Toxic effects of Cd2+on the intestinal structure of Cypridopsis vidua (Ostracoda)
2017-08-07CHENShimeiLiDanniDingQingqingYUNa
CHEN Shi-mei,Li Dan-ni,Ding Qing-qing,YU Na,2
(1.School of Life Science,East China Normal University,Shanghai 200240,China; 2.College of Teacher Education,East China Normal University,Shanghai 200062,China)
Toxic effects of Cd2+on the intestinal structure of Cypridopsis vidua (Ostracoda)
CHEN Shi-mei1,Li Dan-ni1,Ding Qing-qing1,YU Na1,2
(1.School of Life Science,East China Normal University,Shanghai 200240,China; 2.College of Teacher Education,East China Normal University,Shanghai 200062,China)
Cypridopsis vidua is one of the few ostracods which can surrive from heavy pollution water.The toxic eff ects of Cd2+on C.vidua and its intestinalultrastructure were examined using a static renewal system.The LC50values for cadmium in C.vidua were 5.00,2.01,0.46 and 0.14 mg/L at 24,48,72 and 96 h exposure respectively,and the safe concentration of Cd2+for long-term C.vidua survivalwas less than 0.014 mg/L.To observe the structure changes of its intestinal,four Cd2+concentrations were set up,and two of them were below the safe concentration of Cd2+(0.001 and 0.004 mg/L)and the other concentrations were above its safe concentration(0.016 and 0.064 mg/L).The experiment lasted for 7 days.When microstructure of C.vidua was observed,the gastrointestinal organization was not damaged below the safe concentration;while the degree of injury showed a certain amount of time and dose eff ects in 24-72 hours above the safe concentration,and some structures among those surviving animals were slightly recovered in 7 days under same concentration.Sub-microscopic analysis of intestinal cells of C.vidua in two concentrations (0.004 and 0.064 mg/L)groups showed,diff erent degrees of structure damage were found in the cell membrane,cytoplasm and organelles,which worsened with increasing Cd2+concentrations.Among these cellular structures,the damage to the membrane system of the cell was especially serious.
Ostracoda;Cd2+;acute toxicity;intestinal ultrastructure
摘要:Cypridopsis vidua是少数能在重污染水体中生存的介形类之一.本文采用急性毒性实验方法,研究了Cd2+对介形类C.vidua及其肠壁结构的影响,结果表明,24、48、72和96 h时Cd2+对C.vidua的半致死浓度(LC50)分别为5.00、2.01、0.46和0.14 mg/L,安全浓度为0.014 mg/L.在急性毒性试验的基础上,于安全浓度上下分别设置了两个Cd2+实验浓度对介形类进行攻毒研究,目的是进一步探讨在安全浓度附近,Cd2+对C.vidua肠壁细胞的损伤情况,实验持续7 d.显微结果显示,在安全浓度以下时,C.vidua的胃肠道结构基本没有受到损伤,但超过安全浓度后,C.vidua的胃肠道结构损伤程度于96 h以内表现出了一定的时间和剂量效应,但至第7天时部分幸存下来的C.vidua其受损胃肠道结构出现一定程度的恢复,但已无法恢复到最初的状态了;亚显微切片显示,肠壁细胞的膜结构、胞质、胞器等均有不同程度的损伤,且随镉离子浓度的升高损伤明显加剧,其中细胞的膜结构损伤尤为严重.
0 Introduction
In recent years,a large number ofheavy metals have been drained into bodies ofwater with industrial waste water and domestic sewage,causing deterioration in the water quality which threatens the survival and reproduction of aquatic organisms.Heavy metals could accumulate in aquatic organisms,thereby threatening human health when ingested[1].In addition,heavy metals could impact growth and reproduction,damage cellular membrane structures,inhibit celldivision,or even killthe aquatic organisms[1].Cadmium is an environmentalpollutant that is stored long–term in the body[2-3]with a broad distribution in the nature environment,and causes acute and chronic poisoning in animals.While cadmium is deposited into rivers,lakes and other bodies of water by the industrial waste water discharge,the atmospheric deposition of cadmium dust as wellas soilerosion,have a serious impact on the living conditions of nearby humans[4],as well as the fisheries[5].Thus,cadmium pollution research was suggested as a focus for the future by the United Nations Environment Program(UNEP),and cadmium was the first of the 12 dangerous chemicals listed[6].Therefore,the toxic eff ects of cadmium on the structure and physiological and biochemical reactions of an organism have been a hot research topic in environmental biology[7].
Today,the aquatic organisms that are important members of the aquatic ecosystem which are widely used as bioindicators for the polluted water bodies[7-11].Freshwater ostracod,a small crustacean,is the good modeltaxa to study toxicity in aquatic organisms because of their small size,wide distribution and high abundance,and easy collection in the natural environment,as well as suitable maintenance and culture in the laboratory[12-13].Ostracods are widely used in environmental research[13-17].Early studies have shown a close relationship between the ostracod community structure and the ecology of the surrounding aquatic environment[14,17-22]. Of these studies,some ostracods were suggested to serve as the right indicator for heavy metal pollution[23-24],pesticide pollution[15,25-27]and eutrophic waters[17,28].For example,Cypris subglobosa,Physocypria kraepelini and Heterocypris incongruens have an important role in the evaluation of water polluted with heavy metals[23-24,29-30].However,those studies were only concerned with the relationship between the environmental factors and the compositionand distribution of the ostracod,but did not examine damage to the cell tissue and organ of organism.
For aquatic animals,there are usually three ways(namely,gills,skin and digestive)that heavy metals are absorbed and accumulated in the body[1].Ofthese ostracods are diff erent from the others(such as shrimp,oysters,crab and fish)because of their small size,and except the marine genus Astrope,they had no special respiratory organs[31].Ostracods rely on the spread of the water and the penetration of the gas for gas exchange,and only a small amount of metal ions could be absorbed into the organism through the skin due to their chitin exoskeleton,so the intestinalwellis the most important way that Cd2+enters ostracods.In this study,Cypridopsis vidua(O.F.M¨uller,1776),selected as experimental materials,was one of the few ostracods surviving from heavy pollution water[18,32-37].This parthenogenetic species has been used for a long time and has substantial background information[38-41].C.vidua was used to study the acute toxic eff ects of Cd2+and alterations in organism microstructure and ultrastructure,to provide a theoretical basis for water quality assessment using aquatic organisms.
1 Experimental
1.1 Ostracod culture and reagents
C.vidua was cultivated in our laboratory for fifteen years.For culturing,C.vidua were transferred under a dissecting microscope into a beaker of culture solution,consisting of tap water aerated for more than a week,with a few drops of liquid with grated fresh duckweed.For the following experiment,adult ostracods with strong viability and of similar size were selected after 24 hours of acclimation.During acclimation period,these animals were not fed with the grated duckweed to empty the digestive tract[13].
The working Cd2+stock solution was 10 mg/L Cd2+(CdCl2·2.5H2O).This stock was diluted stepwise according to a set concentration gradient for the experiment.NaOH and HCl solutions were used to adjust the pH.
1.2 Toxicity experiment
To determine the concentration ranges for the test solutions in the experiment,repeated preliminary experiments were carried by exposing ostracods to solutions for 24 hours.Our result showed the maximum Cd2+concentration that allowed total survival was 0.04 mg/L,while 5 mg/L was 100%lethal for C.vidua.
To obtain the safe concentration of C.vidua in the Cd2+solutions,six concentrations of cadmium(0.04,0.105,0.276,0.725,1.904 and 5 mg/L)were chosen with geometric spacing between the determined maximum concentration for total survival and the minimum one for total lethality.Three treatment groups and one blank control group were exposed to each cadmium treatment.Each solution(20 mL)was put into a 50 mL-beaker,and then 20 animals were put into the solution.For the cadmium trials,environmentalconditions were kept constant during the experiment(such as water temperature=(25±0.5)°C,pH value=7.0±0.5,salinity= 0.04 g/L,dissolved oxygen=(8.0±0.5)mg/L).The duration of each trial was 24,48,72 and 96 hours.In the process of exposure to cadmium solution,the dead animals were counted andremoved without delay.The parasite criterion for ostracod death was performed as described by Yu et al[13].
Subacute toxicity experiment was divided into two parts.First,to observe the microstructure of the intestinal of C.vidua,four concentrations were chosen,among which 0.001 and 0.004 mg/L of the Cd2+concentrations were below the safe concentration,while 0.016 and 0.064mg/L were above the safe concentration.Second,to observe the submicroscopic structure,two concentrations(0.004 and 0.064 mg/L),close to the safe concentration from the acute toxicity experiment,were chosen.Organisms were incubated for a week.Then the specimens were obtained at 0,24,48,72,96 h and 7 d.The specimens from the first part experiment were fixed with Canoy’s fl uid,and the ones from the second part were fixed with 2.5%glutaraldehyde [pH 7.4,prepared with phosphate–buff ered saline(PBS)].
1.3 Structural and ultrastructural analysis of intestinal
Specimens from the fi rst subacute toxicity experiment were randomly selected and fi xed in Canoy’s fl uid and routinely processed for light microscopy to obtain 6µm-thick paraffi n sections.The damaged intestinal was observed under a phase-contrast OLYMPUS BX51,Japan. Specimens from the second experiment were fixed at 4°C in 2.5%glutaraldehyde[42],rinsed repeatedly in PBS for 2 hours and post–fixed for 1 hour in 2.0%OsO4,and then dehydrated through a graded series of ethanol(30%,50%,70%,90%and 100%;three times)before being embedded in Epon,thin sections(70 nm per section)were cut with Reichert Jung ultramicrotome using Diatome diamond knives.The thin sections were stained with uranyl acetate and lead citrate[43],before being observed under a Zeiss EM 912 transmission electron microscope.
1.4 Statistical methods
The percentage mortality was calculated according to the recorded mortality at 24,48, 72 and 96 h for each experimental group.The resulting data were converted into probits[44]. SPSS 14.0 statistical software was employed to perform the analysis.The log values of cadmium concentration served as the horizontal coordinate and the probit of mortality served as the verticalcoordinate to calculate a regression equation between the probit and concentration of the experimental solutions.The LC50of cadmium,as well as their respective 95%confidence intervals,were calculated using the probit analysis in SPSS 14.0[45].The safe cadmium concentration for C.vidua was calculated using empiricalformulas[13].Safe concentration was defined to be equalto 96 h–LC50multiplied by 0.1[46].
2 Results and Discussion
2.1 Animals mortality and the safe concentration of Cd2+
There was no ostracod mortality observed in the control group.This,therefore,removes the possibility that natural death of C.vidua or other externalenvironment changes influenced the results.The mortality of C.vidua increased with increasing cadmium concentrations and a dose–dependent eff ect was observed.A considerable linear positive correlation was found between the death probit of C.vidua and the cadmium concentrations.The LC50for cadmiumfor acute C.vidua toxicity at 24,48,72 and 96 h was 5.00,2.01,0.46 and 0.14 mg/L,respectively, suggesting that the LC50decreased with increasing exposure time(Table 1).The toxicity of cadmium of C.vidua increased in a time–dependent manner.The observed safe concentration of cadmium was 0.014 mg/L(Table 1).
The results showed that the safe concentration range of Cd2+for the long-term survival of C.vidua was less than 0.014 mg/L.This value is far lower than that of other ostracods, such as Diacypris compacta(0.43 mg/L)[47]and Cypris subglobosa(0.069 mg/L)[48],but much higher than other species such as Stenocypris major(0.013 mg/L)[49]and Physocypria kraepelini (0.004 mg/L)[30],and P.kraepelini is an ostracod species with high tolerance to environmental pollutants[14,17,50].These suggest that C.vidua has a very high tolerance to Cd2+pollution, and the toxic eff ects of Cd2+are slow in this species.
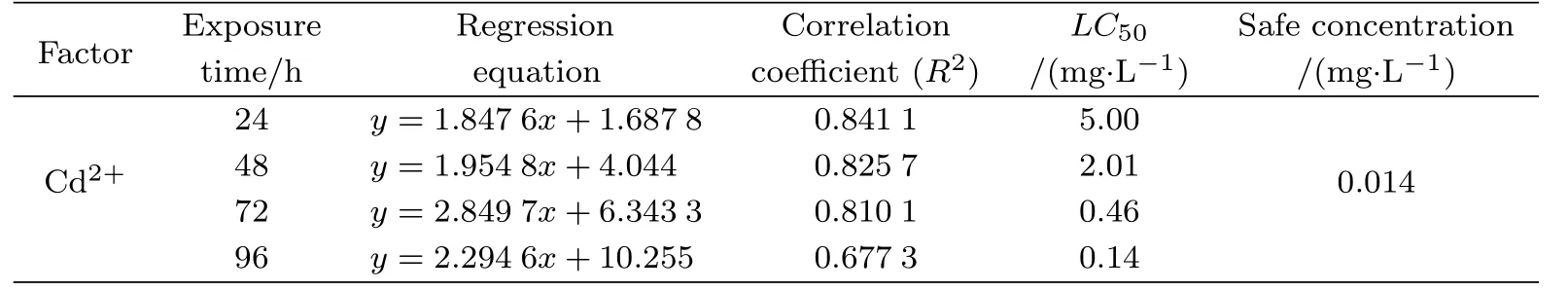
Tab.1 Cypridopsis vidua tolerance to water–borne Cd2+
2.2 The relationship between LC50values and exposure time
No lethal threshold concentration of cadmium was found during 96 hours exposure time (Figure 1),which suggested that the mortality of C.vidua increased continuously with exposure time,and that cadmium accumulated in the body of C.vidua.However,a significant turning pointwasfound at72 h for the half-lethalconcentration ofCd2+on C.vidua(Figure 1).Namely, the change in the LC50curve for Cd2+gradually paralleled the time axis,which indicated that the remaining surviving ostracods might produce a high tolerance to Cd2+.
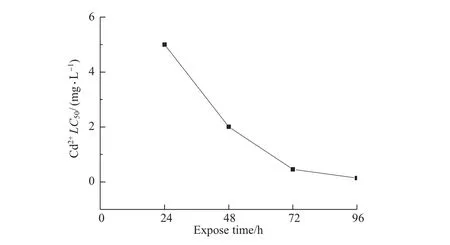
Fig.1 The relationship between LC50values and exposure time
Cadmium can accumulate in the organism ofanimals and human beings through the food chain[51]with a very long biologic half–life of 10–30 years[2-3].When a large amount of Cd2+enters the water,it can cause acute or chronic eff ects,which include organ system toxicity,and even death[52].The toxic eff ects of Cd2+persist for a long time in the organism,which woulddamage important cell,tissue and even organ structures[53].In fact,after the heavy metal toxicity is introduced into the aquatic environment,a three–step process willdevelop in aquatic organisms[54],such as the chemical and physico–chemical processes,physiological process and detoxification process.In this study,no lethal threshold concentration of cadmium found reflected in the first two stages ofthis process,in which the rate ofmortality of C.vidua gradually increased with increasing Cd2+concentration or prolonged experimental time,until all died; and the significant turning point found reflected showed in the third stage,which indicated that the remaining surviving ostracods might have a high tolerance to Cd2+and some detoxification mechanism might be functioning in the organism.It has been confirmed that there were some detoxification processes,which occur through binding between the toxic chemicals and a receptor[55].For example,metallothionein detoxification in animal organism[56-57].
2.3 Structural and ultrastructural changes in C.vidua intestinal
Although digestive tract is the main way that contaminants are absorbed in microcrustaceans(e.g.ostracod)[15],the effects of contaminants to their intestinal have never been concerned.In this study,comparing the microstructure of the intestinal of C.vidua with the blank control group,below the safe concentration(0.001 and 0.004 mg/L),when the experiment has continued for 7 days,there was no ostracod mortality observed and no observable changes in the intestinal structure of C.vidua.Above the safe concentration(0.016 and 0.064 mg/L),although no ostracod mortality was observed in 24-72 hours,multilayer cellular formations(mcfs)located in the foregut and hindgut edges or ventrolateralstructure were minorly injured in 24-48 hours;some organization structure(such as foregut and hindgut,mcfs, liver and pancreas)have been injured in 72 hours.However,there were animals mortality observed for both high Cd2+concentration groups(0.016 and 0.064 mg/L)in 96 hours(5% and 25%,respectively)and 7 days(32%and 49%,respectively).Surprisingly,some structures among those surviving animals were slightly recovered in 7 days under the same concentration, but the initial state has been unable to achieve.
The submicroscopic structure was observed and compared with the controlgroup(Fig.2A), in the low concentration treatment groups(0.004 mg/L)(Fig.2B–H),the cell volume increased as the cells swelled,the gaps between the cells widened.In addition,the bilayer structure of the nuclear envelope was indistinct,the chromatin distribution was not uniform,was more condensed.The number of mitochondria was reduced(Fig.2E).Some of the endoplasmic membrane was damaged,appearing broken,and the ribosomes were disrupted.The cytoplasm contained a large number of lipid droplets(Fig.2F).In the high concentration groups (0.016 mg/L)(Fig.3B–H),the degree of cell swelling increased,and there was further disintegration of the nuclear envelope.At 96 h,the heterochromatin was fractured.Mitochondria were fewer in number.The endoplasmic reticulum was expanded with many vesicles and debris, and no ribosome attached.A large amount of smooth endoplasmic reticulum appeared.The structure ofthe intestinalmicrovilliwas affected by the changes in intestinalcellultrastructure in C.vidua.All intestinal microvilli of C.vidua were neatly arranged in the control group (Fig.3A).Some were deformed in organisms exposed to low concentrations(Fig.2D);and some began to collapse,disintegrate and appear vacuolated when the concentration of Cd2+was high (Fig.3B,E–G).
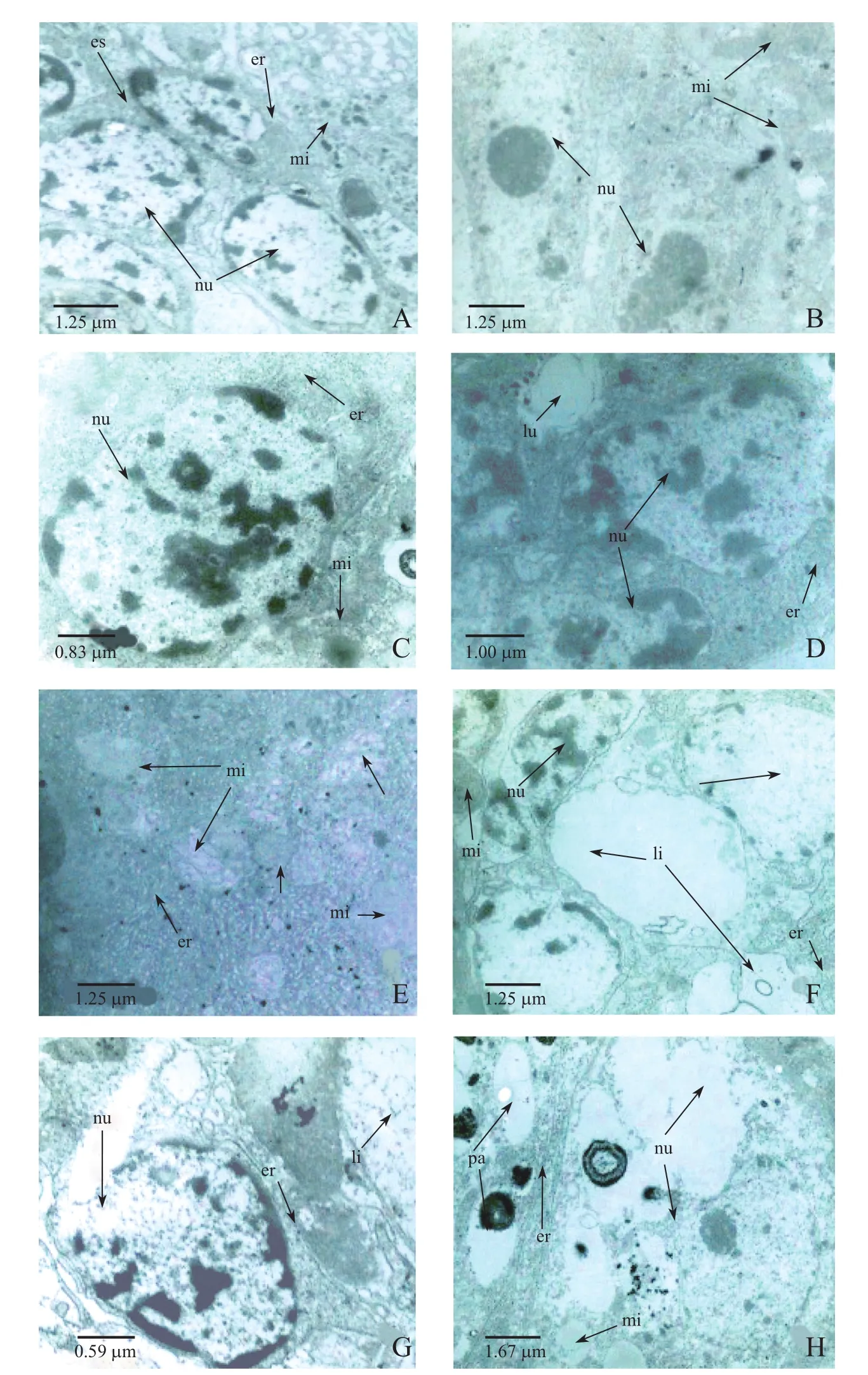
Fig.2 Electron micrographs of intestinal cells from C.vidua exposed to no Cd2+(control)or low Cd2+(0.004 mg/L)
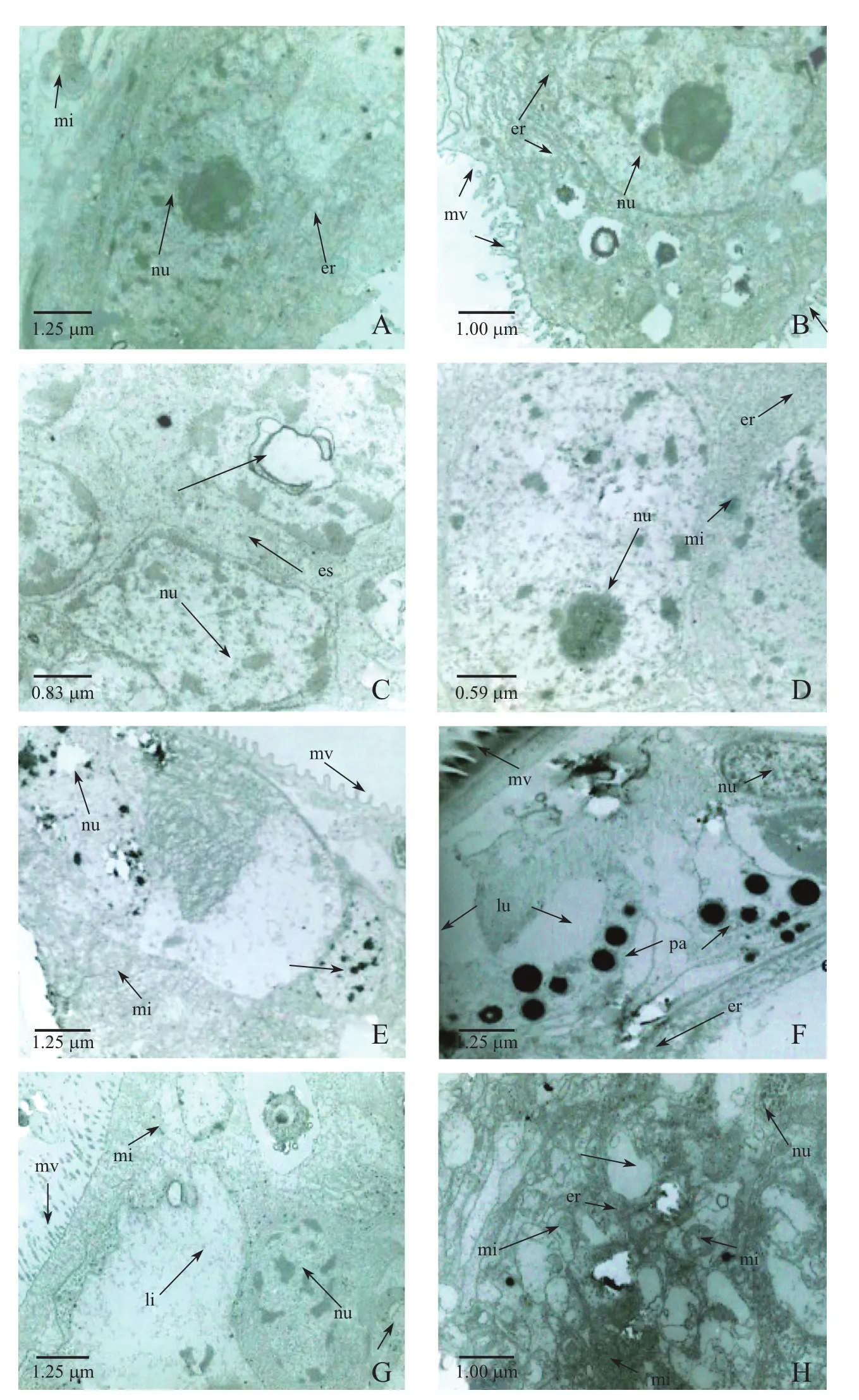
Fig.3 Electron micrographs of intestinal cells from C.vidua exposed to no Cd2+(control)or high Cd2+(0.064 mg/L)
The biological damages caused by Cd2+pollution include:1)excessive reactive oxygen species(ROS)production in the organism;and 2)the metal–dependent enzymes,specifically the metalcofactor ofantioxidant enzyme,might be replaced by combination of Cd2+and intracellular thiolgroups,or through competition or non–competitive effect,which reduces the elimination of free radicals by reducing the activity of antioxidant enzymes.For example,because of the affi nity of Cd2+with thiol,carboxyl and nitrogen is higher than Zn2+and other trace elements,Cd2+can damage enzyme systems which require activation by these trace elements, and make intracellular zinc–containing enzymes useless,reducing the activity of intracellular antioxidant enzymes[58-63]and enhancing the rate of lipid peroxidation.Both of these eff ects lead to the accumulation of lipid peroxides,which then damage the structure of cellular membranes,altering their permeability,and ultimately leading to the decomposition of organelle membrane system[58-59].The results showed that the rough endoplasmic reticulum changed suffi ciently to inhibit the protein synthesis in two Cd2+concentration treatment groups,and the mitochondria were damaged suffi ciently to aff ect oxidative phosphorylation,and the nuclei were damaged enough to aff ect cell integrity and even kill the organism[64].In addition,some researchers reported that the mitochondria was an intracellular organelles which was the most vulnerable to be damaged[65-66],and the view was agreed by the changes ofintestinalultrastructure in this study.In this study,the mitochondrial membrane was severely damaged and even became vacuolated with increasing Cd2+concentrations and prolonged exposure time.Cd2+inhibits the activity ofenzymes that synthesize membrane phospholipids,which lead to damage of the structure of the membrane system,resulting in swelling and eventual disintegration of the organelles[67-68].As the mitochondria supply energy for necessary functions,mitochondrial injury directly impairs the oxidation-antioxidation balance of tissue cells[63,69].
3 Conclusions
The results in this study indicated that C.vidua has a high tolerance to cadmium.And different degrees of structure damage were found in the cell membrane,cytoplasm,and organelles of C.vidua,which worsened with increasing Cd2+concentrations.Among these cellular structures,the damage to the membrane system of the cell was especially serious.These intimated that cadmium could damage or lead to death to the aquatic organisms.The study may provide a theoretical basis for water quality assessment using aquatic organisms.
[1]STANKOVIC S,JOVIC M,STANKOVIC A R,et al.Heavy metals in seafood mussels.Risks for human health. [M]//LICHTFOUSE E,SCHWARZBAUER J,ROBERT D,Environmental chemistry for a sustainable world: Volume 1-Nanotechnology and Health Risk.Netherlands:Springer,2012:311-373.
[2]YANG J,LEWANDROWSKI K B.Trace elements,vitamins,and nutrition[M]//MCCLATCHEY K D.Clinical Laboratory Medicine.2nd ed.Philadelphia:Lippincott Williams and Wilkins,2002:439-462.
[3]GO Y M,ROEDE J R,ORR M,et al.Integrated redox proteomics and metabolomics of mitochondria to identify mechanisms of Cd Toxicity[J].Toxicological Sciences,2014,139(1):59-73.
[4]ZHOU Q,KONG F,ZHU L.Ecotoxicology[M].Beijing:Science Press,2004:56-58.
[5]OMER S A,ELOBEID M A,FOUAD D,et al.Cadmium bioaccumulation and toxicity in tilapia fi sh(Oreochromis niloticus)[J].Journal of Animal and Veterinary Advances,2012,11(10):1601-1606.
[6]IARC(International Agency for Research on Cancer).Beryllium,Cadmium,Mercury,and Exposures in the Glass Manufacturing Industry[J].IARC monographs on the evaluation of carcinogenic risks to humans,1993, 58:41-117.
[7]LIU G,SHENG Z,WANG Y,et al.Glutathione peroxidase 1 expression,malondialdehyde levels and histological alterations in the liver of Acrossocheilus fasciatus exposed to cadmium chloride[J].Gene,2016,578(2):210-218.
[8]DEFUR P L.Use and role of invertebrate models in endocrine disruptor research and testing[J].National Research Council,Institute of Laboratory Animal Resources,2004,45(4):484-493.
[9]MIRTO S,DANOVARO R.Meiofaunal colonisation on artifi cial substrates:A tool for biomonitoring the environmental quality on coastal marine systems[J].Marine Pollution Bulletin,2004,48(9-10):919-926.
[10]SUTHERLAND T F,LEVINGS C D,PETERSEN S A,et al.The use of meiofauna as an indicator of benthic organic enrichment associated with salmonid aquaculture[J].Marine Pollution Bulletin,2007,54(8):1249-1261.
[11]ZHANG Q,HU G.Applications of meiobenthos in marine ecological monitoring[J].Marine Information,2008, 4:28-29.
[12]RUIZ F,GONZ´ALEZ-REGALADO ML,BORREGO J,et al.Ostracoda and Foraminifera as short–term tracers of environmental changes in very polluted areas:The Odiel Estuary(SWSpain)[J].Environment Pollution,2004, 129(1):49-61.
[13]YU N,CHEN S,LI E,et al.Tolerance of Physocypria kraepelini(Crustacean,Ostracoda)to water–borne ammonia,phosphate and pH value[J].Journal of Environmet Science,2009,21:1575-1580.
[14]YU N,CHEN L,ZHAO Q.CCA of ostracod distribution and environmental factors in the Taihu Lake[J].Acta Micropalaeontologica Sinica,2007,24:53-56.
[15]RUIZ F,ABAD M,BODERGAT A M,et al.Freshwater ostracods as environmental tracers[J].International Journal of Environmental Science and Technology,2013(10):1115-1128.
[16]K¨ULK¨OYL¨UO˘GLU O,SARI N,D¨UGEL M,et al.Eff ects of limnoecological changes on the Ostracoda(Crustacea)community in a shallow lake(Lake C¸ubuk,Turkey)[J].Limnologica-Ecology and Management of Inland Waters,2014,46:99-108.
[17]WEI C,YU N,ZHAO Q,et al.Canonical correspondence analysis of modern Ostracoda and environmental factors in the Dishui Lake,Shanghai[J].Acta Micropalaeontological Sinica,2015,32(2):115-124.
[18]K¨ULK¨OYL¨UO˘GLU O.Ecology of freshwater Ostracoda(Crustacea)from lakes and reservoirs in Bolu,Turkey [J].Journal of Freshwater Eecology,2003,18(3):343-347.
[19]PIERI V,VANDEKERKHOVE J,GOI D.Ostracoda(Crustacea)as indicators for surface water quality:A case study from the Ledra River Basin(NE Italy)[J].Hydrobiologia,2012,688:25-35.
[20]K¨ULK¨OYL¨UO˘GLU O,SARI N.Ecological characteristics of the freshwater Ostracoda in Bolu Region(Turkey) [J].Hydrobiologia,2012,688:37-46.
[21]LORENSCHATJ,P´EREZ L,CORREA-METRIO A,et al.Diversity and spatial distribution of extant freshwater ostracodes(Crustacea)in ancient Lake Ohrid(Macedonia/Albania)[J].Diversity,2014,6(3):524-550.
[22]SCHNEIDER A,WETTERICH S,SCHIRRMEISTER L,et al.Freshwater ostracods(Crustacea)and environmental variability of polygon ponds in the tundra of the Indigirka Lowland,north-east Siberia[J].Polar Research,2016,35.DOI:10.3402/polar.v35.25225.
[23]KHANGAROT BS,RAY PK.Sensitivity of midge larvae of Chironomus tentans Fabricius(Diptera:Chironomidae)to heavy metals[J].Bulletin of Environment Contamination and Toxicololgy,1989,42(3):325-330.
[24]BERGIN F,KUCUKSEZGIN F,ULUTURHAN E,et al.The response of benthic Foraminifera and Ostracoda to heavy metal pollution in Gulf of Izmir(Eastern Aegean Sea)[J].Estuarine,Coastal and Shelf Science,2006, 66(3-4):368-386.
[25]RATHORE RS.Studies on the use of some freshwater invertebrates as sensitive test models for the assessment of toxicity of environmental pollutants[D].Lucknow:University of Lucknow,2001:1-196.
[26]BELGIS Z C,PERSOONE G,BLAISE C.Cyst–based toxicity testsⅩⅤⅠ-sensitivity comparision of the solid phase Heterocypris incongruens microbiotest with the Hyalella azteca and Chironomus riparius contact assays on freshwater sediments from Peninsula Harbour(Ontario,Canada)[J].Chemosphere,2003,52(1):95-101.
[27]S´ANCHEZ-BAYO F.From simple toxicological models to prediction of toxic eff ects in time[J].Ecotoxicology, 2009,18:343-354.
[28]BAK M,SZLAUER-LUKASZEWSKA A.Bioindicative potential of diatoms and ostracods in the Odra mouth environment quality assessment[J].Nova Hedwigia,Beiheft,2012,141(3):463-484.
[29]KHANGAROT BS,RAY PK.Response of a freshwater ostracod(Cypris subglobosa Sowerby)exposed to Copper at diff erent pH levels[J].Acta Hydrochimica et Hydrobiologica,1987,15(6):553-558.
[30]CHEN S,YU N,ZHOU Y,et al.Acute toxicity experiment of Cd2+,Zn2+and Cu2+in Physocypria kraepelini (Ostracoda)[J].Acta Micropalaeontologica Sinica,2010,27(2):118-124.
[31]DU N.Crustacean[M].Beijing:Science and Technology Press,1993:137-158.
[32]LIM R P,WONG M C.The eff ects of pesticides on the population dynamics and production of Stenocypris major Baird(Ostracoda)in ricefi elds[J].Archiv f¨ur Hydrobiologie,1986,106:421-427.
[33]KISS A.Limnological investigations of small water bodies in the Pilis Biosphere Reserve,Hungary.Part II. K¨oegyi-t´o and Unk´as-t´ocsa[J].Opuscula Zoologica(Budapest),2001,33:67-74.
[34]SHORNIKOV E I,TREBUKHOVA Y A.Ostracods of brackish and fresh waters of southwestern coast of Peter the Great Bay[M]//KASYANOV V L,VASCHENKO M A,PITRUK D L,The state of environment and biota of the southwestern part of Peter the Great Bay and the Tumen River mouth.Vladivostok:Dalnauka,2001: 56-84.
[35]K¨ULK¨OYL¨UO˘GLU O.On the usage of ostracods(Crustacea)as bioindicator species in diff erent aquatic habitats in the Bolu region,Turkey[J].Ecological Indicators,2004,4(2):139-147.
[36]K¨ULK¨OYL¨UO˘GLUO.Ecology and phenology of freshwater ostracods in Lake G¨olk¨oy(Bolu,Turkey)[J].Aquatic Ecology,2005,39(3):295-304.
[37]K¨ULK¨OYL¨UO˘GLU O,D¨UGEL M,KILIC¸M.Ecological requirements of Ostracoda(Crustacea)in a heavily polluted shallow lake,Lake Yeni¸ca˘ga(Bolu,Turkey)[J].Hydrobiologia,2007,585(1):119-133.
[38]ROCA J R,BALTANAS A,UIBLEIN F.Adaptive responses in Cypridopsis vidua(Crustacea:Ostracoda)to food and shelter off ered by a macrophyte(Chara fragilis)[J].Hydrobiologia,1993,262(2):127-131.
[39]CYWINSKA A,CRUMP D,LEAN D.Infl uence of UV radiation on four freshwater invertebrates[J].Photochemistry and Photobiology,2000,72(5):652-659.
[40]CYWINSKA A,HEBERT P D N.Origins of clonal diversity in the hypervariable asexual ostracode Cypridopsis vidua[J].Journal of Evolutionary Biology,2002,15(1):134-145.
[41]HUNT G,PARK L E,LABARBERA M.A novel crustacean swimming stroke:coordinated four–paddled locomotion in the cypridoidean ostracode Cypridopsis vidua(M¨uller)[J].Biological Bulletin,2007,212(1):267-273.
[42]ARNAUD J,BRUNET M,MAZZA J.Studies on the midgut of Centropages typicus(Copepod,Calanoida).Ⅰ. Structural and Ultrastructural Data[J].Cell and Tissue Research,1978,187(2):333-353.
[43]REYNOLDS E S.The use of lead citrate at high pH as an electron opaque stain in electron microscopy[J]. Journal of Cell Biology,1963,17:208-212.
[44]HUI X.Environmental Toxicology[M].Beijing:Chemical Industry Publishing House,2003,266-276.
[45]REISH D L,OSHIDA P S.Manual of methods in aquatic environment research,part 10:short–term static bioassays[J].FAO Fisheries Technical Paper,1987,247:1-62.
[46]SPRAGUE J B.Measurement of pollutant toxicity to fi sh-III:Sublethal eff ects and“safe”concentrations[J]. Water Research,1971,5(6):245-266.
[47]BROOKS A,WHITE R M,PATON D C.Eff ects of heavy metals on the survival of Diacypris compacta(Herbst) (Ostracoda)from the Coorong,South Australia[J].International Journal of Salt Lake Research,1995,4(2):133-163.
[48]VARDIA H K,RAO P S,DURVE V S.Eff ect of copper,cadmium and zinc on fi sh-food organisms,Daphnia lumholtzi and Cypris subglobosa[J].Proceedings:Animal Sciences,1988,97(2):175-180.
[49]SHUHAIMI-OTHMAN M,NADZIFAH Y,NUR-AMALINA R et al.Toxicity of metals to a freshwater ostracod: Stenocypris major[J].Journal of Toxicology,2011,(3):1-8.
[50]YILMAZ F,K¨ULK¨OYL¨UO˘GLU O.Tolerance,optimum ranges,and ecological requirements of freshwater Ostracoda(Crustacea)in Lake Alada˘g(Bolu,Turkey)[J].Ecological Research,2006,21(2):165-173.
[51]SAIPAN P,TENGJAROENKUL B,PRAHKARNKAEO K.Accumulation of Arsenic and Cadmium in foods of animal origin collected from the local markets in northeastern region Thailand[J].International Journal of Animal&Veterinary Advances,2014,6(4):130-134.
[52]MENKE A,MUNTNER P,SILBERGELD E K,et al.Cadmium levels in urine and mortality among U.S.adults [J].Environmental Health Perspectives,2009,117(2):190-196.
[53]BERNHOFT R A.Cadmium Toxicity and Treatment[J].The Scientifi c World Journal,2013(7):66-67.
[54]TAO S,LIANG T,CAO J,et al.Synergistic eff ect of copper and lead uptake by fi sh[J].Ecotoxicology and Environmental Safety,1999,44(2):190-195.
[55]ZHOU X,ZHU G,SUN J,et al.Toxicity of copper,zinc,lead,cadmium to tissue’s cellular DNA of the fi sh (Carassius auratus)[J].Acta Agriculture Nucleatae Sinica,2001,15(3):167-173.
[56]ZALUPS R K,AHMAD S.Molecular handling of cadmium in transporting epithelia[J].Toxicology and Applied Pharmacology,2003,186(3):163-188.
[57]LOEBUS J,LEITENMAIER B,MEISSNER D,et al.The major function of a metallothionein from the aquatic fungus Heliscus lugdunensis in cadmium detoxifi cation[J].Journal of Inorganic Biochemistry,2013,127:253-260.
[58]SHUKLA G S,HUSAIN T,SRIVASTAVA R S,et al.Glutathione peroxidase and catalase in livers,kidney, testis and brain regions of rats following cadmium exposure and subsequent withdrawal[J].Industrial Health, 1989,27(2):59-69.
[59]LIU R,LIU Y.Study on relationship between lipid perxidation and inviability of isolated rat hepatocytes caused by Cadmium[J].China Environmental Science,1990,10(3):187-191.
[60]VENUGOPAL N,ROMESH T R S L.Eff ects of cadmium on antioxidant enzyme activities and lipid pemxidation in freshwater fi eld crab barytelphusa guerlni[J].Bulletin of Environment Contamination Toxicology,1997,59: 132-138.
[61]SOEGIANTO A,CHAMANTIER-DAURES M,TRILLES J P,et al.Impact of cadmium on the structure of gills and epipodites of the shrimp Penaeus japonicas(Crustacea:Decapoda)[J].Aquatic Living Resources,1999, 12(1):57-70.
[62]LIU X,ZHOU Z,CHEN L.Eff ect of Cadmium on antioxidant enzyme activities of the juvenile Eniocheir sinensis [J].Marine Sciences,2003,27(8):59-63.
[63]LEE S M,KIM H L,LEE S,et al.Toxicogenomic and signaling pathway analysis of low-dose exposure to cadmium chloride in rat liver[J].Molecular&Cellular Toxicology,2013,9(4):407-413.
[64]YANG Y,JIA X.Joint toxicity of Cu2+,Zn2+,and Cd2+to tadpole of Bufo bufo gargarizans[J].Chinese Journal of Applied and Environmental Biology,2006,12(3):356-359.
[65]GOBE G,CRANE D.Mitochondria,reactive oxygen species and cadmium toxicity in the kidney[J].Toxicology Letters,2010,198(1):49-55.
[66]LIU D,YAN B,YANG J,et al.Mitochondrial pathway of apoptosis in the hepatopancreas of the freshwater crab Sinopotamon yangtsekiense exposed to cadmium[J].Aquatic Toxicology,2011,105(3-4):394-402.
[67]CASALINO E,CALZARETTI G,SBLANO C,et al.Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium[J].Toxicology,2002,179(1-2):37-50.
[68]LIU D H,WANG M,ZOU J H,et al.Uptake and accumulation of cadmium and some nutrientions by roots and shoots of maize(Zea mays L.)[J].Pakistan Journal of Botany,2006,38(3):701-709.
[69]WANG L,SUN H.Eff ect of cadmium on ultrastructure of myocardial cell of freshwater crab,Sinopotamon yangtsekiense[J].Acta Hydrobiogica Sinica,2002,26(1):8-13.
(责任编辑:张晶)
Cd2+对Cypridopsis vidua(介形纲)肠壁结构的毒性效应
陈仕梅1,李丹妮1,丁晴晴1,禹娜1,2
(1.华东师范大学生命科学学院,上海200240;2.华东师范大学教师教育学院,上海200062)
介形类;Cd2+;急性毒性;肠壁结构
2016-09-12
公益性行业(农业)科研专项项目(201203065-04);国家自然科学基金(31672263,41372365)
陈仕梅,女,硕士研究生,研究方向动物毒理学.
禹娜,女,教授,博士生导师,研究方向为水生动物生态学.E-mail:nyu@bio.ecnu.edu.cn.
1000-5641(2017)04-0168-12
X592
A
10.3969/j.issn.1000-5641.2017.04.015
