水稻矮化剑叶卷曲突变体dcfl1的鉴定与基因精细定位
2017-06-05张孝波谢佳张晓琼田维江何沛龙刘思岑何光华钟秉强桑贤春
张孝波,谢佳,张晓琼,田维江,何沛龙,刘思岑,何光华,钟秉强,桑贤春
(西南大学水稻研究所/转基因植物与安全控制重庆市重点实验室,重庆 400715)
水稻矮化剑叶卷曲突变体dcfl1的鉴定与基因精细定位
张孝波,谢佳,张晓琼,田维江,何沛龙,刘思岑,何光华,钟秉强,桑贤春
(西南大学水稻研究所/转基因植物与安全控制重庆市重点实验室,重庆 400715)
【目的】对一个水稻矮化剑叶卷曲突变体进行鉴定与基因定位,为水稻等禾谷类作物剑叶形态发育及分子改良奠定基础。【方法】在籼型水稻恢复系缙恢10号的甲基磺酸乙酯(EMS)突变库中筛选到一个隐性矮化剑叶卷曲突变体,命名为dcfl1(dwarf and curled flag leaf 1)。田间小区种植,全生育期内观察dcfl1和野生型的株型变化。苗期利用扫描电镜观察叶鞘内表皮细胞大小;孕穗期和抽穗期利用石蜡切片观察剑叶基部形态;开花期测定剑叶、倒2叶和倒3叶的叶绿素含量;成熟期考查株高、有效穗数、穗实粒数、结实率和千粒重等主要农艺性状。配制西农1A/dcfl1杂交组合,利用F1和F2群体进行遗传分析,并利用F2隐性群体进行基因定位。【结果】生育期内,突变体dcfl1都表现出矮化性状。dcfl1叶鞘内表皮细胞长度明显比野生型要短,达到了极显著水平。与野生型相比,穗长、倒1节间和倒2节间均显著变短,倒3节间和倒4节间无显著变化。抽穗期dcfl1剑叶的叶片和叶鞘连接处硬化,剑叶基部展开受阻,半边叶片向内卷曲,剑叶上部和中部正常,其他叶片也正常。农艺性状调查发现,dcfl1的有效穗数为14.24,极显著高于野生型的11.62,穗粒数、实粒数、结实率和千粒重等则无显著变化。此外,dcfl1的叶色略深,剑叶、倒2叶和倒3叶的叶绿素a含量均极显著高于野生型,类胡萝卜素含量也略有升高,但仅剑叶达到极显著差异水平,叶绿素b的含量则无显著变化。西农1A/dcfl1的F1群体中,株高和剑叶表型与野生型一致。F2群体中分离出正常和突变两种表型,突变表型与dcfl1类似,植株株高变矮,剑叶基部特异卷曲,说明矮化和剑叶基部特异卷曲是一对共分离性状。且两种表型分离比符合3﹕1,表明dcfl1突变型受1对隐性核基因控制。利用620株F2隐性单株,最终将DCFL1精细定位在第3染色体短臂InDel标记Ind03-11和Ind03-6之间78 kb的物理范围内,包含15个注释基因,为DCFL1的克隆和水稻剑叶形态发育机理研究奠定了基础。【结论】dcfl1是一个水稻矮化剑叶基部特异卷曲突变体,基因精细定位在第3染色体78 kb的物理范围内。
水稻;矮化;剑叶卷曲;遗传分析;精细定位
0 引言
【研究意义】叶片形态、大小和颜色直接决定了群体光能利用效率,进而影响作物的产量和品质,受到育种和分子生物学家的广泛关注[1]。剑叶是水稻最重要的功能叶,叶片大小和形态与单株产量、单穗重、穗粒数均呈极显著正相关,同时,剑叶叶型也是水稻理想株型的重要构成部分[2-3]。因此,阐明剑叶发育的分子机理对水稻生产具有重要意义。【前人研究进展】目前,利用突变体已在水稻中克隆了一系列调控叶片大小和形态发育的基因,如窄叶基因 NAL1[4]、NAL2/NAL3[5]、NAL7[6],窄卷叶基因NRL1[7-8],卷叶基因 CFL1[9]、SRL1[10]和 RL14[11]等,为阐释水稻叶片发育的分子机理奠定了基础。然而,这些突变体多表现为阶段性或全生育期所有叶片发育缺陷,剑叶特异异常发育的突变体还鲜有报道。目前普遍认为剑叶发育属于复杂性状,已利用 F2分离群体、双单倍体(DH)、重组自交系(RILs)和单片段代换系(CSSLs)等材料,在水稻12条染色体上定位了系列控制剑叶大小的QTL。YAN等[12]利用IR64/Azucena衍生DH群体在第 4染色体上定位到一个控制剑叶大小的QTL。KOBAYASHI等[13]利用 Milyang23/Akihikari组合衍生的RIL群体(191个家系)定位到7个控制剑叶长度和5个控制剑叶宽度的QTL。近来,BIAN等[14]利用CSSLs群体鉴定了4个控制水稻剑叶宽度、1个控制剑叶面积和 2个控制剑叶角度的 QTL;ZHANG等[3]则利用 RIL群体在海南和杭州两地种植,检测到9个控制剑叶长度和14个控制剑叶宽度的QTL,其中仅7个为先前鉴定的QTL;CHEN等[15]利用 RIL群体也鉴定到 5个控制剑叶叶宽发育的QTL,并认为主效QTL qFLW4是NAL1的同义突变,其通过选择性剪切调控剑叶宽度的发育。【本研究切入点】尽管目前已经鉴定到大量控制剑叶发育的QTL,但这些研究主要集中在叶片大小上,对剑叶形态发育还鲜有报道。利用EMS诱变籼型水稻恢复系缙恢10号,从其后代鉴定到一个矮化,剑叶基部特异卷曲突变体,暂命名为dcfl1(dwarf and curled flag leaf 1)。【拟解决的关键问题】本研究对突变体dcfl1进行了形态鉴定、细胞学观察和基因精细定位等研究,为DCFL1的克隆和功能研究奠定了基础,有利于水稻剑叶形态发育的分子机理的阐释。此外,剑叶基部卷曲导致叶片直立,有利于群体的通透性,dcfl1在水稻育种中也具有重要的应用价值,是一类新型种质资源。
1 材料与方法
1.1 试验材料
突变体 dcfl1来自西南大学水稻所培育的晚籼恢复系缙恢10号EMS诱变库,经过多世代连续种植,突变性状已稳定遗传。配置西农1A/ dcfl1杂交组合,调查西农1A、dcfl1及其杂交组合F1和F2的株高及剑叶形态,进行遗传分析,利用F2群体隐性单株进行基因定位。西农1A是西南大学水稻所选育的不育系,整个生育期株高及叶片均正常。
1.2 主要农艺性状鉴定
田间小区种植dcfl1和野生型缙恢10号,成熟期分别测量10株材料的株高和节间长并调查穗长、有效穗数、穗粒数、穗实粒数、结实率和千粒重等主要农艺性状。
1.3 光合色素含量测定
开花期,参照文献[16]描述的方法测定dcfl1和野生型的光合色素含量,测定部位为剑叶、倒2叶和倒3叶的叶片中部。
1.4 石蜡切片分析
孕穗期和抽穗期分别选取未全展的剑叶基部和全展剑叶基部,根据文献[17]略有改动。用FAA固定液固定后,依次进行乙醇脱水、二甲苯透明、石蜡包埋、切片、番红固绿染色等步骤。并在体视镜下观察叶片形态。
1.5 扫描电镜观察
田间种植三叶期秧苗,移至扫描电镜室,利用日立 SU350型扫描电镜在-20℃冷冻条件下观察第二片叶的叶鞘内表皮细胞。
1.6 DNA的提取
在西农1A/ dcfl1的F2群体中分别选取正常和突变单株各10株,构成正常基因池和突变基因池。采取改良的CTAB法[18]提取基因池DNA,采用碱煮法[19]提取定位群体单株基因组DNA。
1.7 SSR分析
RM系列SSR引物来源于http://www.gramene.org/网站。根据缙恢10号和西农1A的DNA序列差异,利用Vector 10软件设计InDel标记。SSR引物及InDel标记均由上海英俊生物公司合成。PCR反应总体积12.5 μL,包括1.25 μL的10×PCR缓冲液、0.65 μL的25 mmol·L-1MgC12、0.5 μL 2.5 mmol·L-1dNTPs、8.0 μL的ddH2O、1.0 μL的10 μmol·L-1引物、1.0 μL的模板DNA和0.1 μL的5 U·μL-1Taq酶。PCR反应程序为94℃ 5 min;94℃ 20 s,55℃ 20 s,72℃ 20 s,35个循环; 72℃ 10 min。PCR产物用10%的非变性聚丙烯酰胺凝胶电泳后,0.1%AgNO3染色10 min,去离子水漂洗2次,1%质量浓度的氢氧化钠和0.1%甲醛混合液显色,观察照相。
1.8 遗传图谱构建
西农 1A/dcfl1杂交组合的 F2群体中,具有西农1A带型的单株记为A,具有dcfl1带型的单株记为B,具有 F1杂合体带型的单株记为 H。利用公式[(H+2A)/2n]×100%计算遗传重组率,其中,H代表定位群体中杂合体带型单株的数量,A代表正常株带型单株的数量,n表示F2群体隐性单株总株数。
2 结果
2.1 dcfl1的形态鉴定
突变体dcfl1整个生育期都表现出矮化性状。播种后5 d的dcfl1籽苗地上部长度只有野生型的一半,二叶期也明显矮于野生型。dcfl1的倒1节间、倒2节间和穗长显著短于野生型,进而导致株高半矮化(图1)。扫描电镜观察野生型和 dcfl1叶鞘内表皮细胞,发现dcfl1的细胞长度明显比野生型短,达到了极显著水平,而细胞宽度并无显著差异(图 2),暗示突变体的矮化性状可能是由于细胞长度变短而导致的。农艺性状分析(表1)发现,dcfl1的有效穗数为14.24,极显著高于野生型的11.62,穗粒数、实粒数、结实率和千粒重等则无显著变化。此外,dcfl1的叶片呈深绿色(图1-D),光合色素测定表明剑叶、倒2叶和倒3叶的叶绿素a含量均极显著增加,叶绿素b含量无显著变化,进而导致dcfl1的叶绿素a/b值极显著增加;dcfl1的类胡萝卜素含量虽略有升高,但仅剑叶中的含量极显著高于野生型。
抽穗期dcfl1剑叶的叶片和叶鞘连接处硬化,叶基部展开受阻,半边叶片向内卷曲(图3-A),而剑叶上部和中部无卷曲现象(图3-B)。为进一步观察剑叶基部形态,将未展开的剑叶和全展开时期的剑叶基部进行石蜡切片。发现在剑叶发育前期,野生型的剑叶有规律地向内卷曲排列(图3-C),而dcfl1的内卷叶排列不规则(图3-D)。剑叶全展开后,野生型的剑叶基部呈向外展开状(图3-E),而dcfl1的剑叶基部展开受阻,一半叶片呈向外展开状,但内卷叶始终无法向外展开(图3-F),从而形成了剑叶基部半边叶片向内卷曲这一突变表型。尽管目前已经报道了许多矮化突变体,但剑叶基部特异卷曲的水稻矮化突变体还没有报道。
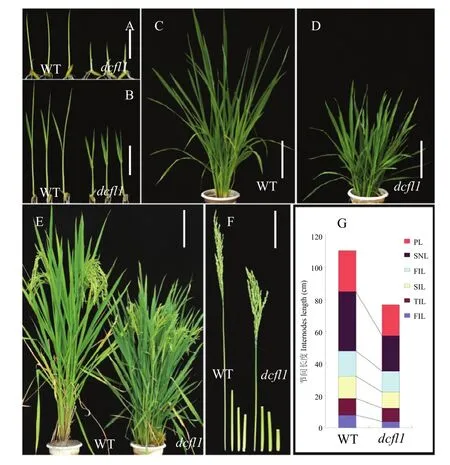
图1 突变体dcfl1和野生型(WT)缙恢10号形态鉴定Fig. 1 Morphology identification of the dcfl1 and the wild type

表1 突变体dcfl1与野生型的农艺性状Table 1 Agronomic traits of dcfl1 mutant and the wild type
2.2 遗传分析
西农 1A/dcfl1杂交组合的 F1代植株剑叶基部均无卷曲现象,且株高正常。F2群体中分离出正常和突变2种类型,正常型1 940株,突变型620株,突变型植株也表现为剑叶基部卷曲和矮化现象,与dcfl1类似,表明剑叶基部卷曲和矮化是1对共分离性状。χ2测验显示正常植株与突变植株的分离比符合 3﹕1(χ2=0.79<χ20.05=3.84),暗示dcfl1的突变性状受1对隐性核基因控制。

图2 突变体dcfl1和野生型(WT)缙恢10号细胞大小比较Fig. 2 Comparison of cell length and width between the dcfl1 and the wild type
2.3 基因定位
利用平均分布在水稻12条染色体上、在西农1A和野生型缙恢10号间呈多态性的96对SSR标记筛选正常基因池和突变基因池,发现第3染色体上的SSR标记RM6297、RM14347、RM5474和RM5955在基因池间呈现多态性,暗示可能与 DCFL1连锁。利用140个 F2隐性单株进行验证,确定了连锁关系并将DCFL1初步限定在RM14347和RM5474之间,遗传距离分别为4.32和3.81 cM。
为进一步确定DCFL1的物理位置,在DCFL1初步定位区间内设计开发了20对InDel标记,多态性筛选发现 4对在亲本之间具有多态性,分别命名为Ind03-8、Ind03-11、Ind03-6和Ind03-4(表2)。利用这些多态性标记对620株西农1A/dcfl1杂交组合的F2隐性单株进行分析,结果表明,Ind03-8、Ind03-11、Ind03-6和Ind03-4的交换株分别为9、2、3和7个,且前 2个标记的交换株不同于后者,从而将 DCFL1精细定位在InDel标记Ind03-11和Ind03-6之间,物理距离约为78 kb(图4)。

图3 突变体dcfl1和野生型(WT)缙恢10号的剑叶形态Fig. 3 Flag leaf phenotype of the dcfl1 and the wild type
根据Gramene(http://ensembl.gramene.org/ Oryza_ sativa/Info/Index)和 Rice genome annotation project(http://rice.plantbiology.msu.edu/)提供的信息,发现在DCFL1精细定位区间内,包含15个注释基因(表3),5个编码逆转录转座子蛋白,6个编码表达蛋白,4个为功能基因,分别编码细胞色素P450蛋白、60S核糖体蛋白L21-2、β-淀粉酶和RNA基序识别蛋白。

表2 基因定位InDel引物Table 2 Primers of InDel markers for gene mapping
3 讨论

图4 DCFL1在水稻第3染色体上的分子定位Fig. 4 Molecular mapping of DCFL1 gene on rice chromosome 3
在水稻生产中,叶片适度卷曲有利于叶的挺直,从而改善了群体结构、提高了光能利用率,在水稻高产育种中具有重要的应用价值。因此,揭示水稻叶片卷曲的遗传机制不仅有利于叶片发育的分子机理阐释,也为水稻株型育种提供了基础材料和理论支撑。目前,在水稻中至少报道了20多份卷叶突变体,这些突变体的形成多受植物叶片极性建成、泡状细胞大小和数量变化以及环境因素的影响。葱状卷曲突变体sll1[20]是由于叶片远轴面厚壁组织细胞发育异常而表现出卷曲。位于第3染色体上的突变体srl2[21]表现出叶片半卷,叶片变窄,株高降低。类似于 sll1,srl2也是由于远轴面厚壁组织细胞异常导致叶片卷曲。突变体adl1[22]表现为下表皮泡状细胞异位发育,从而导致叶片向远轴面卷曲。另一个外卷突变体oul1[23]来源于水稻最外层细胞特异基因 Roc5的敲除,表现出近轴侧泡状细胞体积变大而引起叶片外卷。SRL1编码糖基磷脂酰肌醇固定蛋白,调控叶片上表皮泡状细胞数量从而控制叶片卷曲[10]。突变体rl14[11]卷叶表型是由于近轴面泡状细胞萎缩引起,RL14通过调控次生细胞壁组分合成从而影响叶片水分运输,并进一步影响泡状细胞形态。环境诱导型卷叶突变体rl15(t)叶片卷曲行为受环境诱导,湿度是诱导突变体卷曲的主要因素[25]。值得注意的是突变体cfl1[9],由于剑叶卷曲,所以该突变体被命名为 curly flag leaf1,但其他叶片也受到影响,因此,cfl1也属于所有叶片全卷突变体。此外,rl12(t)叶片卷曲特性随着发育进程而发生变化,卷曲表型主要发生在叶片中上部1/3处,中下部正常,剑叶亦是如此[24]。本文报道的 dcfl1突变体,仅剑叶基部卷曲,明显不同于已报道的水稻卷叶突变体,也不同于剑叶发育缺陷相关突变体,因此,dcfl1是一类新型剑叶基部特异卷曲突变体。

表3 定位区间内注释基因Table 3 Annotated genes of the gene mapping range
目前报道的叶片发育调控基因,多具有“一因多效”性。如,Ghd7调控抽穗期和产量性状,同时也影响剑叶的叶面积[26-27];dtl1是一个矮化突变体,同时也表现叶片卷曲、分蘖减少和不育等性状[28]。dcf1并不是传统的叶卷曲突变体,只是剑叶基部特异卷曲,剑叶中上部和其他叶片均正常,因此它的育性并没有受影响。另外,尽管dcfl1的穗长变短了,但dcfl1的穗型更紧凑,使dcfl1穗粒数无明显变化,而且有效穗增加了,从而暗示该突变体的产量有所提高。突变体dcfl1除剑叶特异卷曲外,还表现植株的矮化和叶片颜色深绿。这可能是由于 DCFL1的多效性造成的,引起这些突变表型的原因还有待进一步研究。
利用SSR等分子标记最终将DCFL1定位在第3染色体InDel标记Ind03-11和Ind03-6之间78 kb物理距离内,包含15个注释基因,其中4个为功能基因。细胞色素 P450编码基因已克隆,可能通过脂类代谢途径调控细胞伸长,其突变体oscyp96b4主要表现为植株半矮化和育性降低。它的矮化表型并不受激素的调控,而是通过转录剂量的方式来降低水稻株高[29]。ZHANG等[30]鉴定到一个 oscyp96b4的等位突变体sd37,除全生育期植株矮化外,还表现稻穗和花轴变短,籽粒变小。另一个等位突变体dss1萌发以及早期生长均延迟,它的矮化表型也不受外源激素影响。但内源ABA的积累和GA的缺陷可能是dss1矮化的原因,而且其耐旱性增强[31]。此外,WANG等[32]鉴定了oscyp96b4第三个等位突变体bsh1,其表现出株高、千粒重以及每株产量均显著降低,叶鞘角质层蜡质含量降低,暗示BSH1可能参与蜡质生物合成。其他3个基因则没有克隆:β-淀粉酶是一个非生物胁迫蛋白,响应磷和钾的缺失[33-34];60S核糖体蛋白L21-2编码基因在不育系和保持系之间具有表达差异[35];RNA基序识别蛋白则尚没有描述。从表型和定位结果推测,DCFL1可能是一个调控剑叶发育的新基因。
4 结论
EMS诱变获得一个矮化和剑叶基部卷曲的新型水稻突变体dcfl1,其表现为分蘖数增多、叶色深绿。产量性状除有效穗极显著升高外,其他无明显变化。dcfl1的叶绿素a含量极显著高于野生型,导致叶绿素a/b比值极显著增加。剑叶基部卷曲和植株矮化受同1对隐性核基因调控,利用西农1A和dcfl1杂交组合的F2分离群体,最终将DCFL1定位在水稻第3染色体InDel标记Ind03-11和Ind03-6之间78 kb的物理距离内,包含15个注释基因。
[1] 徐静, 王莉, 钱前, 张光恒. 水稻叶片形态建成分子调控机制研究进展. 作物学报, 2013, 39(5): 767-774.
XU J, WANG L, QIAN Q, ZHANG G H. Research advance in molecule regulation mechanism of leaf morphogenesis in rice (Oryza sativa L.). Acta Agronomica Sinica, 2013, 39(5): 767-774. (in Chinese)
[2] YUE B, XUE W Y, LUO L J, XING Y Z. QTL analysis for flag leaf characteristics and their relationships with yield and yield traits in rice. Acta Genetica Sinica, 2006, 33(9): 824-832.
[3] ZHANG B, YE W J, REN D Y, TIAN P, PENG Y L, GAO Y, RUAN B P, WANG L, ZHANG G H, GUO L B, QIAN Q, GAO Z Y. Genetic analysis of flag leaf size and candidate genes determination of a major QTL for flag leaf width in rice. Rice, 2015, 8: 2.
[4] QI J, QIAN Q, BU Q Y, LI S Y, CHEN Q, SUN J Q, LIANG W X, ZHOU Y H, CHU C C, LI X G, REN F G, PALME K, ZHAO B R, CHEN J F, CHEN M S, LI C Y. Mutation of rice Narrow leaf1 gene, which encodes a novel protein, affects vein patterning and polar auxin transport. Plant Physiology, 2008, 147(4): 1947-1959.
[5] CHO S H, YOO S C, ZHANG H, PANDEYA D, KOH H J, HWANG J Y, KIM G T, PAEK N C. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytologist, 2013, 198(4): 1071-1084.
[6] FUJINO K, MATSUDA Y, OZAWA K, NISHIMURA T, KOSHIBA T, W. FRAAIJE M, SEKIGUCHI H. NARROW LEAF 7 controls leaf shape mediated by auxin in rice. Molecular Genetic Genome, 2008, 279(5): 499-507.
[7] HU J, ZHU L, ZENG D, GAO Z, GUO L, FANG Y, ZHANG G, DONG G, YAN M, LIU J, QIAN Q. Identification and characterization of NARROW AND ROLLED LEAF 1, a novel gene regulating leaf morphology and plant architecture in rice. Plant Molecular Biology, 2010, 73(3): 283-292.
[8] WU C, FU Y P, HU G C, SI H M, CHENG S H, LIU W Z. Isolation and characterization of a rice mutant with narrow and rolled leaves. Planta, 2010, 232(2): 313-324.
[9] WU R H, LI S B, HE S, WAßMANN F, YU C H, QIN G J, SCHREIBER L, QU L J, GU H Y. CFL1, a WW domain protein, regulates cuticle development by modulating the function of HDG1, a class IV homeodomain transcription factor, in rice and Arabidopsis. The Plant Cell, 2011, 23(9): 3392-3411.
[10] XIANG J J, ZHANG G H, QIAN Q, XUE H W. Semi-rolled leaf1 encodes a putative glycosylphosphatidylinositol-anchored protein and modulates rice leaf rolling by regulating the formation of bulliform cells. Plant Physiology, 2012, 159(4): 1488-1500.
[11] FANG L K, ZHAO F M, CONG Y F, SANG X C, DU Q, WANG D Z, LI Y F, LING Y H, YANG Z L, HE G H. Rolling-Leaf14 is a 2OG-Fe (II) oxygenase family protein of unknown function that modulates rice leaf rolling by affecting secondary cell wall formation in leaves. Plant Biotechnology Journal, 2012, 10(5): 524-532.
[12] YAN J P, ZHU J, HE C X, BENMOUSSA M, WU P. Molecular marker-assisted dissection of genotype× environment interaction for plant type traits in rice (Oryza sativa L.). Crop Science, 1999, 39(2): 538-544.
[13] KOBAYASHI S, FUKUTA Y, MORITA S, SATO T, OSAKI M, KHUSH G S. Quantitative trait loci affecting flag leaf development in rice (Oryza sativa L.). Breed Science, 2003, 53(3): 255-262.
[14] BIAN J M, HE H H, SHI H, ZHU G Q, LI C J, ZHU C L, PENG X S, YU Q Y, FU J R, HE X P, CHEN X R, HU L F, OUYANG L J. Quantitative trait loci mapping for flag leaf traits in rice using a chromosome segment substitution line population. Plant Breeding, 2014, 133(2): 203-209.
[15] CHEN M L, LUO J, SHAO G N, WEI X J, TANG S Q, SHENG Z H, SONG J, HU P S. Fine mapping of a major QTL for flag leaf width in rice, qFLW4, which might be caused by alternative splicing of NAL1. Plant Cell Reports, 2012, 31(5): 863-872.
[16] LICHTENTHALER H K. Hlorophylls and carotenoids pigments of photosynthetic biomembranes. Methods in Enzymology, 1987, 48: 350-382.
[17] SANG X C, LI Y F, LUO Z K, WANG N, LING Y H, ZHAO H M, YANG Z L, LUO H F, LIU Y S, HE G H. CHIMERIC FLORAL ORGANS 1, encoding a monocot-specific MADS box protein, regulates floral organ identity in rice. Plant Physiology, 2012, 160(2): 788-807.
[18] MURRAY M G, THOMPSON W F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research, 1980, 8(19): 4321-4325.
[19] 桑贤春, 何光华, 张毅, 杨正林, 裴炎. 水稻PCR扩增模板的快速制备. 遗传, 2003, 25(6): 705-707.
SANG X C, HE G H, ZHANG Y, YANG Z L, PEI Y. The simple gain of templates of rice genomes DNA for PCR. Hereditas, 2003, 25(6): 705-707. (in Chinese)
[20] ZHANG G H, XU Q, ZHU X D, QIAN Q, XUE H W. SHALLOT-LIKE1 is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. The Plant Cell, 2009, 21(3): 719-735.
[21] LIU X F, LI M, LIU K, TANG D, SUN M F, LI Y F, SHEN Y, DU G J, CHENG Z K. Semi-Rolled Leaf 2 modulates rice leaf rolling by regulating abaxial side cell differentiation. Journal of Experimental Botany, 2016, 67(8): 2139-2150.
[22] HIBARA K, OBARA M, HAYASHIDA E, ABE M, ISHIMARU T, SATOH H, ITOH J, NAGATO Y. The ADAXIALIZED LEAF 1 gene functions in leaf and embryonic pattern formation in rice. Developmental Biology, 2009, 334(2): 345-354.
[23] ZOU L P, SUN X H, ZHANG Z G, LIU P, WU J X, TIAN C J, QIU J L, LU T G. Leaf rolling controlled by the homeodomain leucine zipper class IV gene Roc5 in rice. Plant Physiology, 2011, 156(3): 1589-1602.
[24] 罗远章, 赵芳明, 桑贤春, 凌英华, 杨正林, 何光华. 水稻新型卷叶突变体 rl12(t)的遗传分析和基因定位. 作物学报, 2009, 35(11): 1967-1972.
LUO Y Z, ZHAO F M, SANG X C, LING Y H, YANG Z L, HE G H. Genetic analysis and gene mapping of a novel rolled leaf mutant rl12(t) in rice. Acta Agronomica Sinica, 2009, 35(11): 1967-1972. (in Chinese)
[25] 张礼霞, 刘合芹, 于新, 王林友, 范宏环, 金庆生, 王建军. 水稻卷叶突变体 rl15(t)的生理学分析及基因定位. 中国农业科学, 2014, 47(14): 2881-2888.
ZHANG L X, LIU H Q, YU X, WANG L Y, FAN H H, JIN Q S, WANG J J. Molecular mapping and physiological characterization of a novel mutant rl15(t) in rice. Scientia Agricultura Sinica, 2014, 47(14): 2881-2888. (in Chinese)
[26] XUE W Y, XING Y Z, WENG X Y, ZHAO Y, TANG W J, WANG L, ZHOU H J, YU S B, XU C G, LI X H, ZHANG Q F. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nature Genetics, 2008, 40(6): 761-767.
[27] 谈聪, 翁小煜, 鄢文豪, 白旭峰, 邢永忠. 多效性基因Ghd7调控水稻剑叶面积. 遗传, 2012, 34(7): 901-906.
TAN C, WENG X Y, YAN W H, BAI X F, XING Y Z. Ghd7, a pleiotropic gene controlling flag leaf area in rice. Hereditas, 2012, 34(7): 901-906. (in Chinese)
[28] 张帆涛, 方军, 孙昌辉, 李润宝, 罗向东, 谢建坤, 邓晓建, 储成才. 水稻矮秆突变体dtl1的分离鉴定及其突变基因的精细定位. 遗传, 2012, 34(1): 79-86.
ZHANG F T, FANG J, SUN C H, LI R B, LUO X D, XIE J K, DENG X J, CHU C C. Characterization of a rice dwarf and twist leaf 1 (dtl1) mutant and fine mapping of DTL1 gene. Hereditas, 2012, 34(1): 79-86. (in Chinese)
[29] RAMAMOORTHY R, JIANG S Y, RAMACHANDRAN S. Oryza sativa cytochrome P450 family member OsCYP96B4 reduces plant height in a transcript dosage dependent manner. PLoS ONE, 2011, 6(11): e28069.
[30] ZHANG J, LIU X Q, LI S Y, CHENG Z K, LI C Y. The rice semi-dwarf mutant sd37, caused by a mutation in CYP96B4, plays an important role in the fine-tuning of plant growth. PLoS ONE, 2014, 9(2): e88068.
[31] TAMIRU M, UNDAN J R, TAKAGI H, ABE A, YOSHIDA K, UNDAN J Q, NATSUME S, UEMURA A, SAITOH H, MATSUMURA H, URASAKI N, YOKOTA T, TERAUCHI R. A cytochrome P450, OsDSS1, is involved in growth and drought sress responses in rice (Oryza sativa L.). Plant Moecular Biology, 2015, 88(1): 85-99.
[32] WANG X L, CHENG Z J, ZHAO Z C, GAN L, QIN R Z, ZHOU K N, MA W W, ZHANG B C, WANG J L, ZHAI H Q, WAN J M. BRITTLE SHEATH1 encoding OsCYP96B4 is involed in secondary cell wall formation in rice. Plant Cell Reports, 2016, 35: 745-755.
[33] PARK M R, BAEK S H, DE LOS REYES B G, YUN S J, HASENSTEIN K H. Transcriptome profiling characterizes phosphate deficiency effects on carbohydrate metabolism in rice leaves. Journal of Plant Physiology, 2012, 169(2): 193-205.
[34] SHANKAR A, SINGH A, KANWAR P, SRIVASTAVA A K, PANDEY A, SUPRASANNA P, KAPOOR S, PANDEY G K. Gene expression analysis of rice seedling under potassium deprivation reveals major changes in metabolism and signaling components. PLoS ONE, 2013, 8(7): e70321.
[35] YANA J J, TIAN H, WANG S Z, SHAO J Z, ZHENG Y Z, ZHANG H Y, GUO L, DING Y. Pollen developmental defects in ZD-CMS rice line explored by cytological, molecular and proteomic approaches. Journal of Proteomics, 2014, 108: 110-123.
(责任编辑 李莉)
Identification and Gene Mapping of a Dwarf and Curled Flag Leaf Mutant dcfl1 in Rice (Oryza sativa L.)
ZHANG XiaoBo, XIE Jia, ZHANG XiaoQiong, TIAN WeiJiang, HE PeiLong, LIU SiCen, HE GuangHua, ZHONG BingQiang, SANG XianChun
(Rice Research Institute of Southwest University/Chongqing Key Laboratory of Application and Safety Control of Genetically Modified Crops, Chongqing 400715)
【Objective】Leaf blade is an important factor of plant type, which is directly related to leaf photosynthetic area and light energy utilization. Flag leaf is most prominently in the formation of rice production. Study of the genes which regulate flag leaf development in rice is of very significance in rice functional genomics research and molecular breeding. A novel flag leaf mutant has been identified and the results of study will provide a foundation for the research of leaf morphological formation and plant type breeding in Oryza sativa L.【Method】A dwarf and curled flag leaf mutant (dcfl1) was discovered from the progeny of indica restorerline Jinhui10 with seeds treated by ethyl methane sulfonate (EMS) and the traits of dwarf and curled flag leaf base inherited steadily after multi-generations’ self-fertility. The second leaf sheath was observed by scanning electron microscopy (SEM) at the three-leaf stage. The flag leaf base was used for paraffin section at the booting and heading stages. At the blooming stage, the characteristics of chloroplast pigment of the flag, second and third leaf blades were measured. At the maturity stage, agronomic traits such as plant height, panicle length, efficient panicle per plant, seed number per panicle, filled grain number per panicle, seed setting ratio, and 1000-seed weight were measured. The dcfl1 was crossed with indica sterile line Xinong 1A, and the F1and F2generations were used for genetic analysis. Additionally, gene mapping was performed based on the recessive individuals of the F2generation of Xinong 1A/ dcfl1.【Result】The dcfl1 was dwarf in all phases of plant development. The cell length of the 2nd leaf sheath surface of the dcfl1 was significantly shorter than the wild type. The panicle length, the first and the second internode of the dcfl1 were all significantly shorter than those of the wild type. The dcfl1 displayed a severe curl at the base of flag leaf blade after the heading stage, while the upper of flag leaf blade was nearly normal in the flag leaf. Meanwhile, the other leaf blades appeared as normal as the wild type. No significant differences were detected in grain number per panicle, filled grain number per panicle, seed setting rate and 1000-seed weight between the dcfl1 and the wild type. However, the number of the tiller in the dcfl1 was more than the wild type and the efficient panicle per plant was increased significantly than the wild type. Having the dark green leaves, the contents of chlorophyll a and total chlorophyll in the dcfl1 increased significantly compared with those of the wild type for the flag leaves, the second and the third leaves. Genetic analysis indicated that the dwarf and curled flag leaf traits of dcfl1 were controlled by a recessive nuclear gene. Based on the F2population derived from a cross between the dcfl1 and an indica sterile line, Xinong 1A, the gene was fine mapped on chromosome 3 between InDel marker Ind03-11 and Ind03-6 with the physical distance 78 kb, containing fifteen annotated genes.【Conclusion】The dcfl1 is a novel recessive dwarf and curled flag leaf mutant coming from EMS-inducement. The DCFL1 was mapped on chromosome 3 with 78 kb physical distance. These results will provide a foundation for map-based cloning of DCFL1 gene and understanding of the molecular mechanism of the rice flag leaf.
rice (Oryza sativ L.); dwarf; curled flag leaf; genetic analysis; gene mapping
2016-11-09;接受日期:2017-01-04
中央高校基本科研业务费项目(XDJK2013A023)、重庆市研究生科研创新项目(CYS14043)
联系方式:张孝波,E-mail:zhangxiaobo@163.com。通信作者桑贤春,E-mail:sangxianchun@163.com
