木质素增量取食对台湾乳白蚁消化系统的影响
2017-08-02曾文慧钟俊鸿刘炳荣李秋剑
曾文慧,钟俊鸿,刘炳荣,李秋剑
木质素增量取食对台湾乳白蚁消化系统的影响
曾文慧,钟俊鸿,刘炳荣,李秋剑*
(广东省生物资源应用研究所,广东省动物保护与资源利用重点实验室,广东省野生动物保护与利用公共实验室,广州 510260)
木质素代谢是白蚁消化木质纤维素的关键环节之一。本文利用木质素增量喂食台湾乳白蚁CoptotermesformosanusShiraki,通过测定其取食量、体内酚氧化酶含量、纤维素酶活性的变化及后肠微生物群落16s rRNA基因Illumina HiSeq高通量测序比较分析来研究木质素取食对低等白蚁消化系统的影响。结果表明:木质素取食组内源性(前肠/唾液腺)酚氧化酶含量的显著大于大于对照组,内源及外源性纤维素酶活性均小于对照组,尤其BG酶活性显著小于对照组;1%木质素对乳白蚁取食有一定的抑制作用,其后肠细菌多样性及丰度较对照组有所下降,但无显著的组间群落结构差异;Metastat、LDA(LDA Effect Size)及组间T-检验属水平群落差异分析显示,乳白蚁后肠核心菌群(相对丰度>1%)Dysgonomonas属、弧菌属在木质素取食组中相对丰度显著小于对照组,CandidatusAzobacteriodes属相对丰度则显著大于对照组,它们在木质素增量取食引起的细菌群落变化中起关键作用。
台湾乳白蚁;酚氧化酶;纤维素酶;肠道微生物群落;16S rRNA基因高通量测序
台湾乳白蚁CoptotermesformosanusShiraki广泛分布于我国南方地区,是一种偏好以木质纤维素为主食的低等白蚁。木质纤维素是植物细胞壁的核心成分,主要由约50%纤维素、25%半纤维素以及约20%木质素构成。台湾乳白蚁通过以下两个系统来消化木质纤维素:1)前肠/唾液腺及中肠组成的内源性消化系统;2)后肠鞭毛虫以及共生细菌群落组成的外源性消化系统(Nakashima, 2002;Brune, 2014;Karl and Scharf, 2015)。其中,降解纤维素酶主要包括以下几种:内切葡聚糖酶endo-β-1,4-glucanase(EC.3.2.1.4,简称EG),纤维二糖水解酶β-1,4-cellobiohydrolase(EC.3.2.1.91,简称CBH)以及β-糖苷酶β-glucosidase(EC.3.2.1.21,简称BG)(Zhouetal., 2007)。木质素在低等白蚁肠道中未检测到大量的降解,而主要以苯环支链氧化、甲氧基的去甲基化以及木质素的空间结构重排等修饰形式来促进纤维素、半纤维素与消化酶的接触(Lietal., 2012)。涉及木质素代谢酶类称为木质素酶/酚氧化酶(phenoloxidase,PO),是一种含铜的酶的总称,能够催化单酚羟化成二酚(如多巴),并把二酚氧化成醌(刘丽萍等,2013)。值得引起注意的是,白蚁体内木质素代谢起始于唾液腺,唾液腺中下唇腺所分泌的主要成分为1,4-苯二酚(对苯二酚)的诱食信息素,不仅属于酚氧化酶即木质素酶底物的一种,亦能够促进白蚁聚集,增加其取食量(Reinhardetal., 1997;Reinhard and Kaib, 2001;Reinhardetal., 2002a)。
到目前为止,只在美洲散白蚁ReticulitermesflavipesKollar 转录组序列中克隆并表达出了酚氧化酶活性的虫漆酶基因RflacA以及RflacB(Tartaretal., 2009;Coyetal., 2010;Qiuetal., 2015)。白蚁食物中木质素含量对台湾乳白蚁取食量、消化系统影响还鲜有研究。本实验通过木质素含量的差异取食,一方面,研究由此引起的对酚氧化酶浓度、白蚁取食量、相关纤维素酶活力的响应;另一方面,由于白蚁取食状态是其后肠微生物群落结构的重要影响因子(Mikaelyanetal., 2014;Otanietal., 2014;Rahmanetal., 2015),实验亦对后肠细菌群落结构进行了16S rRNA高通量测序,以此研究木质素取食对后肠细菌群落的影响。
1 材料与方法
1.1 实验白蚁
供试白蚁工蚁取自本实验饲养的五个独立的台湾乳白蚁巢。供试工蚁在28℃,相对湿度70%±5%条件下,以蛭石保湿饥饿72 h均一化肠道、减小原始误差,选择个体大小相近的3-4龄幼虫进行试验。
1.2 主要试剂
本实验选用主要试剂包括:羧甲基纤维素钠(CMC-Na,天津福晨化学试剂),葡萄糖(Glucose,广州化学试剂),滤纸(双圈定性滤纸,直径9 cm),木质素(lignin,Genview),微晶纤维素(microcellulose,Genview),电泳琼脂糖(agarose,Genview),对硝基苯-8-D-纤维二糖苷(p-NPC,Sigma),对硝基苯酚(p-NP,Genview),牛清血蛋白组分V(BSA,Albumin,Fraction V,Genview),3,5-二硝基水杨酸(DNS,Genview),考马斯亮蓝G-250(Coomassie brilliant blue G-250,Genview)。
1.3 实验方法
1.3.1 乳白蚁的诱导喂养
(1)取肠道均一化后的五巢白蚁均分别放置于铺有双蒸水或1%木质素湿润的双层滤纸培养皿中,在28℃,相对湿度70%±5%条件下避光培养12 d,每天补充双蒸水0.5 mL,每皿放入乳白蚁0.40 g,以供取食量分析及以及肠道微生物群落总DNA的提取; (2) 随机选取肠道均一化后的3巢白蚁均分别放置于双蒸水琼脂糖平板(1%微晶纤维素,1% 琼脂糖)及木质素琼脂糖平板(1%微晶纤维素,1%琼脂糖,1%木质素)中,在28℃,相对湿度70%±5%条件下避光培养3 d,每皿放入乳白蚁0.25g以供提取粗酶液。
1.3.2 纤维素酶粗酶液制取
取琼脂糖平板培养乳白蚁,每皿各25头置于0.9%(W/V)灭菌生理盐水中漂洗净,在实体解剖镜下将白蚁肠道解剖,分成前肠/ 唾液腺,表皮以及后肠三部分,转移至醋酸钠缓冲液(SAB,0.1 M,pH5.6)中冰浴研磨;使用冷冻离心机(Sigma,3K15)将匀浆液在12000 rpm,4°C下冷冻离心(Sigma,3K15)15 min,离心两次,取上清即为粗酶液用于昆虫酚氧化酶酶联免疫吸附测定(enzyme linked immunosorbent assay,ELISA);同方法解剖白蚁肠道,分成前肠/唾液腺+中肠与后肠两部分,制取粗酶液用于白蚁纤维素酶活性测定。每组3巢平行。
1.3.3 蛋白质浓度测定
采用考马斯亮蓝法进行测定。将粗酶液按一定的比例进行稀释,取50 μL稀释后粗酶液,加入250 μL考马斯亮蓝G-250显色试剂,多功酶标仪测定OD595,用BSA制作标准曲线求蛋白质含量(Zengetal., 2016)。
1.3.4纤维素酶活力及昆虫酚氧化酶浓度测定
(1)β-1,4-内切葡聚糖酶(EG)以及β-糖苷酶(BG)活力的测定,分别以120 μL 1% CMC与120 μL 1%水杨苷(salicine)为底物,酶液12 μL,37℃反应60 min,采用DNS显色法,使用多功能酶标仪(Victor 3 Multi-label Microplate Reader,Perkin Elmer,美国)在540 nm测定光吸收值以葡萄糖为标准物计算产物还原糖含量。EG/BG酶活力U定义为,每毫克蛋白质在37℃,pH5.6反应条件下分解1% CMC/1%水杨苷,每分钟可以产生还原糖的量,表示为U/mmol(Zengetal., 2016)。(2)纤维二糖水解酶(CBH)活力测定:以120 μL 1mmolpNPC底物,酶液12 μL,37℃反应60 min,加入120 μL Na2CO3(0.6 mol/L)溶液终止反应,使用酶标仪测定OD405,从p-NP标准曲线求得p-NP含量,计算酶活力单位。CBH酶活力U定义为:每毫克蛋白质在37°C,pH 5.6反应条件下分解pNPC,每分钟可以产生p-NP的量,表示为U/mmol(Zengetal., 2016);(3)参照昆虫酚氧化酶(PO)ELISA试剂盒(上海江莱科技)实验手册,采用双抗体一步夹心法,HPP标记检测抗体,底物TMB显色,使用酶标仪测定OD450光吸收值,计算样品酚氧化酶浓度。以双蒸水组为对照组,计算纤维素酶相对活力(%)以及酚氧化酶相对浓度(%)。每组3巢平行。
1.3.5 取食量测定
将蒸馏水与1%木质素组白蚁啃食12 d的滤纸用蒸馏水洗净,放置烘箱中60°C,18 h过夜干燥,称量干燥后的滤纸重量。每组5巢平行。
1.3.6 肠道微生物群落16s RNA测序分析
取滤纸喂养12 d的乳白蚁工蚁,每皿20头置于0.9%(W/V)灭菌生理盐水中漂洗净,去除头胸部,使用TaKaRa MiniBEST Bacteria Genomic DNA Extraction Kit Ver.3.0试剂盒(天津,中国)提取腹部肠道微生物群落总DNA。将达到测序标准的DNA样品依托测序公司(诺禾致源,北京)对肠道细菌16s rRNA 基因V4区域进行PCR扩增、文库构建以、Illumina HiSeq高通量测序及后续数据分析。测序原始数据经过拆分、拼接、过滤、去嵌合体最终得到有效数据。基于有效数据对97%一致性的OTUs(Operational Taxonomic Units )进行聚类和物种分类分析,并将OUTs和物种注释结合,对OTUs进行丰度、Alpha多样性指数等分析,同时对物种注释在各个分类水平上进行群落结构的统计分析、样本间群落结构差异进行比较。以上分析主要通过FLASH V1.2.7、QiimeV1.7.0、R软件、UCHIME Algorithm、Uparse v7.0.1001、RDP Classifier 、Version 2.2 和MUSCLE Version 3.8.31计算程序以及Gold database、GreenGene数据库完成。每组3巢平行。
1.3.7 统计学方法
采用SPSS 17.0(SPSS Inc.Chicago,IL,美国)软件对酶活力、ELISA、取食量的结果差异均采用LSD-t检验(LSD,α=0.05)。所有误差线为均值的标准误差(SEM)。
依托测序公司诺进行了以下组间物种差异统计学分析:MetaStat、LEfSe(LDA Effect Size)以及t检验分析组间微生物群落中显著差异物种,非参数检验ANOSIM (Analysis of similarities)和MRPP(Multi-response permutation procedure)来分析两组白蚁微生物整体群落组成的差异。
2 结果与分析
2.1 木质素取食对台湾乳白蚁滤纸取食量的影响
分别用双蒸水和木质素溶液浸润的滤纸喂食台湾乳白蚁12 d后,取食量统计结果表明,1%木质素与双蒸水对照组乳白蚁对滤纸的取食量分别为0.04±0.003(mg/d·g白蚁)与0.02±0.008(mg/d·g白蚁),1%木质素表现出了对乳白蚁滤纸取食的抑制作用,但无统计学显著性(图1)。
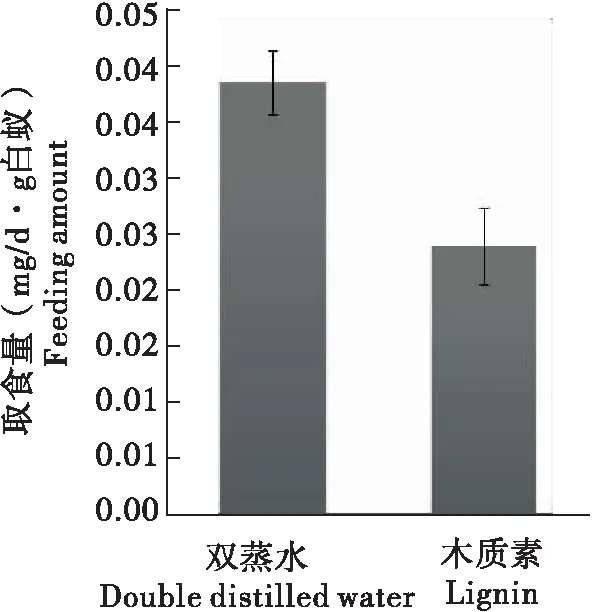
图1 木质素对台湾乳白蚁滤纸取食量的影响Fig.1 Effect of lignin on filter paper feeding amount of Coptotermes formosanus注:数值为平均值±标准误差。Note: Error bars represent standard error of the mean (SEM).
2.2 木质素取食对台湾乳白蚁消化酶的影响
昆虫酚氧化酶的ELISA实验结果表明:1%木质素取食组酚氧化酶浓度在前肠/唾液腺为双蒸水对照组的160.71%±16.71%,显著大于对照组;其后肠酚氧化酶浓度则显著小于对照组,为对照组的58.65%±10.81%;表皮酚氧化酶浓度两组之间未见显著性差异(图2)。
纤维素酶活力测定结果表明:前肠/唾液腺+中肠与后肠的EG、BG及CBH活力均小于对照组。其中,木质素取食组BG活性在两组之间存在显著差异,其前肠/唾液腺+中肠BG酶活性为对照的62.1%±7.1%,后肠为对照的33.8%±7.0%(图3)。
2.3 木质素取食对台湾乳白蚁肠道细菌群落的影响
2.3.1 样品细菌群落丰度及多样性分析
6个乳白蚁后肠微生物群落样品经测序共获得475497条16S rRNA基因V4高变区有效序列,6个样品测序覆盖率均达到99%以上,说明被测序深度足够覆盖样品的核心细菌群落。将序列以97%的相似性归并后得到OUT数量显示,对照组细菌种类大于木质素取食组。其次,对照组菌群丰度指数Shannon、Simpson、Chao及ACE均表明双蒸水对照组菌群丰度和多样性略高于木质素取食组(表1)。
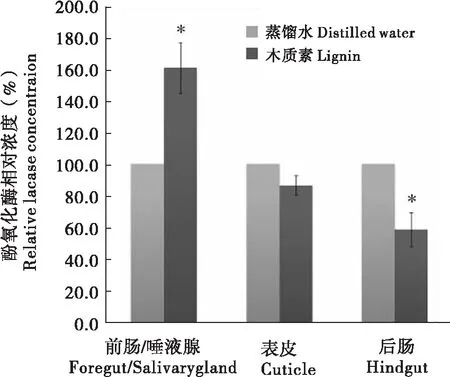
图2 木质素对台湾乳白蚁酚氧化酶浓度的影响Fig.2 Effect of lignin on phenoloxidase concentration of Coptotermes formosanus注:数值为平均值±标准误差,以双蒸水处理组为对照(100%)。*(P<0.05),表示木质素取食组与对照组之间存在显著差异(独立样品t-检验),下同。Note: Error bars represent standard error of the mean (SEM), the double distilled water treated group was the negative control (100%).Data with the “*” (P<0.05) indicate the significance between two groups (independent samples t- test), the same below.
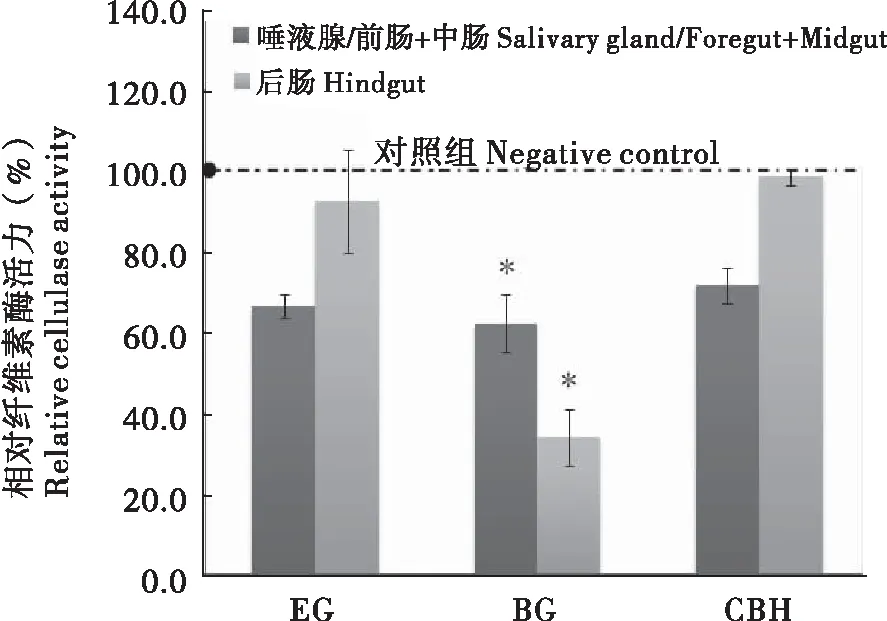
图3 木质素对台湾乳白蚁纤维素酶活力的影响Fig.3 Effect of lignin on cellulase activity of Coptotermes formosanus注:EG、BG与CBH分别为内切葡聚糖酶,β-糖苷酶以及纤维二糖水解酶缩写。Note: The endo-β-1, 4-glucanase, β-1, 4-cellobiohydrolase and β-glucosidase are abbreviated to EG, BG and CBH.

表1 样本细菌群落丰度及多样性指数预测Table 1 The estimated richness and alpha diversity of sampling bacteria community
注:HO为双蒸水处理组,Lig为1%木质素取食组。香浓指数、辛普森指数、赵氏菌种丰富度指数、ACE指数越大表明群落多样性越高。Note: Double distilled treated samples are labeled as HO, 1% lignin feeding samples are labeled as Lig.The higher Shannon, Simpson, Chao 1and ACE index are, the higher richness of the sample is.
2.3.2 细菌群落组成整体结构比较
乳白蚁后肠细菌群落结构组间差异Anosim(表2)及MRPP(表3)统计分析均表明,双蒸水对照组与木质素取食组的组内摆动性小于组间差异,但组间群落结构均未表现统计学显著差异。
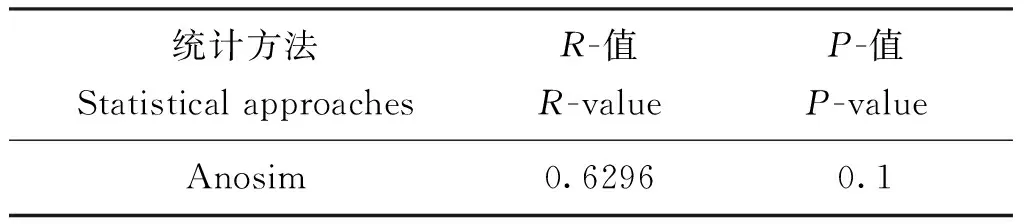
表2 微生物群落结构组间差异Anosim统计学分析Table 2 Anosim difference analysis of microbial community constructure between groups
注:R-value介于(-1,1)之间,R-value大于0,说明组间差异显著,R-value小于0,说明组内差异大于组间差异,统计分析的可信度用P-value 表示,P小于0.05表示统计具有显著性。Note:R-value ranges from-1 to 1, with a value greater than 0 indicate significant with-group difference.R-value less than 0 indicate the within-group difference is greater than the with-group difference.P-value less than 0.05 indicate the statistical significant.
2.3.3 组间显著差异细菌群落统计分析
以属水平为目标,分析具有组间显著差异的细菌群落。Metastat分析结果显示,以群落相对百分含量大于0.5%为丰度阈值,对照组Dysgonomonas属及弧菌属相对丰度显著大于木质素组(表4);LDA分析(显著差异阈值LDA值大于4)结果亦显示,Dysgonomonas属在照组中的丰度显著大于木质素取食组,CandidatusAzobacteriodes属则显著小于木质素取食组(图4);组间属水平T-检验分析(P<0.05)结果显示,对照组、弧菌属、耶尔森菌属及西瓦式菌属相对丰度显著大于木质素组(图5)。
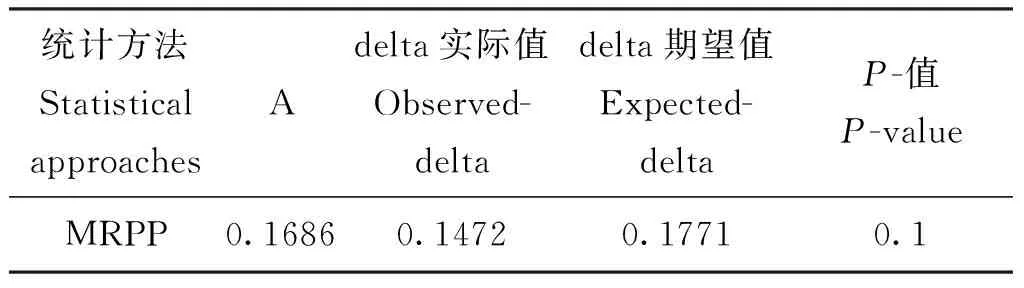
表3 微生物群落结构组间差异MRPP统计学分析Table 3 MRPP difference analysis of microbial community constructure between groups
注:Observe delta值越小说明组内差异小,Expect delta值越大说明组间差异大。A值大于0说明组间差异大于组内差异,A值小于0说明组内差异大于组间差异。P-值小于0.05说明差异显著。Note: Delta is the weighted mean within-group distance.The A value is the chance-corrected with-group agreement, ranging from 0 to 1, with higher value indicating higher with-group homogeneity.P-values less than 0.05 are deemed statistically significant.
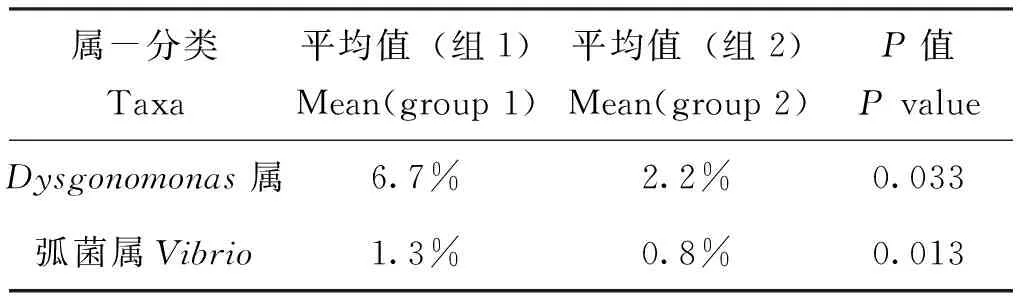
表4 组间属水平Metastat分析Table 4 Metastat analysis between groups in genus level
注:平均值表示该属在样品属水平的相对丰度,筛选阈值为P<0.05,属水平丰度大于0.5%。组1为蒸馏水对照组,组2为木质素取食组。Note: The mean of group indicates the relative richness of this genus, the screening threshold arePvalue less than 0.05 and relative richness greater than 0.5%.Group 1 is negative control (distilled water), group 2 is lignin feeding group.

图4 组间属水平LEfSe分析Fig.4 LDA Effect Size analysis between groups in genus level注:HO、Lig分别表示蒸馏水对照组与木质素取食组。显著差异阈值LDA值大于4。Note: Distilled water control and lignin feeding group are labeled with HO and Lig, respectively.LDA value greater than 4 is the screening threshold.

图5 组间属水平T-检验分析Fig.5 T- test analysis between groups in genus level注:HO、Lig分别表示蒸馏水对照组与木质素取食组。差异显著阈值,P<0.05。Note: Distilled water control and lignin feeding group are labeled with HO and Lig, respectively.P value less than 0.05 is the screening threshold.
3 结论与讨论
台湾乳白蚁不仅是我国南方地区森林及城市中分布最广泛及破坏力最为强大的白蚁种类,因其对木质纤维素的高效降解能力,亦使其成为研究食木白蚁木质纤维素降解系统的模式白蚁种类。天然木质纤维素的重要组成部分—木质素氧化分解相关的酚氧化酶可能涉及白蚁取食、免疫等多种生理功能(Reinhardetal., 2002a;庞秋香,2008)。
木质素是一种分子结构中含有氧代苯丙醇或其衍生物结构单元的芳香性高聚物。低等白蚁木质素的降解起始于唾液腺,并在进入后肠之前即完成了一系列苯环氧化修饰,解聚化等修饰过程(Sharyetal., 2007;Keetal., 2010;Lietal., 2012)。本研究中1%木质素取食诱导了台湾乳白蚁唾液腺/前肠中酚氧化酶浓度显著高于对照组,后肠酚氧化酶浓度则显著小于对照组。目前发现的白蚁酚氧化酶均来源于唾液腺分泌(Reinhardetal., 2002a,2002b),因此酚氧化酶上游底物木质素含量的增加自然促进了唾液腺酚氧化酶的分泌。唾液腺中的诱食信息素(phagostimulating pheromone)对苯二酚亦为酚氧化酶的底物,被认为来源于食物,但所需浓度极低;唾液腺中多余的对苯二酚以熊果苷形式储存,并被BG酶所诱导释放(Itakuraetal., 1997;Coyetal., 2010;Dittmer and Kanost, 2010)。本研究中,内源性(前肠/唾液腺+中肠)与外源性(后肠)纤维素酶BG与CBH活性均显著小于对照组。唾液腺中BG酶活力的下降可能导致白蚁自身释放的对苯二酚量的下降。由于木质素的代谢副产物,以及包括对苯二酚在内的多种单酚/多酚类物质能够被酚氧化酶催化形成生理毒性苯醌类物质(Keetal., 2011)。因此BG酶分泌的减少可能是白蚁自身对苯醌类毒理物质的代谢应激,同理亦导致了木质素取食组的取食量下降。取食是白蚁后肠微生物群落丰度与结构的重要影响因子之一,取食量下降直接会影响后肠微生物的数量与种类(Otanietal., 2014;Mikaelyanetal., 2015)。肠道细菌群落16s rRNA高通量测序分析结果显示,木质素取食组后肠细菌群落丰度及多样性小于蒸馏水对照组,而乳白蚁后肠共生微生物是其木质纤维素代谢酶类的主要提供者(Brune and Dietrich, 2015),这则可能是导致后肠3种纤维素酶活性出现了不同程度下降的原因。
乳白蚁后肠的细菌及古细菌承担了聚合物降解、氢代谢、氧气合成、等糖酵解等代谢功能为共生鞭毛虫及白蚁提供了生存所需能量,并通过固氮等作用合成大量营养成份及活性物质(Brune and Dietrich, 2015)。综合Metastat、LDA及组间T-检验在属水平的细菌群落差异分析结果,CandidatusAzobacteriodes属、Dysgonomonas属、弧菌属、耶尔森菌属及西瓦式菌属为木质素取食组与蒸馏水对照取食组肠道微生物群落差异的标记性菌属。拟杆菌门(Bacteroides)是乳白蚁后肠绝对优势菌群,通常能占到整个肠道细菌量的75%以上。而CandidatusAzobacteriodes与Dysgonomonas则为拟杆菌门核心菌群(Nodaetal., 2005; Inoueetal., 2015)本研究中,CandidatusAzobacteriodes属在蒸馏水对照组的相对丰度(55.7%)显著小于在木质素取食组中的相对丰度(66.8%)。CandidatusAzobacteroides属不仅是台湾乳白蚁最重要的固氮酶者亦能够合成大量的氨基酸以及各种营养因子(Hongohetal., 2008;Brune, 2014),并且在其基因组中发现了大量糖基水解酶基因(Brune and Dietrich, 2015)。Dysgonomonas属亦具有固氮及营养因子合成能力(Hussenederetal., 2009;Inoueetal., 2015;Pramonoetal., 2015),它在蒸馏水对照组中的相对丰度(6.7%)显著大于在木质素取食组中的相对丰度(2.2%)。但是到目前为止,只在后肠共生密螺旋体属Treponema中发现了苯环降解相关基因(Lucey and Leadbetter, 2014)。本实验中两组白蚁后肠密螺旋体属细菌的丰度并无显著差异。因此木质素在经过前肠/唾液腺、中肠的支链修饰后,在后肠是如何影响CandidatusAzobacteriodes与Dysgonomonas等核心菌群的丰度还需要进一步的研究。弧菌属与耶尔森菌属均属于变形菌门Proteobacteria,对pH值非常敏感(Nackeetal., 2011;Makondeetal., 2015),但木质素取食是否会引起肠道内pH值的变化还未有研究。
综上所述,本研究通过乳白蚁木质素含量差异取食实验,结果表明木质素增量取食可以引起内源性(前肠/唾液腺)酚氧化酶含量的显著增加,内源及外源性纤维素酶活性的下降,特别是BG酶的大幅度下降;1%木质素对乳白蚁取食有一定的抑制作用,同时伴随后肠细菌群落丰度与多样性的下降;乳白蚁后肠核心菌群(相对丰度>1%)CandidatusAzobacteriodes、Dysgonomonas以及弧菌属的相对丰度在木质素含量差异取食中出现了显著差异,它们对白蚁取食中木质素含量改变引起的细菌群落变化起关键的作用。
References)
Brune A.Symbiotic digestion of lignocellulose in termite guts [J].NatureReviewsMicrobiology, 2014, 12 (3): 168-180.
Brune A, Dietrich C.The gut microbiota of termites: Digesting the diversity in the light of ecology and evolution [J].AnnualReviewofMicrobiology, 2015, 6 9(1): 145-166.
Coy MR, Salem TZ, Denton JS,etal.Phenol-oxidizing laccases from the termite gut [J].InsectBiochemistryandMolecularBiology, 2010, 40 (10): 723-732.
Dittmer NT, Kanost MR.Insect multcopper oxidases: Diversity, properties, and physiological roles [J].InsectBiochemistryandMolecularBiology, 2010, 40 (3): 179-188.
Hongoh Y, Sharma VK, Prakash T,etal.Genome of an endosymbiont coupling N (2) fixation to cellulolysis within protist cells in termite gut [J].Science, 2008, 322 (5904): 1108-1109.
Husseneder C, Berestecky JM, Grace JK.Changes in composition of culturable bacteria community in the gut of the Formosan subterranean termite depending on rearing conditions of the host [J].AnnalsoftheEntomologicalSocietyofAmerica, 2009, 102 (3): 498-507.
Inoue J-i, Oshima K, Suda W,etal.Distribution and evolution of nitrogen fixation genes in the Phylum Bacteroidetes [J].MicrobesandEnvironments, 2015, 30 (1): 44-50.
Itakura S, Matsumura M, Tanaka H,etal.Evidence of occurrences of pyruvate dehydrogenase complexes in the gut-free bodies of termitesCoptotermesformosanus[J].MokuzaiGakkaishi, 1997, 43 (9): 800-802.
Karl ZJ, Scharf ME.Effects of five diverse lignocellulosic diets on digestive enzyme biochemistry in the termiteReticulitermesflavipes[J].ArchivesofInsectBiochemistryandPhysiology, 2015, 90 (2): 89-103.
Ke J, Laskar DD, Singh D,etal.In situ lignocellulosic unlocking mechanism for carbohydrate hydrolysis in termites: crucial lignin modification [J].BiotechnologyforBiofuels, 2011, 4 (1): 1-12.
Ke J, Sun JZ, Nguyen HD,etal.In-situ oxygen profiling and lignin modification in guts of wood-feeding termites [J].InsectScience, 2010, 17 (3): 277-290.
Li HJ, Lu JR, Mo JC.Physiochemical lignocellulose modification by the formosan subterranean termiteCoptotermesFormosanusShiraki (Isoptera: Rhinotermitidae) and its potential uses in the production of biofuels [J].Bioresources, 2012, 7 (1): 675-685.
Liu LP,Xi GS,Wang F,etal.Research progress of phenoloxidase in insects [J].ChineseBulletinofLifeSciences, 2013, 25 (4): 383-287.[刘丽萍, 奚耕思, 王芳等.昆虫酚氧化酶的研究进展[J].生命科学, 2013, 25 (4): 383-287]
Lucey KS, Leadbetter JR.Catechol 2,3-dioxygenase and other meta-cleavage catabolic pathway genes in the ‘anaerobic’ termite gut spirocheteTreponemaprimitia[J].MolecularEcology, 2014, 23 (6): 1531-1543.
Makonde HM, Mwirichia R, Osiemo Z,etal.454 Pyrosequencing-based assessment of bacterial diversity and community structure in termite guts, mounds and surrounding soils [J].SpringerPlus, 2015, 4 (1): 471.
Mikaelyan A, Dietrich C, Kohler T,etal.Diet is the primary determinant of bacterial community structure in the guts of higher termites [J].MolecularEcology, 2015, 24 (20): 5284-5295.
Mikaelyan A, Strassert JFH, Tokuda G,etal.The fibre-associated cellulolytic bacterial community in the hindgut of wood-feeding higher termites (Nasutitermesspp.) [J].EnvironmentalMicrobiology, 2014, 16 (9): 2711-2722.
Nacke H, Thurmer A, Wollherr A,etal.Pyrosequencing-Based assessment of bacterial community structure Aalong different management types in German forest and grassland soils [J].PLoSONE, 2012, 6 (6): e17000.
Noda S, Iida T, Kitade O,etal.Endosymbiotic bacteroidales bacteria of the flagellated protistPseudotrichonymphagrassiiin the gut of the termiteCoptotermesformosanus[J].AppliedandEnvironmentalMicrobiology, 2005, 71 (12): 8811-8817.
Otani S, Mikaelyan A, Nobre T,etal.Identifying the core microbial community in the gut of fungus-growing termites [J].MolecularEcology, 2014, 23 (18): 4631-4644.
Pang QX, Pang SX, Zhao FS.Research progress of biochemical characteristics and molecular biology of phenoloxidase and prophenoloxidase [J].ProgressinModernBiomedicine, 2008, 8 (1): 196-200.[庞秋香, 庞书香, 赵博生.酚氧化酶及其酶原的生化特征与分子生物学研究进展[J].现代生物医学进展, 2008, 8 (1): 196-200]
Pramono AK, Sakamoto M, Iino T,etal.Dysgonomonas termitidis sp.nov., isolated from the gut of the subterranean termiteReticulitermessperatus[J].InternationalJournalofSystematicandEvolutionaryMicrobiology, 2015, 65 (2): 681-685.
Qiu H, Geng A, Zhu D,etal.Purification and characterization of a hemocyanin (Hemo1) with potential lignin-modification activities from the wood-feeding termite,Coptotermesformosanus Shiraki [J].AppliedBiochemistryandBiotechnology, 2015, 175 (2): 687-697.
Rahman NA, Parks DH, Willner DL,etal.A molecular survey of Australian and North American termite genera indicates that vertical inheritance is the primary force shaping termite gut microbiomes [J].Microbiome, 2015, 3.
Reinhard J, Hertel H, Kaib M.Feeding stimulating signal in labial gland secretion of the subterranean termiteReticulitermessantonensis[J].JournalofChemicalEcology, 1997, 23 (10): 2371-2381.
Reinhard J, Kaib M.Thin-layer chromatography assessing feeding stimulation by labial gland secretion compared to synthetic chemicals in the subterranean termiteReticulitermessantonensis[J].JournalofChemicalEcology, 2001, 27 (1): 175-187.
Reinhard J, Lacey MJ, Ibarra F,etal.Hydroquinone: A general phagostimulating pheromone in termites [J].JournalofChemicalEcology, 2002a, 28 (1): 1-14.
Reinhard J, Lacey MJ, Lenz M.Application of the natural phagostimulant hydroquinone in bait systems for termite management (Isoptera) [J].Sociobiology, 2002b, 39 (2): 213-229.
Shary S, Ralph SA, Hammel KE.New insights into the ligninolytic capability of a wood decay ascomycete [J].AppliedandEnvironmentalMicrobiology, 2007, 73 (20): 6691-6694.
Tartar A, Wheeler MM, Zhou X,etal.Parallel metatranscriptome analyses of host and symbiont gene expression in the gut of the termiteReticulitermesflavipes[J].BiotechnologyforBiofuels, 2009, 2 (1): 25.
Zeng WH, Li ZQ, Liu BR,etal.Correlation between rearing temperature and the dual cellulolytic system ofCoptotermesformosanusShiraki and its intestinal microenvironment [J].JournalofAsia-PacificEntomology, 2016, 19 (1): 209-215.
Zhou X, Smith JA, Oi FM,etal.Correlation of cellulase gene expression and cellulolytic activity throughout the gut of the termiteReticulitermesflavipes[J].Gene, 2007, 395 (1-2): 29-39.
The effect of incremental lignin feeding on digestive system ofCoptotermesformosanusShiraki
ZENG Wen-Hui, ZHONG Jun-Hong, LIU Bing-Rong, LI Qiu-Jian*
(Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Laboratory of Wild Animal Conservation and Utilization, Guangdong Institute of Applied Biological Resources, Guangzhou 510260, China)
The lignin metabolism is one of the key steps in lignocelluloses digestion of termites.The effect of incremental lignin feeding on the digestive system of lower termiteCoptotermesformosanusShiraki was investigated by comparative assays of the feeding amount, the phenoloxidase concentration, the cellulase activity and the 16s rRNA gene Illumina HiSeq sequencing of symbiotic bacteria community.The results showed that the concentration of the endogenous (foregut/salivary gland) phenoxidase was significantly higher in lignin feeding group; the activities of both the endogenous and exogenous (hindgut) cellulases were lower in lignin feeding group, especially the β-glucosidase activity was significantly lower in lignin feeding group.The 1% (W/V) lignin had feeding inhibition onC.formosanus, and the estimated richness and diversity of bacteria community was comparatively lower in lignin feeding group, but no significant difference was showed in bacteria community structure between groups.The bacteria community difference between groups was statistically analyzed by Metastat、LDA Effect Size and T-test methods.And the result of dominant bacteria population (relative abundance > 1%) in genus level ofC.formosanushindgut suggested that the relative abundances of genusDysgonomonaandVibrowere significantly higher in lignin feeding group; the relative abundance of genusCandidatusAzobacteriodeswas significantly lower in lignin feeding group.These three bacteria genera played key role in symbiotic bacteria community variation induced by incremental lignin feeding.
CoptotermesformosanusShiraki; phenoloxidase; cellulase; intestinal symbiont; 16S rRNA gene Illumina HiSeq;
曾文慧,钟俊鸿,刘炳荣,等.木质素增量取食对台湾乳白蚁消化系统的影响[J].环境昆虫学报,2017,39(3):632-639.
广东省自然科学基金(2015A030313817);广东省科学院科技发展专项(2017GDASCX-0107)
曾文慧,1984年,女,江西人,硕士,助理研究员,研究方向为白蚁生理生化与分子生物学, E-mail: zengwenhuigz@hotmail.com
*通讯作者Author of correspondence,E-mail: Liqj73@aliyun.com
Received: 2016-12-21;接受日期Accepted: 2017-01-18
Q965;S433
A
1674-0858(2017)03-0632-08
