处于起步阶段。河蚬在自然水体污染状况评估、污染水体的生物修复、水体毒性预测等方面具有较高适用性,但河蚬在沉积物毒性鉴定评估(TIE, Toxicity Identification and Evaluation)中的应用研究依然较为缺乏,有待进一步开展。
水体污染物;氨;重金属;有机物;河蚬;生物毒性响应;生物累积
Received12 October 2016accepted16 December 2016
Abstract: As a typical benthonic species, Corbicula fluminea is widely used for biomonitoring freshwater pollution due to its sessile and filter-feeding characteristics. However, there is few reports reviewing the progress about biological toxic response and bioaccumulation for contaminants of C. fluminea in aquatic environment. Therefore, this study summarized the main achievements about the effects of pollutants (ammonia, trace metals, organic contaminants) on freshwater invertebrates C. fluminea in terms of pollutant species, test index, test medium and test duration. The results indicated that the common biomonitoring techniques applied in C. fluminea study could be classified as bioaccumulation, morphology, behavior observation, biochemical index alterations, metabonomics measure and genetic integrity changes. With the development of molecular biology, the use of single biomarker is gradually replaced by multi-biomarker approach. Furthermore, existing researches mainly focus on heavy metals and persistent organic pollutants, whereas studies on ammonia, emerging contaminants and nanomaterials are still in the initial stage. Overall, C. fluminea is a useful species for evaluating aquatic pollution, bioremediation, toxicity prediction. Studies on the application of C. fluminea in the area of sediment Toxicity Identification and Evaluation (TIE), by contrast, are still scarce and need extensive investigations.
Keywords: water pollutants; ammonia; heavy metal; organic pollutants; Corbicula fluminea; biological toxicity response; bioaccumulation
水体沉积物中具有明显生物和生态效应的污染物种类繁多,近年来,污染物的生物毒性响应特征、过程与机制逐渐成为国内外研究的热点。生物毒性测试由于可反映污染物的生物可利用性,是评估水体综合污染的有效手段之一,是化学分析和底栖生物群落结构评价方法的有益补充[1]。双壳贝类作为一类世界性分布的沿岸底栖水生动物,在污染物的生物富集和传递过程中起着重要作用,可通过食用影响人体健康,已成为环境毒理学研究关注的对象[20]。
河蚬作为一种典型淡水底栖双壳贝类,具有活动性低、易于培养、经济实用、分布广泛等特征,对污染物具有较强的富集性,广泛应用于水体尤其是沉积物毒性效应评价和鉴定研究[2]。河蚬原产于亚洲、非洲以及澳大利亚[3],由于其入侵性,目前亦广泛分布于北美、南美以及欧洲等地[4-5]。美国[11,34,42,53-60,88,93,95-101]、中国[18-19,29,40,45-52,61-62,92]、法国[2,6,13-16,28,33,35,37-38,43-44,63-77,86,107-108]、德国[30,32]、英国[12]、葡萄牙[7-10,21,39,41,94,102]、阿根廷[79-80,90,103-104]、巴西[17,36,81,85,105]、西班牙[83,87]、塞尔维亚[91]、马来西亚[82]、伊拉克[78]、菲律宾[106]等国家均开展了河蚬在污染物生物毒性响应的研究。
河蚬对污染物生物毒性响应研究主要包括生物富集、生化指标改变、形态学、行为学观察、野外种群及群落水平分布调查等[1]。也有研究将其作为哨兵生物(sentinel organism),应用于贻贝污染物监测项目(NCCOS)等常规生物监测程序探讨[60]。整体上,河蚬作为水体尤其是沉积物污染受试生物的指示性应用研究尚处于发展阶段。近几年,相关研究逐渐涉及到组织学、组织化学、组织病理学[7-11],代谢组学[12]和基因表达[13-19]等分子生物学方面。各种生物效应、毒理学指标也随着分子生物学及相关分析检测仪器技术的发展而快速更新。
为系统认识河蚬对污染物的生物毒性响应特征,本文基于1983—2016年国外文献中有关河蚬研究成果,结合前期已发表研究成果[20],从水体污染物类型、研究方法、指标体系等方面综合归纳整理河蚬的污染物生物富集及生物毒性效应研究现状及发展历程。结果可为河蚬对水体及沉积物中污染物的生物富集、生物毒性鉴定研究提供参考。
研究过程中,根据污染物种类、试验参数、测试指标进行分类,具体技术路线见图-1。
1 氨污染水体中河蚬的生物毒性响应研究(The biological toxicity response of Corbicula fluminea in ammonia contaminated water)
研究表明,氨对不同水生无脊椎动物具有不同程度的毒性,但是氨毒性可能是致命的[21]。有学者对多种淡水贝类进行氨毒性实验,得出不同贝类的96 h半致死浓度(11.53~23.1 mg·L-1)[22-24]。Cooper等[25-27]曾研究人工模拟河流中河蚬大面积死亡时软体组织腐败所释放氨对本土物种淡水贻贝的影响。但是氨对河蚬本身的急慢性毒性效应却报道较少。据作者检索,国外报道中仅发现一篇文献,论述长期慢性氨污染对河蚬的多种生物标志物产生的亚致死影响研究(表1)[21]。研究发现,采自污染河口区的河蚬由于长期处于污染环境中,其各项酶活性指标均表现出较高背景值,因此受1 mg·L-1含氨水污染后,各项指标并未表现出明显差异。这说明长期处于污染水域会增强河蚬对氨的耐受性,有利于其入侵行为。由于水生生物的内源排放及人类工农业快速发展所引起的外源污染,氨氮已成为水环境中最普遍的污染物之一,但是目前水体中氨污染引起的河蚬毒性响应研究甚少,此类研究有待进一步加强。
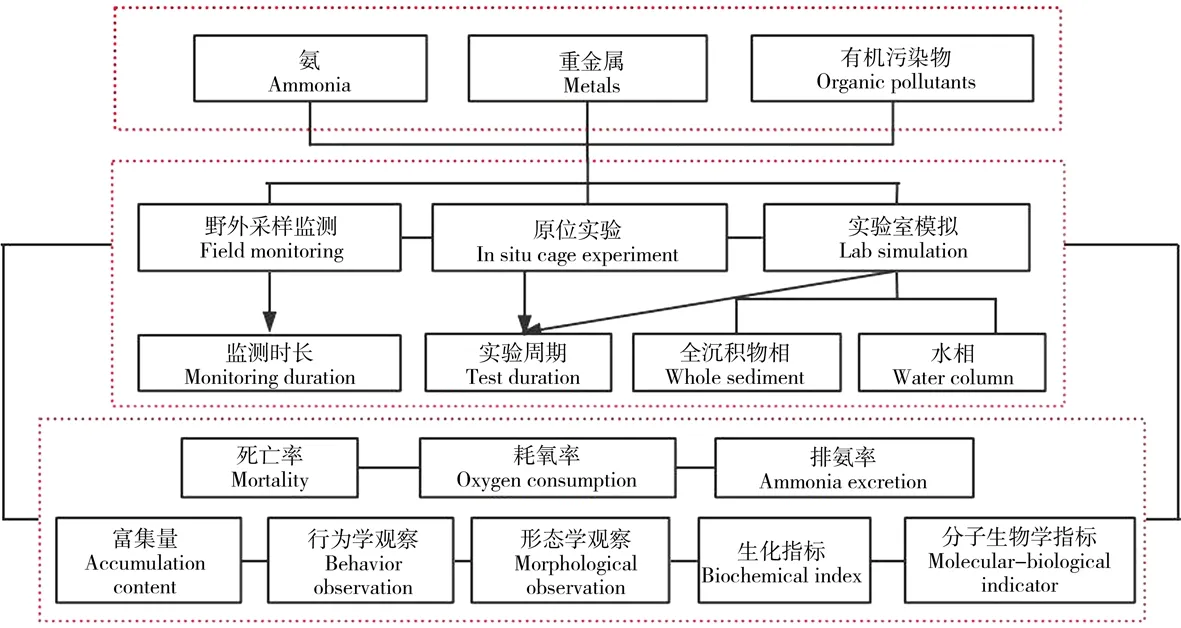
图1 研究过程技术路线Fig. 1 The technology roadmap of research
2 重金属污染水体中河蚬的生物毒性响应研究(The biological toxicity response of Corbicula fluminea in metal contaminated water)
表2汇总了1983—2016年间的65篇在国外期刊发表的有关河蚬对重金属的生物毒性响应研究进展。从表2可以看出,已有大量研究探讨了重金属污染水体中河蚬的生物毒性响应。在研究所属国家分类上,法国有相对较全面的研究。波尔多大学研究团队从1997年到2014年间发表27篇文献。美国、中国、德国、英国、阿根廷、巴西、马来西亚、伊拉克、西班牙等在河蚬对污染物生物毒性响应方面也均有研究。研究过程经历了从早期的河蚬不同软体组织对重金属的富集量、行为学特征(闭壳响应、呼吸活性)[34,37,44,53-54,58,63,66-71,78-80],到后期的生物标志物多指标综合研究,如:生化指标中丙二醛(MDA)、金属硫蛋白(MT)、谷胱甘肽(GSH)含量测定[7-10],抗氧化系统中过氧化氢酶(CAT)、超氧化物歧化酶(SOD)等酶活性的测定[9-10,43,61],蛋白定量验证中MXR蛋白、热休克蛋白Hsp60等的测定[36,72-74],再到分子标记物中基因表达水平的测定研究[13-16]。也有很多研究,探讨了不同非生物因子如pH、温度、溶解氧(DO)、CO2含量等因素影响下不同重金属对河蚬的生物毒性效应[37,43,66-68,70-71]。除以上指标外,还对污染物影响下河蚬消化腺病变、消化腺细胞内污染物颗粒及溶酶体系统变化和中性脂质含量等组织学、组织化学及组织病理学进行研究[7-10,15]。由于双壳类消化腺在调整和消除外源有害物质中起到关键作用,且消化道管壁上皮细胞对许多金属及准金属污染物的损害影响较敏感[9],因此以往研究常对该器官进行组织学分析。英国学者Nicole等[12]还对河蚬代谢组学进行分析,探究Cd和Zn对不同大小河蚬代谢物的影响。整体而言,除法国、葡萄牙、巴西学者涉及到一些组织学观察、生化指标响应、蛋白定量验证、及基因表达方面的研究,其他国家研究多处于基础阶段,多采用河蚬体内污染物富集量、死亡率、掘穴行为等常规指标进行表征。我国学者对重金属污染水体中河蚬的生物毒性响应开展了大量研究[20,29,40,61-62,109-110],对河蚬的金属富集、行为学观察、生化指标改变3个方面进行了污染物生物毒性响应研究,但是对于重金属污染,还未展开组织学、代谢组学、基因水平的研究。
在重金属种类上,常见重金属Cd、Hg、Cu、Zn、Se、U、Ni、Pb等对河蚬的生物毒性效应均有研究,而Cd作为对淡水生物群落毒性最强[28]的污染物,研究文献最多。在79篇文献中有46篇将其列为研究对象(表2)。还有少量研究探讨金属放射性同位素[28-29]、稀土元素[30]的生物毒性。对于近年来新的环境挑战,也有学者对纳米材料尤其是纳米金属进行双壳类生物毒性实验。葡萄牙学者对纳米金刚石及纳米TiO2的河蚬生物毒性研究[9-10]表明,纳米材料影响河蚬的氧化应激系统且引起了消化腺上皮细胞的组织学变化。整体上纳米材料对河蚬生物毒性研究较少。随着纳米材料应用的增多,此类研究有必要继续完善,为环境风险评估提供可靠信息[31]。
在基质选取上,现有研究主要以现场监测和室内模拟模式开展水相(含沉积物孔隙水、沉积物上覆水)、全沉积物相实验研究(表2)。其中,水相研究中包括天然水体的原位水相实验和实验室配水的室内模拟实验。相对而言,水相实验易于操作控制,现有研究多采用水相加标进行模拟实验研究,针对全沉积物的毒性效应研究较少(约占6%)。然而,河蚬作为底栖生物直接接触沉积物,可通过鳃摄取沉积物间隙水中游离态污染物,也可通过摄食途径取食富含污染物的颗粒物而累积污染物。因此,水相实验难以全面、直接反映沉积物的污染和生物毒性。在生物毒性效应研究中,实验基质的选取还需以全沉积物为主要基质。
从实验周期角度来分析,根据研究类别的不同,野外监测及原位试验研究通常周期较长。野外监测考虑河蚬富集的时空分布,通常进行不同季节多次采样[32-34];原位试验[35]由于污染物浓度的不可控性而需进行长期富集实验。由于河蚬对重金属具有较强的富集能力,除少数研究对河蚬进行体外急性(96 h)毒性实验外[36],重金属对河蚬的生物毒性及河蚬对重金属的解毒机制[14, 37-38]研究多采用慢性(1个月~1年)毒性实验。
整体上,河蚬在水体重金属污染中的应用主要集中在4个方面:(1)通过野外监测评估实际水生环境中重金属污染情况;(2)研究河蚬对富含重金属水体的生物修复能力,探讨重金属污染区域的生物修复技术;(3)重金属对河蚬健康影响的毒理学机理研究;(4)毒性预测——依据河蚬生物监测得出的毒理学数据建立生物模型来评估及预测污染物潜在生态风险。
在野外监测上,大量学者通过野外监测分析河蚬体内重金属含量与环境介质(如水体、沉积物)中重金属含量关系,发现痕量重金属在河蚬体内富集量比周围环境介质中含量高几个数量级[34, 39]。虽然河蚬体内污染物含量不能反映环境水体的真实污染水平,但是河蚬对重金属的累积特征与环境中重金属量分布具有很好的相关性[13, 32-33, 40]。迄今为止,体内污染物含量监测是河蚬在天然水体环境污染研究中唯一的实际应用。
在生物修复应用上,葡萄牙学者采用室内模拟实验研究河蚬对酸性矿排水的生物修复效果,发现河蚬对水体中重金属有较强的去除率,证明了河蚬用于生物修复的适用性[41]。但目前仍未发现有研究采用河蚬对实际重金属污染区域进行生物修复,而室内模拟研究仅此一篇文献报道,相关研究有待继续开展。
在河蚬的生物毒理学研究上,河蚬在重金属加标介质中的生化响应[7, 10, 36, 42-43]、基因损伤[13-15]、解毒机制[14, 38, 44]等都有助于研究污染物对河蚬的潜在毒性影响。因为双壳类底栖生物在污染物的影响下早期的指标响应表现在生物标志物层面。毒性响应首先会在亚细胞水平表现出明显响应,随后才会在更高生物水平显现。与软体组织内污染物富集量测定相比,生物标志物能够提供更加完整的、生物学上更可靠的信息[31]。然而,受生物和非生物因素影响,生物标志物亦无法绝对准确地表征污染物生物毒性。此外,由于单生物标志物无法充分反映生物体的健康损害程度,大量学者采用多标志物[7-10, 43]监测河蚬对污染物的生物毒性响应。同时,生物标志物与一般急性毒性实验评价指标——死亡率相比,更加困难、昂贵且耗时。综上所述,河蚬毒理学机制研究仍亟需经济、快捷、准确的测试指标,评估污染物对生物的健康损伤。
在生物毒性预测方面,我国台湾学者进行了大量的探索[45-52]。研究表明,闭壳响应可作为一项生物监测指标研究金属污染水体对双壳贝类的健康影响。闭壳响应模型技术可为将来生态预警系统的建立提供一个风险管理框架。
3 有机物污染水体中河蚬的生物毒性响应研究(The biological toxicity response of Corbicula fluminea in organic contaminated water)
天然水体受人类活动影响,接纳了大量外源性有机污染物,如有机农药、多氯联苯、多环芳烃等持久性有机污染物,烷基酚类,内分泌干扰物、抗生素等多种药物。河蚬在欧美等国被广泛用作指示生物研究多种有机污染物的生物有效性。表3综述了从1986到2015年间的31篇在国外期刊发表的有关河蚬对有机污染物的生物毒性响应报道。
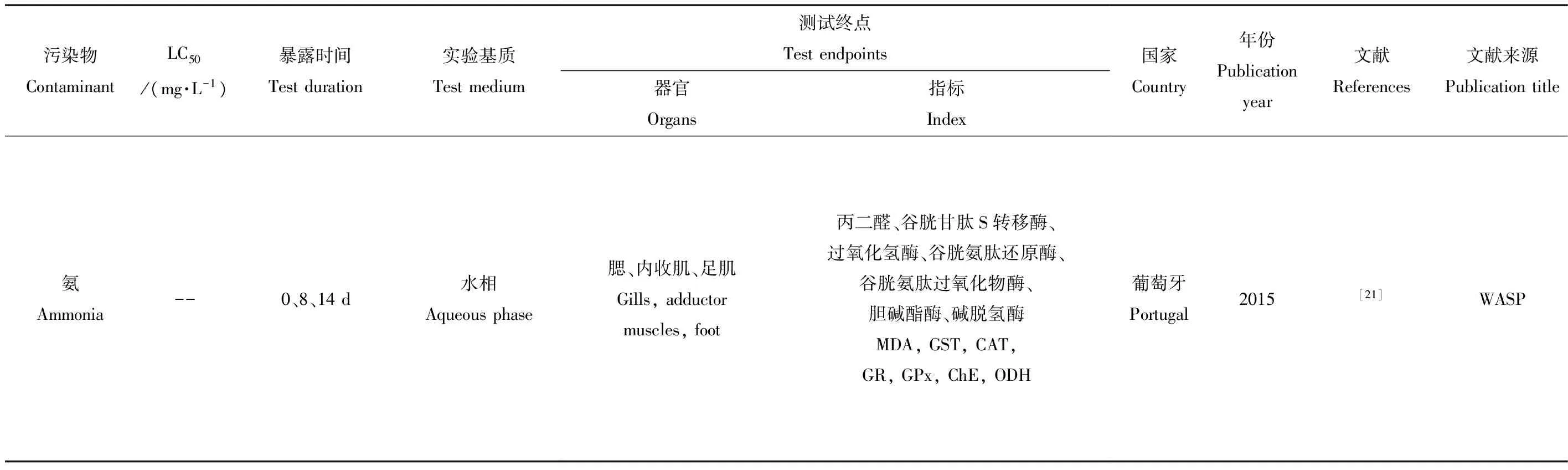
表1 河蚬在氨生物毒性效应中的应用Table 1 Corbicula fluminea in ammonia biological toxicity test
期刊名注释(Journal Title Abbreviations): WASP-Water, Air, & Soil Pollution.
测试指标名称注释(Index Abbreviations): MDA-malondialdehyde, CAT-catalase, GPx-glutathione peroxidase, GST-glutathione S-transferase, GR-glutathione reductase, ChE-cholinesterase, ODH-octopine dehydrogenase.
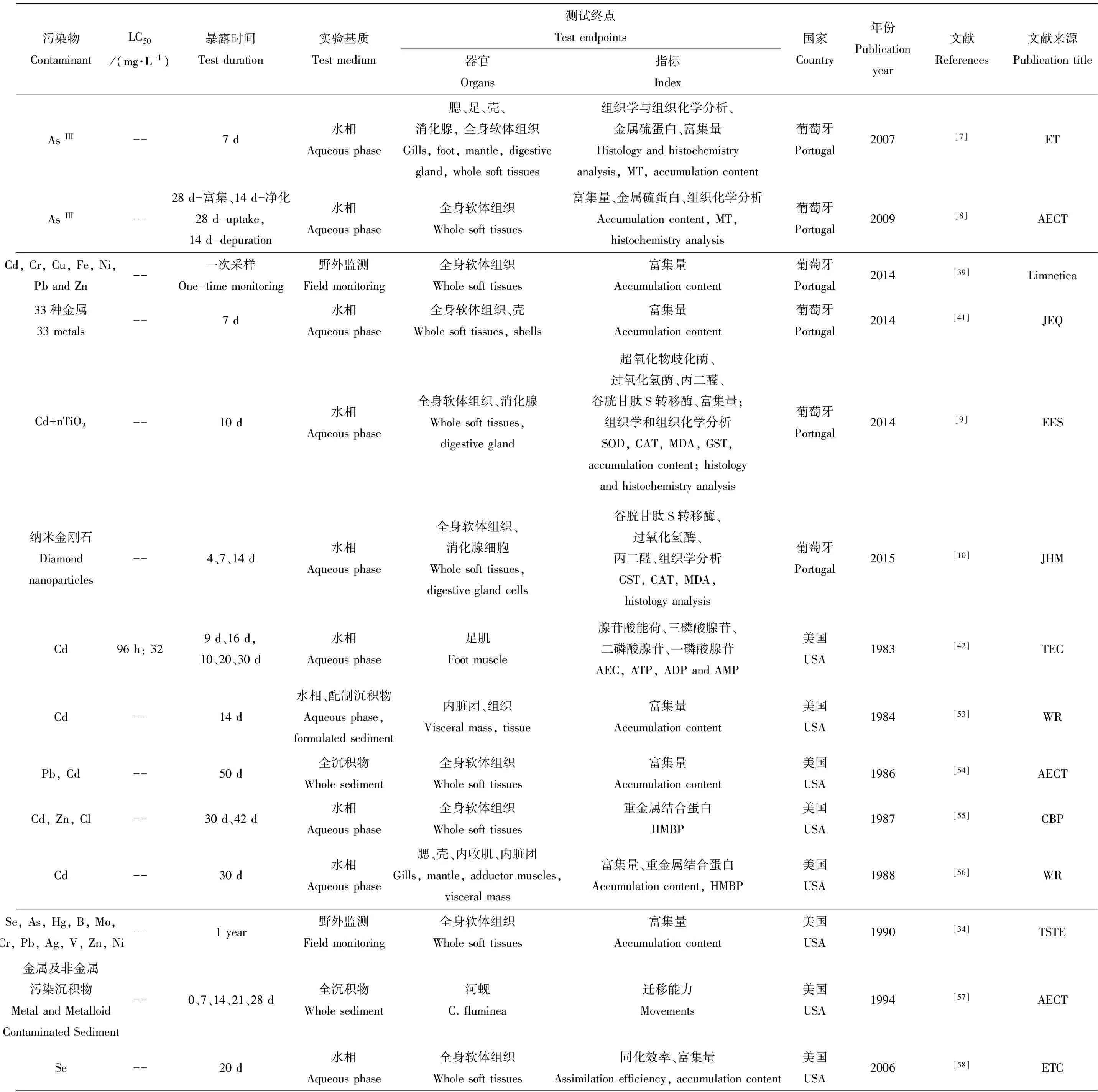
表2 河蚬在金属污染物生物监测中的应用Table 2 Corbicula fluminea in metal contamination biomonitoring
期刊名注释(Journal Title Abbreviations): WASP-Water, Air, & Soil Pollution, STE-Science of the Total Environment, JEQ-Journal of Environmental Quality, EES-Ecotoxicology and Environmental Safety, TEC-Toxicological and Environmental Chemistry, WR-Water Research, CBP-Comparative Biochemistry & Physiology Part C Comparative Pharmacology, TSTE-The Science of the Total Environment, ET-Environmental Toxicology, EMA-Environmental Monitoring and Assessment, ESPI- Environmental Science: Processes & Impacts, ESPR-Environmental Science and Pollution Research, TE-Toxicology and Endocrinology, BECT- Bulletin of Environmental Contamination and Toxicology, JER-Journal of Environmental Radioactivity, JEM-Journal of Environmental Monitoring, WST-Water Science & Technology, BTER- Biological Trace Element Research, JHM-Journal of Hazardous Materials, AJE-Asian Journal of Ecotoxicology, JAES-Journal of Agro-Environment Science, JSCNU-Journal of South China Normal University (Natural Science Edition), ES-Environmental Science, OELS-Oceanologia Et Limnologia Sinica, JLS-Journal of Lake Science, CJAEB- Chinese Journal of Applied & Environmental Biology, CJAE-Chinese Journal of Applied Ecology, Eco-Ecologic Science, RF-Reservoir Fisheries, CJE-Chinese Journal of Ecology, JAAS-Journal of Anhui Agricultural Science.
测试指标名称注释(Index Abbreviations): SOD-superoxide dismutase, CAT- catalase, MT-metallothionein, LPO-lipid peroxidation, ChE-cholinesterase, ODH-octopine dehydrogenase, AEC-adenylate energy charge, ATP-adenosinetriphosphate, CA-carbonic anhydrase, HMBP-heavy metal binding protein, MXR-multixenobiotic resistance protein, MDA-malondialdehyde, GPx-glutathione peroxidase, GSTpi-glutathione S-transferase pi class, GST-glutathione S-transferase, GSH-glutathione, GR-glutathione reductase.
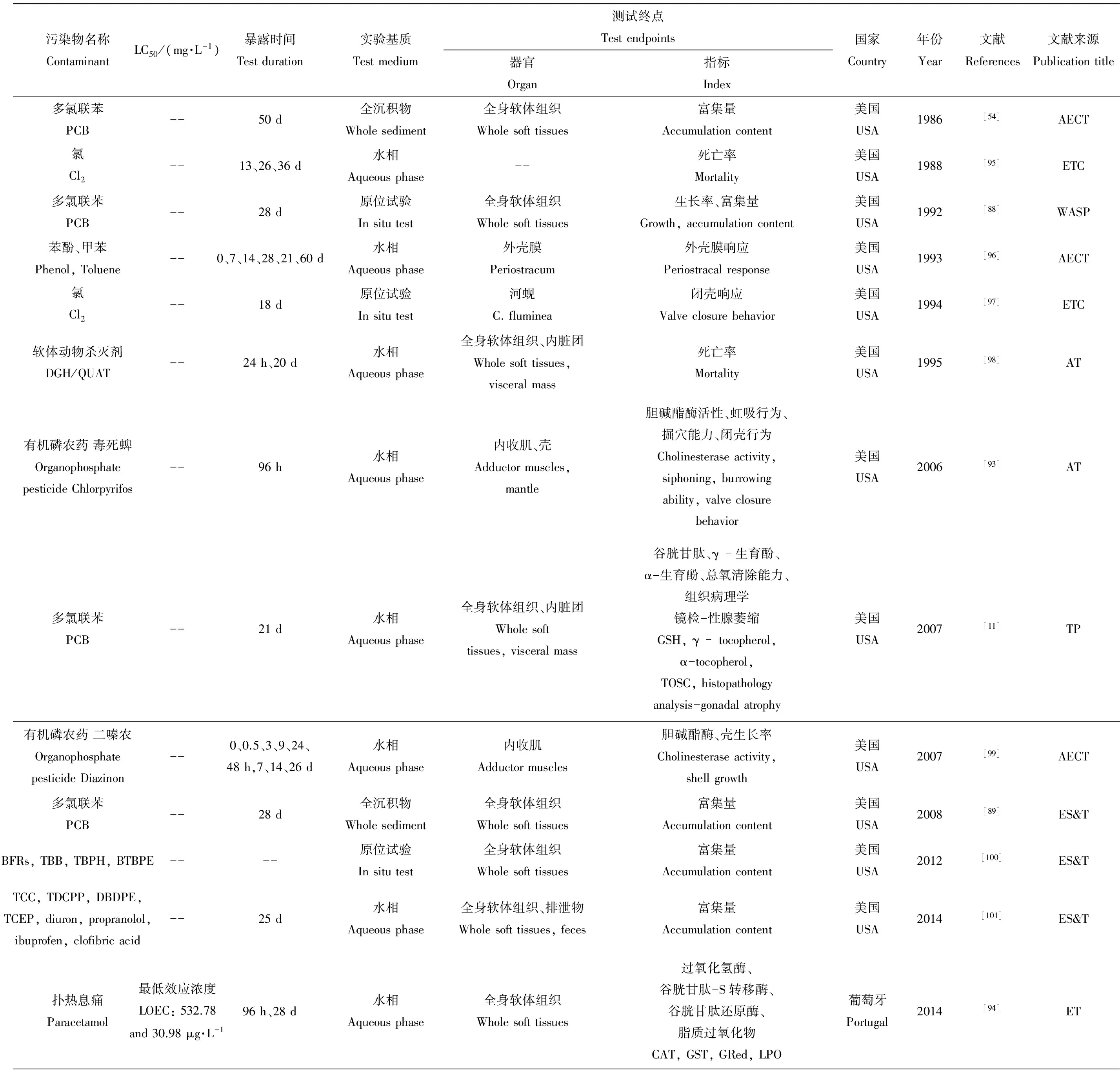
表3 河蚬在有机污染物生物监测中的应用Table 3 Corbicula fluminea in organic contamination biomonitoring
期刊名注释(Journal Title Abbreviations): WASP- Water, Air, & Soil Pollution, AECT-Archives of Environmental Contamination and Toxicology, EC- Environmental Chemistry, EMA-Environmental Monitoring and Assessment, AT-Aquatic Toxicology, BECT- Bulletin of Environmental Contamination and Toxicology, EES-Ecotoxicology and Environmental Safety, ETP-Environmental Toxicology and Pharmacology, TP-Toxicologic Pathology, MR-GTEM--Mutation Research/Genetic Toxicology and Environmental Mutagenesis, MF-Marine Fishers, CJPS-Chinese Journal of Pesticide Science, JRE-Journal of Resources and Ecology, ASC-Acta Scientiae Circumstantiae, CJEE- Chinese Journal of Environmental Engineering.
污染物名称注释(Contaminants Abbreviation): BFRs—brominated flame-retardants, TBB—2,3,4,5-tetrabromobenzoate, TBPH—2-ethylhexyl 2,3,4,5-etrabromophthalate, BTBPE—1,2-bis(2,4,6-tribromophenoxy) ethane, DBDPE—decabromodiphenyl ethane, DGH/QUAT—dodecylguanidine hydrochloride (DGH), QUAT—n-alkyl dimethylbenzyl ammonium chloride, polyDADMAC—poly diallyl dimethyl ammonium chloride, HCH—hexachlorocyolohexane (666), DDT—dichlorodiphenyltri-chloroe-thane.
测试指标名称注释(Index Abbreviations): P450, P418—cytochromes, NADH-red—NADH-cytochrome C reductase, CAT—catalase, PL—peroxidizable lipids, NP—net peroxidation, TOSC—total oxidant scavenging capacity, SOD—superoxide dismutase, GR—glutathione reductase, TR—thioredoxin reductase, MDA—malondialdehyde, TR—thioredoxin reductase, GPx—glutathione peroxidase, GSTpi—glutathione S-transferase pi class, EROD—ethoxyresorufin-O-deethylase, GST—glutathione S-transferase, BSAF—biota-sediment accumulation factor, DBF—dibenzylfluorescein dealkylase, AO—antioxidant enzymes.
从研究区分布看,美国学者用河蚬做指示生物,表征有机污染物生物毒性的研究较多,其次为法国及阿根廷。我国学者在河蚬对有机污染物的生物毒性效应研究中亦开展了大量工作[20,110-111]。测试指标的采用及发展与重金属污染的生物毒性研究相似。早期研究多偏重于河蚬体内有机污染物富集量测定[30,32,88,90,95,96-98,103,107];后期逐步发展为多指标联用,如行为学指标(虹吸行为、掘穴行为、闭壳响应)、形态学指标(组织学、组织化学、组织病理学分析)[11,93]、生化指标(SOD、CAT、GR、GPx、GST、胆碱酯酶、AO酶、EROD等氧化还原系统的多种酶学指标)[2,85,87,91,94,108]、分子生物学相关方法(转录组测序和分析[18-19]、DNA完整性[11, 17, 85-87])等。
从污染物角度,有机污染物种类繁多,研究者多围绕重点关注的持久性有机污染物、药物、苯酚类物质及用于船舶防污漆的三丁基锡,亦有少量学者进行重金属与多氯联苯(PCB)、多环芳烃(PAH)、有机氯农药(OCP)复合污染的研究[54, 80]。河蚬对有机污染物生物毒性效应研究的试验基质选取情况与重金属污染研究情况相同,多数研究采用水相加标实验。对于试验周期,多以有机物毒性强弱及实验浓度为依据,确定不同试验时长。持久性有机污染物及抗生素、雌激素等药物多采用慢性毒性实验[2, 11, 18-19, 54, 86-89]。
整体上,与重金属相比,河蚬在有机污染物生物毒性效应中的研究报道相对较少,主要集中在2个方面:(1)采用野外监测评估实际水生环境中有机污染物污染情况;(2)有机污染物对河蚬健康影响的毒理学机理研究。河蚬体内有机污染物含量的野外监测分析结果表明,河蚬对疏水性有机污染物有较强富集能力[90-92]。河蚬体内有机污染物累积量与水体、沉积物中含量存在显著相关性,且污染水域对河蚬氧化应激系统有不良影响。室内模拟试验及原位试验发现,低剂量长期暴露于有机污染物可引起行为学响应[93]、形态学变化[11]、生化响应[18-19, 85-87, 94]、基因损伤[17-19, 85, 87]等。由此可知,河蚬对有机污染物[19]有较强敏感性和生物累积能力,可以作为哨兵生物对有机物污染区域进行生物监测。以往研究也从细胞水平验证了河蚬用于环境风险评估的适用性。然而,目前沉积物毒性鉴定评估(TIE)中所采用的生物响应指标多为受试生物死亡率,随着环境治理的推进,极毒性污染逐渐减少,采用生物慢性毒性响应指标进行沉积物TIE显得尤为重要。
4 结论与展望(Conclusion and prospect)
经过30多年的探索,国内外在污染水体的河蚬生物毒性响应研究上已有较为全面的发展。大量学者采用河蚬生物体内累积、形态学及行为学观察、生化指标改变、代谢组学、基因完整性等生物监测技术对氨、重金属、有机污染物的生物毒性效应进行表征,认证河蚬对实际水生环境污染状况的评估能力,对污染水体的生物修复能力,对水体的毒性预测能力等。这些研究全面认证了河蚬作为指示生物对于环境风险评估的适用性。
河蚬的生物毒性效应研究多偏重于重金属污染水体,对有机污染物研究相对较少且多局限于持久性有机污染物,对于氨、新型污染物及纳米材料的研究尚处于起步阶段,这些污染物对河蚬毒性影响有待进一步开展。且多种污染物对河蚬的复合毒性研究有待进一步加强。研究中多采用水相进行模拟实验,对于全沉积物的研究仍需加强。
河蚬测试指标从早期的生物富集量测定、行为学形态学观察,到后期出现的生化指标测定,再到分子生物学指标测定。不同测试指标的选取针对不同研究目的。生物富集是污染物生物毒性效应研究的重要过程,污染物在生物体内的富集是一个包含摄食、排泄、储存、降解的动态平衡过程;行为学及形态学观察在污染物生物毒性响应研究中可以提供最直观快捷的反映;随着生物技术的发展,污染物对蛋白质、酶、核酸等生物大分子的毒性作用机理逐渐被关注,因此可以在不同水平进行毒性预测,近年发展的许多生物标志物如氧化应激酶、基因毒性、溶酶体改变、免疫活性以及胆碱酯酶活性等被用于毒理学研究。生物富集及形态学行为学观察可以提供直接、基础的研究,而生化指标及分子生物学指标可以提供更多生物水平层面的毒性预测,与软体组织内污染物富集量测定相比,生物标志物能够提供更加完整的、生物学上更可靠的信息。河蚬测试指标随着分子生物学及相关分析检测仪器技术的飞速发展而快速更新,且多指标全面表征逐步代替单一指标测试,以更全面准确评估污染物对河蚬的毒性影响。然而对于不同特定毒物的特异性生物标志物的筛选研究比较匮乏,有待进一步研究。
目前对河蚬在沉积物毒性鉴定评估(TIE)中的应用研究较为缺乏,尤其将河蚬的行为学及形态学变化、生化响应、基因损伤等指标全面结合用于沉积物毒性鉴定评估(TIE)的研究鲜有报道。而河蚬作为广泛分布且对污染物具有较强富集性及敏感性的双壳类底栖生物,具有较全面的生物监测背景研究,对于其在沉积物毒性鉴定评估中(TIE)的应用研究工作有待开展。
[1] Zhou Q F, Zhang J B, Fu J J, et al. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem [J]. Analytica Chimica Acta, 2008, 606(2): 135-150
[2] Bonnafé E, Sroda S, Budzinski H, et al. Responses of cytochrome P450, GST, and MXR in the mollusk Corbicula fluminea to the exposure to hospital wastewater effluents [J]. Environmental Science and Pollution Research, 2015, 22(14): 11033-11046
[3] Sousa R, Antunes C, Guilhermino L. Ecology of the invasive Asian clam Corbicula fluminea (Müller, 1774) in aquatic ecosystems: An overview [J]. Annales de Limnologie - International Journal of Limnology, 2008, 44(2): 85-94
[4] Hubenov Z, Trichkova T, Kenderov L, et al. Distribution of Corbicula fluminea (Mollusca: Corbiculidae) over an eleven-year period of its invasion in Bulgaria [J]. Acta Zoologica Bulgarica, 2013, 65(3): 315-326
[5] Ilarri M I, Freitas F, Costa-Dias S, et al. Associated macrozoobenthos with the invasive Asian clam Corbicula fluminea [J]. Journal of Sea Research, 2012, 72: 113-120
[6] Fournier E, Adam C, Massabuau J-C, et al. Bioaccumulation of waterborne selenium in the Asiatic clam Corbicula fluminea: Influence of feeding-induced ventilatory activity and selenium species [J]. Aquatic Toxicology, 2005, 72(3): 251-260
[7] Santos H M, Diniz M S, Costa P M, et al. Toxicological effects and bioaccumulation in the freshwater clam (Corbicula fluminea) following exposure to trivalent arsenic [J]. Environmental Toxicology, 2007, 22(5): 502-509
[8] Costa P M, Santos H M, Peres I, et al. Toxicokinetics of waterborne trivalent arsenic in the freshwater bivalve Corbicula fluminea [J]. Archives of Environmental Contamination and Toxicology, 2009, 57(2): 338-347
[9] Vale G, Franco C, Diniz M S, et al. Bioavailability of cadmium and biochemical responses on the freshwater bivalve Corbicula fluminea—The role of TiO2nanoparticles [J]. Ecotoxicology and Environmental Safety, 2014, 109: 161-168
[10] Cid A, Picado A, Correia J B, et al. Oxidative stress and histological changes following exposure to diamond nanoparticles in the freshwater Asian clam Corbicula fluminea (Müller, 1774) [J]. Journal of Hazardous Materials, 2015, 284: 27-34
[11] Lehmann D W, Levine J F, McHugh Law J M. Polychlorinated biphenyl exposure causes gonadal atrophy and oxidative stress in Corbicula fluminea clams [J]. Toxicologic Pathology, 2007, 35(3): 356-365
[12] Spann N, Aldridge D C, Griffin J L, et al. Size-dependent effects of low level cadmium and zinc exposure on the metabolome of the Asian clam, Corbicula fluminea [J]. Aquatic Toxicology, 2011, 105(3-4): 589-599
[13] Arini A, Daffe G, Gonzalez P, et al. What are the outcomes of an industrial remediation on a metal-impacted hydrosystem? A 2-year field biomonitoring of the filter-feeding bivalve Corbicula fluminea [J]. Chemosphere, 2014, 108: 214-224
[14] Arini A, Daffe G, Gonzalez P, et al. Detoxification and recovery capacities of Corbicula fluminea after an industrial metal contamination (Cd and Zn): A one-year depuration experiment [J]. Environmental Pollution, 2014, 192: 74-82
[15] Bigot A, Minguez L, Giambérini L, et al. Early defense responses in the freshwater bivalve Corbicula fluminea exposed to copper and cadmium: Transcriptional and histochemical studies [J]. Environmental Toxicology, 2011, 26(6): 623-632
[16] Simon O, Floriani M, Cavalie I, et al. Internal distribution of uranium and associated genotoxic damages in the chronically exposed bivalve Corbicula fluminea [J]. Journal of Environmental Radioactivity, 2011, 102(8): 766-773
[17] Fedato R P, Simonato J D, Martinez C B R, et al. Genetic damage in the bivalve mollusk Corbicula fluminea induced by the water-soluble fraction of gasoline [J]. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2010, 700(1-2): 80-85
[18] Chen H H, Zha J M, Yuan L L, et al. Effects of fluoxetine on behavior, antioxidant enzyme systems, and multixenobiotic resistance in the Asian clam Corbicula fluminea [J]. Chemosphere, 2015, 119: 856-862
[19] Chen H H, Zha J M, Liang X F, et al. Effects of the human antiepileptic drug carbamazepine on the behavior, biomarkers, and heat shock proteins in the Asian clam Corbicula fluminea [J]. Aquatic Toxicology, 2014, 155: 1-8
[20] 郭晓宇, 李茹枫, 冯成洪. 河蚬在我国沉积物毒性评价与鉴定中的应用研究[J]. 生态毒理学报, 2016, 11(2): 89-100
Guo X Y, Li R F, Feng C H. Corbicula fluminea in sediment toxicity evaluation and identification studies in China [J]. Asian Journal of Ecotoxicology, 2016, 11(2): 89-100 (in Chinese)
[21] Costa S, Guilhermino L. Influence of long-term exposure to background pollution on the response and recovery of the invasive species Corbicula fluminea to ammonia sub-lethal stress: A multi-marker approach with field estuarine populations [J]. Water, Air, & Soil Pollution, 2015, 226(4): 95
[22] Mummert A K, Neves R J, Newcomb T J, et al. Sensitivity of juvenile freshwater mussels (Lampsilis fasciola, Villosa iris) to total and un-ionized ammonia [J]. Environmental Toxicology & Chemistry, 2003, 22(11): 2545-2553
[23] Newton T J, Bartsch M R. Lethal and sublethal effects of ammonia to juvenile Lampsilis mussels (Unionidae) in sediment and water-only exposures [J]. Environmental Toxicology & Chemistry, 2007, 26(10): 2057-2065
[24] Montresor L C, Miranda-Filho K C, Paglia A, et al. Short-term toxicity of ammonia, sodium hydroxide and a commercial biocide to golden mussel Limnoperna fortunei (Dunker, 1857) [J]. Ecotoxicology and Environmental Safety, 2013, 92: 150-154
[25] Ilarri M I, Antunes C, Guilhermino L, et al. Massive mortality of the Asian clam Corbicula fluminea in a highly invaded area [J]. Biological Invasions, 2011, 13(2): 277-280
[26] Cooper N L, Cherry D S. Potential effects of Asian clam (Corbicula fluminea) die-offs on native freshwater mussels (Unionidae) II: Porewater ammonia [J]. Journal of the North American Benthological Society, 2014, 24(2): 381-394
[27] Cherry D S, Scheller J L, Cooper N L, et al. Potential effects of Asian clam (Corbicula fluminea) die-offs on native freshwater mussels (Unionidae) I: Water-column ammonia levels and ammonia toxicity [J]. Journal of the North American Benthological Society, 2014, 24(2): 369-380
[28] Fraysse B, Baudin J P, Garnier-Laplace J, et al. Effects of Cd and Zn waterborne exposure on the uptake and depuration of 57Co, 110mAg and 134Cs by the Asiatic clam (Corbicula fluminea) and the zebra mussel (Dreissena polymorpha)—Whole organism study [J]. Environmental Pollution, 2002, 118(3): 297-306
[29] Fan W H, Ren J Q, Wu C G, et al. Using enriched stable isotope technique to study Cu bioaccumulation and bioavailability in Corbicula fluminea from Taihu Lake, China [J]. Environmental Science and Pollution Research, 2014, 21(24): 14069-14077
[30] Merschel G, Bau M. Rare earth elements in the aragonitic shell of freshwater mussel Corbicula fluminea and the bioavailability of anthropogenic lanthanum, samarium and gadolinium in river water [J]. Science of The Total Environment, 2015, 533: 91-101
[31] Zuykov M, Pelletier E, Harper D A T. Bivalve mollusks in metal pollution studies: From bioaccumulation to biomonitoring [J]. Chemosphere, 2013, 93(2): 201-208
[32] Sebesvari Z, Ettwig K F, Emons H. Biomonitoring of tin and arsenic in different compartments of a limnic ecosystem with emphasis on Corbicula fluminea and Dikerogammarus villosus [J]. Journal of Environmental Monitoring, 2005, 7(3): 203-207
[33] Baudrimont M, Lemaire-Gony S, Ribeyre F, et al. Seasonal variations of metallothionein concentrations in the Asiatic clam (Corbicula fluminea) [J]. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, 1997, 118(3): 361-367
[34] Leland H V, Scudder B C. Trace elements in Corbicula fluminea from the San Joaquin River, California [J]. Science of the Total Environment, 1990, 97-98(7): 641-672
[35] Marie V, Baudrimont M, Boudou A. Cadmium and zinc bioaccumulation and metallothionein response in two freshwater bivalves (Corbicula fluminea and Dreissena polymorpha) transplanted along a polymetallic gradient [J]. Chemosphere, 2006, 65(4): 609-617
[36] Rocha C T, Souza M M. The influence of lead on different proteins in gill cells from the freshwater bivalve, Corbicula fluminea, from defense to repair biomarkers [J]. Archives of Environmental Contamination and Toxicology, 2012, 62(1): 56-67
[37] Inza B, Ribeyre F, Boudou A. Dynamics of cadmium and mercury compounds (inorganic mercury or methylmercury): Uptake and depuration in Corbicula fluminea. Effects of temperature and pH [J]. Aquatic Toxicology, 1998, 43(4): 273-285
[38] Baudrimont M, Andres S, Durrieu G, et al. The key role of metallothioneins in the bivalve Corbicula fluminea during the depuration phase, after in situ exposure to Cd and Zn [J]. Aquatic Toxicology, 2003, 63(2): 89-102
[39] Reis P A, Guilhermino L, Antunes C, et al. Assessment of the ecological quality of the Minho estuary (Northwest Iberian Peninsula) based on metal concentrations in sediments and in Corbicula fluminea [J]. Limnetica, 2014, 33(1): 161-173
[40] Kong M, Hang X, Wang L, et al. Accumulation and risk assessment of heavy metals in sediments and zoobenthos (Bellamya aeruginosa and Corbicula fluminea) from Lake Taihu [J]. Water Science and Technology, 2016, 73(1): 203-214
[41] Rosa I C, Costa R, Gonçalves F, et al. Bioremediation of metal-rich effluents: Could the invasive bivalve work as a biofilter? [J]. Journal of Environment Quality, 2014, 43(5): 1536-1545
[42] Giesy J P, Duke C S, Bingham R D, et al. Changes in phosphoadenylate concentrations and adenylate energy charge as an integrated biochemical measure of stress in invertebrates: The effects of cadmium on the freshwater clam Corbicula fluminea [J]. Toxicological & Environmental Chemistry, 2008, 6(4): 259-295
[43] Legeay A, Achard-Joris M, Baudrimont M, et al. Impact of cadmium contamination and oxygenation levels on biochemical responses in the Asiatic clam Corbicula fluminea [J]. Aquatic Toxicology, 2005, 74(3): 242-253
[44] Simon O, Garnier-Laplace J. Kinetic analysis of uranium accumulation in the bivalve Corbicula fluminea: Effect of pH and direct exposure levels [J]. Aquatic Toxicology, 2004, 68(2): 95-108
[45] Liao C-M, Jou L-J, Chen B-C. Risk-based approach to appraise valve closure in the clam Corbicula fluminea in response to waterborne metals [J]. Environmental Pollution, 2005, 135(1): 41-52
[46] Jou L-J, Liao C-M. A dynamic artificial clam (Corbicula fluminea) allows parsimony on-line measurement of waterborne metals [J]. Environmental Pollution, 2006, 144(1): 172-183
[47] Liao C-M, Lin C-M, Jou L-J, et al. Linking valve closure behavior and sodium transport mechanism in freshwater clam Corbicula fluminea in response to copper [J]. Environmental Pollution, 2007, 147(3): 656-667
[48] Liao C-M, Jou L-J, Lin C-M, et al. Predicting acute copper toxicity to valve closure behavior in the freshwater clam Corbicula fluminea supports the biotic ligand model [J]. Environmental Toxicology, 2007, 22(3): 295-307
[49] Liao C-M, Jau S-F, Chen W-Y, et al. Acute toxicity and bioaccumulation of arsenic in freshwater clam Corbicula fluminea [J]. Environmental Toxicology, 2008, 23(6): 702-711
[50] Liao C-M, Jau S-F, Lin C-M, et al. Valve movement response of the freshwater clam Corbicula fluminea following exposure to waterborne arsenic [J]. Ecotoxicology, 2009, 18(5): 567-576
[51] Jou L J, Chen W Y, Liao C M. Online detection of waterborne bioavailable copper by valve daily rhythms in freshwater clam Corbicula fluminea [J]. Environmental Monitoring and Assessment, 2009, 155(1-4): 257-272
[52] Chen W-Y, Liao C-M, Jou L-J, et al. Predicting bioavailability and bioaccumulation of arsenic by freshwater clam Corbicula fluminea using valve daily activity [J]. Environmental Monitoring and Assessment, 2010, 169(1-4): 647-659
[53] Graney R L, Cherry D S, Cairns J. The influence of substrate, pH, diet and temperature upon cadmium accumulation in the Asiatic clam (Corbicula fluminea) in laboratory artificial streams [J]. Water Research, 1984, 18(7): 833-842
[54] Tatem H E. Bioaccumulation of polychlorinated biphenyls and metals from contaminated sediment by freshwater prawns, Macrobrachium rosenbergii and clams, Corbicula fluminea [J]. Archives of Environmental Contamination & Toxicology, 1986, 15(2): 171-183
[55] Doherty F G, Failla M L, Cherry D S. Identification of a metallothionein-like, heavy metal binding protein in the freshwater bivalve, Corbicula fluminea [J]. Comparative Biochemistry & Physiology Part C Comparative Pharmacology, 1987, 87(1): 113-120
[56] Doherty F G, Failla M L, Cherry D S. Metallothionein-like heavy metal binding protein levels in Asiatic clams are dependent on the duration and mode of exposure to cadmium [J]. Water Research, 1988, 22(7): 927-932
[57] Mccloskey J T, Dixon P M, Newman M C. Effect of metal and metalloid contaminated sediment on the spatial distribution of Asiatic clams (Corbicula fluminea) [J]. Archives of Environmental Contamination & Toxicology, 1995, 28(2): 203-208
[58] Lee B G, Lee J S, Luoma S N. Comparison of selenium bioaccumulation in the clams Corbicula fluminea and Potamocorbula amurensis: A bioenergetic modeling approach [J]. Environmental Toxicology & Chemistry, 2006, 25(7): 1933-1940
[59] Peltier G L, Wright M S, Hopkins W A, et al. Accumulation of trace elements and growth responses in Corbicula fluminea downstream of a coal-fired power plant [J]. Ecotoxicology and Environmental Safety, 2009, 72(5): 1384-1391
[60] Shoults-Wilson W A, Unrine J M, Rickard J, et al. Comparison of metal concentrations in Corbicula fluminea and Elliptio hopetonensis in the Altamaha River system, Georgia, USA [J]. Environmental Toxicology and Chemistry, 2010, 29(9): 2026-2033
[61] Ren J, Luo J, Ma H, et al. Bioavailability and oxidative stress of cadmium to Corbicula fluminea [J]. Environmental Science: Processes & Impacts, 2013, 15(4): 860-869
[62] Geng N, Wang C, Wang P F, et al. Cadmium accumulation and metallothionein response in the freshwater bivalve Corbicula fluminea under hydrodynamic conditions [J]. Biological Trace Element Research, 2015, 165(2): 222-232
[63] Inza B, Ribeyre F, Maury-Brachet R, et al. Tissue distribution of inorganic mercury, methylmercury and cadmium in the Asiatic clam (Corbicula fluminea) in relation to the contamination levels of the water column and sediment [J]. Chemosphere, 1997, 35(12): 2817-2836
[64] Bregni M, Ueno N T, Childs R. Bioaccumulation and metallothionein response in the Asiatic clam (Corbicula fluminea) after experimental exposure to cadmium and inorganic mercury [J]. Environmental Toxicology & Chemistry, 1997, 16(16): 2096-2105
[65] Andrès S, Baudrimont M, Lapaquellerie Y, et al. Field transplantation of the freshwater bivalve Corbicula fluminea along a polymetallic contamination gradient (River Lot, France): I. Geochemical characteristics of the sampling sites and cadmium and zinc bioaccumulation kinetics [J]. Environmental Toxicology & Chemistry, 1999, 18(11): 2462-2471
[66] Fraysse J P, Baudin J. Cadmium uptake by Corbicula fluminea and Dreissena polymorpha: Effects of pH and temperature [J]. Bulletin of Environmental Contamination and Toxicology, 2000, 65(5): 638-645
[67] Tran D, Boudou A, Massabuau J C. How water oxygenation level influences cadmium accumulation pattern in the Asiatic clam Corbicula fluminea: A laboratory and field study [J]. Environmental Toxicology & Chemistry, 2001, 20(9): 2073-2080
[68] Tran D, Boudou A, Massabuau J C. Relationship between feeding-induced ventilatory activity and bioaccumulation of dissolved and algal-bound cadmium in the Asiatic clam Corbicula fluminea [J]. Environmental Toxicology & Chemistry, 2002, 21(2): 327-333
[69] Tran D, Fournier E, Durrieu G, et al. Copper detection in the Asiatic clam Corbicula fluminea: Optimum valve closure response [J]. Aquatic Toxicology, 2003, 65(3): 317-327
[70] Tran D, Massabuau J C, Garnier-Laplace J. Effect of carbon dioxide on uranium bioaccumulation in the freshwater clam Corbicula fluminea [J]. Environmental Toxicology & Chemistry, 2004, 23(3): 739-747
[71] Fournier E, Tran D, Denison F, et al. Valve closure response to uranium exposure for a freshwater bivalve (Corbicula fluminea): Quantification of the influence of pH [J]. Environmental Toxicology & Chemistry, 2004, 23(5): 1108-1114
[72] Achard M. Induction of a multixenobiotic resistance protein (MXR) in the Asiatic clam Corbicula fluminea after heavy metals exposure [J]. Aquatic Toxicology, 2004, 67(4): 347-357
[73] Tran D, Bourdineaud J P, Massabuau J C, et al. Modulation of uranium bioaccumulation by hypoxia in the freshwater clam Corbicula fluminea: Induction of multixenobiotic resistance protein and heat shock protein 60 in gill tissues [J]. Environmental Toxicology & Chemistry, 2005, 24(9): 2278-2284
[74] Achard-Joris M, Gonzalez P, Marie V, et al. cDNA cloning and gene expression of ribosomal S9 protein gene in the mollusk Corbicula fluminea: A new potential biomarker of metal contamination up-regulated by cadmium and repressed by zinc [J]. Environmental Toxicology & Chemistry, 2006, 25(2): 527-533
[75] Fournier E, Adam C, Massabuau J C, et al. Selenium bioaccumulation in Chlamydomonas reinhardtii and subsequent transfer to Corbicula fluminea: Role of selenium speciation and bivalve ventilation [J]. Environmental Toxicology & Chemistry, 2006, 25(10): 2692-2699
[76] Tran D, Fournier E, Durrieu G, et al. Inorganic mercury detection by valve closure response in the freshwater clam Corbicula fluminea: Integration of time and water metal concentration changes [J]. Environmental Toxicology & Chemistry, 2007, 26(7): 1545-1551
[77] Tran D, Massabuau J C, Garnier-Laplace J. Impact of hypoxia on hemolymph contamination by uranium in an aquatic animal, the freshwater clam Corbicula fluminea [J]. Environmental Pollution, 2008, 156(3): 821-826
[78] Abaychi J K, Mustafa Y Z. The Asiatic clam, Corbicula fluminea: An indicator of trace metal pollution in the Shatt al-Arab River, Iraq [J]. Environmental Pollution, 1988, 54(2): 109-122
[79] Bilos C, Colombo J C, Presa M J. Trace metals in suspended particles, sediments and Asiatic clams (Corbicula fluminea) of the Río de la Plata Estuary, Argentina [J]. Environmental Pollution, 1998, 99(1): 1-11
[80] Cataldo D, Colombo J C, Boltovskoy D, et al. Environmental toxicity assessment in the Paraná River Delta (Argentina): Simultaneous evaluation of selected pollutants and mortality rates of Corbicula fluminea (Bivalvia) early juveniles [J]. Environmental Pollution, 2001, 112: 379-389
[81] De Oliveira L F, Dos Reis Martinez C B. Chromium accumulation in the Asian clam, Corbicula fluminea (Müller, 1774), as an indicative of landfill leachate contamination [J]. Bulletin of Environmental Contamination and Toxicology, 2014, 93(2): 149-153
[82] Ismail F A, Aris A Z, Latif P A. Dynamic behaviour of Cd2+adsorption in equilibrium batch studies by CaCO3-rich Corbicula fluminea shell [J]. Environmental Science and Pollution Research, 2014, 21(1): 344-354
[83] Bonnail E, Sarmiento A M, DelValls T A, et al. Assessment of metal contamination, bioavailability, toxicity and bioaccumulation in extreme metallic environments (Iberian Pyrite Belt) using Corbicula fluminea [J]. Science of The Total Environment, 2016, 544: 1031-1044
[84] Oneto M L, Basack S B, Casabe N B, et al. Biological responses in the freshwater bivalve Corbicula fluminea and the earthworm Eisenia fetida exposed to fenitrothion [J]. Fresenius Environmental Bulletin, 2005, 14(8): 716-720
[85] Dos Santos K C, Martinez C B R. Genotoxic and biochemical effects of atrazine and Roundup®, alone and in combination, on the Asian clam Corbicula fluminea [J]. Ecotoxicology and Environmental Safety, 2014, 100: 7-14
[86] Champeau O, Narbonne J-F. Effects of tributyltin and 17β-estradiol on immune and lysosomal systems of the Asian clam Corbicula fluminea (M.) [J]. Environmental Toxicology and Pharmacology, 2006, 21(3): 323-330
[87] Aguirre-Martínez G V, DelValls A T, Laura Martín-Díaz M. Yes, caffeine, ibuprofen, carbamazepine, novobiocin and tamoxifen have an effect on Corbicula fluminea (Müller, 1774) [J]. Ecotoxicology and Environmental Safety, 2015, 120: 142-154
[88] Peterson M J, Southworth G R, Ham K D. Effect of sublethal chlorinated discharges on PCB accumulation in transplanted Asiatic clams (Corbicula fluminea) [J]. Water Air & Soil Pollution, 1994, 73(73): 169-178
[89] McLeod P B, Luoma S N, Luthy R G. Biodynamic modeling of PCB uptake by Macoma balthica and Corbicula fluminea from sediment amended with activated carbon [J]. Environmental Science & Technology, 2008, 42(2): 484-490
[90] Colombo J C, Bilos C, Campanaro M, et al. Bioaccumulation of polychlorinated biphenyls and chlorinated pesticides by the Asiatic clam Corbicula fluminea; its use as sentinel organism in the Rio de La Plata Estuary, Argentina. [J]. Environmental Science & Technology, 1995, 29(4): 914-927
[92] Lee C-C, Jhuang Y-F, Liu L-L, et al. The major source and impact of phenyltin contamination on freshwater aquaculture clam Corbicula fluminea and wild golden apple snail Pomacea canaliculata [J]. Environmental Chemistry, 2009, 6(4): 341-349
[93] Cooper N L, Bidwell J R. Cholinesterase inhibition and impacts on behavior of the Asian clam, Corbicula fluminea, after exposure to an organophosphate insecticide [J]. Aquatic Toxicology, 2006, 76(3-4): 258-267
[94] Brandão F P, Pereira J L, Gonçalves F, et al. The impact of paracetamol on selected biomarkers of the mollusc species Corbicula fluminea [J]. Environmental Toxicology, 2014, 29(1): 74-83
[95] Ramsay G G, Tackett J H, Morris D W. Effect of low-level continuous chlorination on Corbicula fluminea [J]. Environmental Toxicology & Chemistry, 1988, 7(10): 855-856
[96] Rollins H B, Hutchinson P J, Prezant R S. Detection of xenophobic response in the periostracum of the bivalve, Corbicula fluminea, through laser-induced mass spectrometry [J]. Archives of Environmental Contamination & Toxicology, 1993, 24(2): 258-267
[97] Ham K D, Peterson M J. Effect of fluctuating low-level chlorine concentrations on valve-movement behavior of the Asiatic clam (Corbicula fluminea) [J]. Environmental Toxicology & Chemistry, 1994, 13(3): 493-498
[98] Bidwell J R, Farris J L, Cherry D S. Comparative response of the zebra mussel, Dreissena polymorpha, and the Asian clam, Corbicula fluminea, to DGH/QUAT, a nonoxidizing molluscicide [J]. Aquatic Toxicology, 1995, 33(3-4): 183-200
[99] Bouldin J L, Farris J L, Moore M T, et al. Assessment of diazinon toxicity in sediment and water of constructed wetlands using deployed Corbicula fluminea and laboratory testing [J]. Archives of Environmental Contamination and Toxicology, 2007, 53(2): 174-182
[100] La Guardia M J, Hale R C, Harvey E, et al. In situ accumulation of HBCD, PBDEs, and several alternative flame-retardants in the bivalve (Corbicula fluminea) and gastropod (Elimia proxima) [J]. Environmental Science & Technology, 2012, 46(11): 5798-5805
[101] Ismail N S, Müller C E, Morgan R R, et al. Uptake of contaminants of emerging concern by the bivalves Anodonta californiensis and Corbicula fluminea [J]. Environmental Science & Technology, 2014, 48(16): 9211-9219
[102] Rosa I C, Garrido R, Ré A, et al. Sensitivity of the invasive bivalve Corbicula fluminea to candidate control chemicals: The role of dissolved oxygen conditions [J]. Science of The Total Environment, 2015, 536: 825-830
[103] Basack S B, Oneto M L, Verrengia Guerrero N R, et al. Accumulation and elimination of pentachlorophenol in the freshwater bivalve Corbicula fluminea [J]. Bulletin of Environmental Contamination & Toxicology, 1997, 58(3): 497-503
[104] Basack S B, Oneto M L, Fuchs J S, et al. Esterases of Corbicula fluminea as biomarkers of exposure to organophosphorus pesticides [J]. Bulletin of Environmental Contamination & Toxicology, 1998, 61(5): 569-576
[105] Jacomini A E, Avelar W E P, Martinêz A S, et al. Bioaccumulation of atrazine in freshwater bivalves Anodontites trapesialis (Lamarck, 1819) and Corbicula fluminea (Müller, 1774) [J]. Archives of Environmental Contamination and Toxicology, 2006, 51(3): 387-391
[106] Beltran K S, Pocsidio G N. Acetylcholinesterase activity in Corbicula fluminea Mull, as a biomarker of organophosphate pesticide pollution in Pinacanauan River, Philippines [J]. Environmental Monitoring and Assessment, 2010, 165(1-4): 331-340
[107] Narbonne J F, Djomo J E, Ribera D, et al. Accumulation kinetics of polycyclic aromatic hydrocarbons adsorbed to sediment by the mollusk Corbicula fluminea [J]. Ecotoxicology & Environmental Safety, 1999, 42(1): 1-8
[108] Vidal M, Basseres A, Narbonne J. Potential biomarkers of trichloroethylene and toluene exposure in Corbicula fluminea [J]. Environmental Toxicology and Pharmacology, 2001, 9(3): 87-97
[109] 韩雨薇, 张彦峰, 陈萌, 等. 沉积物中重金属Pb和Cd对河蚬的毒性效应研究[J]. 生态毒理学报, 2015, 10(4): 129-137
Han Y W, Zhang Y F, Chen M, et al. Toxicity of Pb/Cd-spiked freshwater sediments to Corbicula fluminea [J]. Asian Journal of Ecotoxicology, 2015, 10(4): 129-137 (in Chinese)
[110] 邱昕晔, 俞爽, 刘红玲. 河蚬(Corbicula fluminea)在生态毒理学研究中的应用与评价[J]. 生态毒理学报, 2016, 11(1): 80-93
Qiu X Y, Yu S, Liu H L. A review of ecotoxicological studies of Corbicula fluminea [J]. Asian Journal of Ecotoxicology, 2016, 11(1): 80-93 (in Chinese)
[111] 金小伟, 查金苗, 许宜平, 等. 3种氯酚类化合物对河蚬的毒性和氧化应激[J]. 生态毒理学报, 2009, 4(6): 816-822
Jin X W, Zha J M, Xu Y P, et al. Toxicity and oxidative stress of three chlorophenols to freshwater clam Corbicula fluminea [J]. Asian Journal of Ecotoxicology, 2009, 4(6): 816-822 (in Chinese)
◆
AReviewofBiologicalToxicityResponseofAsianClamCorbiculaflumineatoContaminatedEnvironment
Guo Xiaoyu1, Li Rufeng2, Feng Chenghong1,2,*, Han Zhihua3
1. State Key Laboratory of Water Environment Simulation, School of Environment, Beijing Normal University, Beijing 100875, China2. Key Laboratory for Water and Sediment Science of Ministry of Education, School of Environment, Beijing Normal University, Beijing 100875, China3. Nanjing Institute of Environmental Science, Ministry of Environmental Protection, Nanjing 210042, China
10.7524/AJE.1673-5897.20161012002
2016-10-12录用日期2016-12-16
1673-5897(2017)3-086-24
X171.5
A
冯成洪(1978-),男,博士,副教授,研究方向为污染物迁移转化及环境效应。
环境保护部公益性行业科研专项(No.201409040);北京市高等学校青年英才计划项目(No.YETP0235);环境模拟与污染控制国家重点联合实验室联合基金(16L01ESPC)
郭晓宇(1988-),女,博士研究生,研究方向为污染物迁移转化及环境效应,E-mail: guoxiaoyu@mail.bnu.edu.cn
*通讯作者(Corresponding author), E-mail: fengchenghong@bnu.edu.cn
郭晓宇, 李茹枫, 冯成洪, 等. 污染水体中河蚬的生物毒性响应研究进展[J]. 生态毒理学报,2017, 12(3): 86-109
Guo X Y, Li R F, Feng C H, et al. A review of biological toxicity response of Asian clam Corbicula fluminea to contaminated environment [J]. Asian Journal of Ecotoxicology, 2017, 12(3): 86-109 (in Chinese)
