Effect of Potassium Chloride on the Detonation Performance and Thermal Stability of Ammonium Nitrate
2017-05-07TANLiuLIUDabinXUSenXIALianghongWUQiujie
TAN Liu, LIU Da-bin, XU Sen,2, XIA Liang-hong, WU Qiu-jie,2
(1. School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China; 2. National Quality Supervision and Inspection Center for Industrial Explosive Materials, Nanjing 210094, China; 3. Jiangxi Guotai Industrial Explosive Group Co.,LTD, Nanchang 330096, China)
1 Introduction
Ammonium nitrate(AN) is widely usedin nitrogen fertilizers and energetic material[1-2]. AN has many advantages: for example, it is inexpensive, releases almost 100% gaseous products and has a positive oxygen balance[3-4]. However, the applications of AN are limited by phase transition, hygroscopicity and unlawful use. AN can exist in five polymorphic forms (designated as phases Ⅴ, Ⅳ, Ⅲ, Ⅱ and Ⅰ) under ambient pressure, particularly the Ⅳ↔Ⅲ transition observed near the room temperature. The phase Ⅳ (density=1.72 g·cm-3) to phase Ⅲ(density=1.66 g·cm-3) can cause variation in density and volume change (3.8%), which can lead to the formation of a porous structure and cracked crystals with poor mechanical strength and unwanted explosive performance[5-6].
AN is mostly mixed with fuel oil to form the most popular commercial explosive: ammonium nitrate fuel oil(ANFO)[7-9]. The knowledge of preparing explosives from AN and the common availability of ingredient is used by offenders. Meanwhile many large accidents associated with AN happened frequently[10-11], the harm brought by the explosion characteristics of AN has aroused great attention. There has been a growing demand for detailed information on its detonation characteristics. Vytenis Babrauskas[12]indicated that AN was found to be the cause of explosions in transport or storage. Richard[9]et al observed that the presence of pyrite reduces the temperature and accelerates the rate of decomposition of AN. Anuj A[13]et al proved that LiF can inhibit AN thermal decomposition. Tang[14]used compound fertilizer raw material diammonium phosphate and ammonium sulfate improved the thermal stability of AN. Bernard[15]and Oxley[16]et al, they indicated the inorganic acid and the halogen atom to the thermal decomposition of AN with a great role in promotion. Zhe Han[17]et al, they proved that presence of sodium sulfate can increase the “onset” temperature of AN decomposition, while potassium chloride(KCl) tends to decrease the “onset” temperature thus acting as AN thermal decomposition promoter.
Most studies focused on the thermal decomposition of AN, one hypothesis is that diluting AN with a chemically inert material or the incorporation of small amounts of a material that increases the chemical reaction zone could diminish the explosivity of AN. However, whether the AN-additive mixtures exhibit explosivity or not needs to be confirmed. KCl is one of suppressor agent in mine safety explosive and a fertilizer raw material. In addition, some researchers[16, 18-19]found that chlorine ions can promote thermal decomposition of AN. Thus the safety performance of AN/KCl mixtures is important for industrial handing, transportation and use. In this study, the safety performance of AN/KCl mixtures prepared by using different mixing methods(mechanical mixing method and solution mixing method) was investigated, with an emphasis being placed on analyzing the thermal decomposition and the explosive performance of AN.
2 Experimental
2.1 Preparation of Modified AN Sample
AN and KCl were of technical grade and used without further purification in the experiments, from Kailong Chemical (China) and Huarui Chemical (China) respectively. The modified AN stored in a vacuum desiccator and dried in an oven under 80 ℃ for 120 min before the test to prevent moisture absorption. The water content of the mixtures was identified using a Sartorius-MA35, and the moisture was maintained at approximately 0.06% to ensure the consistency of the experiment.
2.1.1 Mechanical Mixing Method
To prevent moisture absorption, AN, KCl and the mill pot were preheated at 80 ℃ for 30 min. 1000 g AN was mixed with different mass faction of additive by ball mill at a speed of 120 r·min-1for 30 min, then stored in a desiccator before use.
2.1.2 Solution Mixing Method
AN solution was prepared by dissolving 500 g AN in 200 mL of distilled water at 80 ℃. The solution was kept in a water bath for 30 min, after complete dissolution of the salt, different mass faction of additive were added to the solution. After heating and complete dissolution, the solution is drying in the vacuum oven at the temperature of 80 ℃, then modified AN was milled by ball mill. The milled AN dried in an oven under 80 ℃ for 120 min and stored in a desiccators before use.
2.2 Test Methods
2.2.1 DSC Thermal Analysis
The thermal stabilityof modified AN was studied using a Mettler-Toledo DSC 1 instrument. Modified AN of 1-2 mg were heated from 25 ℃ to 400 ℃ in a sealed crucible at a heating rate of 10 ℃·min-1. The reaction was studied in nitrogen atmospheres with a constant flow rate of 30 mL·min-1. The water content of the mixtures was identified before use.
2.2.2 ARC Experiments
In this study, an adiabatic calorimeter called ARC was used. This instrument was used for acquiring thermodynamic and kinetic information for runaway reactions in order to evaluate thermal hazards associated with the reactive material[22-23]. The main components and the detailed description of principle of ARC can be found elsewhere[24]. The main operation indexes of the ARC are temperature range 0-500 ℃; pressure range 0-20 MPa; sample mass range 0.01-10 g; slope sensitivity 0.02 ℃·min-1.
2.2.3 The Koenen Test
According to “The United Nations proposals on the transport of dangerous goods-Testing and Standards Manual” (the fifth revised edition), the Koenen test is used to measure the sensitivity of solid substances to intense heat with varied confinement. The sample is placed in a specified sample tube. Test using a phthalic acid dibutyl adjusts the heating rate to (3.3±0.3) K·s-1. The tube with 1.0 mm orifice plate was exposed to direct flame and heated for 5min or until an earlier event occurred[20-21]. The test was measured for two runs for each sample.
2.2.4 The UN Gap Test
By the UN gap test, the propagate detonation ability of the modified AN was tested. Test device schematic diagram is shown in Fig.1. The test pipe was made of steel tube, with an inner diameter of 40 mm and an outer diameter of (48±2) mm, and the total length of the tube was 400 mm. 160 g of booster explosive (50/50-PETN/TNT, diameter (50±1) mm, density 1.60 g·cm-3) was used for the booster charge and initiated with a No.8 electric detonator. The edge length of the witness plate was (150±10) mm, and the thickness was (3.2±0.2) mm. The thickness of the steel spacer was (1.6±0.2) mm, and the plastic membrane was made of polyethylene sheet. The mass of typical sample mass is 500 g[20-21]. The test was measured for two runs for each sample.

a. diagrammatic sketch

b. photograph
Fig.1 Schematic showing of the UN gap test
3 Results and Discussion
3.1 Thermal Analysis Results
3.1.1 Analysis of DSC Measurements
The DSC results are shown in Fig.2 and Table 1. The DSC curve of AN is presented in Fig.2a. AN shows four endothermic peaks and one exothermic peak. As shown in Table 1, the onset temperature of the AN/KCl mixtures was moved to high temperature with increasing the content of additive. Fig.2b and Fig.2c show the DSC curves of AN/KCl mixtures that prepared by different mixing methods. The onset temperature of AN/20% KCl mixture (solution mixing method) was increased from 204.33 ℃ to 235.15 ℃, and the AN/20% KCl mixture (mechanical mixing method) was increased to 244.50 ℃. While the peak temperature was decreased from 286.75 ℃ to 271.66 ℃ (solution mixing method) and 258.31 ℃ (mechanical mixing method) respectively. It can be concluded that KCl can increase the onset temperature of thermal decomposition and has a role in promoting the thermal decomposition of AN.


a. neat AN

b. AN /KCl (mechanical mixing)

c. AN/KCl (solution mixing)
Fig.2 DSC curves of samples
Table 1 The results of DSC thermal analysis

materialsmixingmethodTo/℃Tp/℃AN-204.33286.75AN/10%KClsolution220.17250.84AN/15%KClsolution223.68261.48AN/20%KClsolution235.15271.66AN/10%KClmachinery223.14263.12AN/15%KClmachinery228.31260.46AN/20%KClmachinery244.50258.31

(1)
(2)
(3)
(4)
(5)

(6)
(7)
(8)
(9)
(10)
(11)
Many researchers[18, 26]also have noted that acid is essential for chloride catalysis. There is no acidic substance in AN/KCl mixtures, but the peak temperature of AN/KCl mixtures was decreased. The peak temperature of mixtures (mechanical mixing method) was around 260 ℃. Interestingly, even the KCl reached 20%, the temperature was just decreased to 258.31 ℃. For the solution mixing method, the peak temperature of AN/10% KCl mixtures was decreased to 250.84 ℃, but the temperature was moved to high temperature with increasing the KCl content. As shown in the Fig.3, the exothermic maximum of the AN/30% KCl mixture was increased to 294.30 ℃ (solution mixing method) and 288.56 ℃ (mechanical mixing method). This result shows that the acid may come from the decomposition of AN. A small amount of KCl will promote thermal decomposition of AN. However, the catalytic effect would be limited by the content of acid. On the other hand, the chlorine ions does not affect the first step of thermal decomposition of AN. KCl acts as a diluents can isolate the heat exchange of AN and absorb heat from the reaction zone, so KCl can improve the onset temperature of AN. Meanwhile the results show that the mixing methods just have a little effect on thermal stability of AN. The main reason we think is the melting point. The third exothermic peak at 158.33 ℃ is the melting point of AN, but the melting point of KCl is 770 ℃. When temperature reached the melting point of AN, KCl will be separated from AN.

Fig.3 DSC curves of AN/30%KCl(solution mixing and mechanical mixing)
3.1.2 ARC Results and Discussions
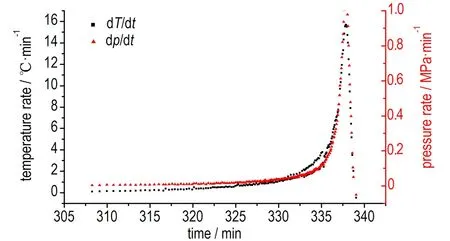
The ARC curves of temperature-time and pressure-time for samples are shown in Figs.8-10, and measured data are listed in Table 2. Fig.4a shows the ARC curves of 1#AN (0.196 g), no exothermic reaction was detected in this test. Heat accumulation has an important influence on thermal stability, especially in the materials with poor explosive properties. That no exothermic reaction was observed in 1#AN maybe can explain by the small sample size. As shown in the Fig.4b, the onset exothermic reaction of 2#AN (0.372 g) appeared at 205.33 ℃, and the initial self-heating rate was 0.037 ℃·min-1. Temperature and pressure rise slowly, this phenomenon illustrated that thermal decomposition of AN is relatively mild. Compared to AN, the onset temperature of AN/10% KCl (mechanical mixing method) was 204.36 ℃ (Fig.5), and the initial self-heating rate was 0.06 ℃·min-1, which was far larger than detection sensitivity (0.02 ℃·min-1). The pressure has a sharp increase, and the maximum reaction pressure is 3.943 MPa. And not only that, the last heat release lasted shorter than AN, which means that AN decomposed very fast with KCl. This phenomenon illustrated that KCl can accelerate the decomposition of AN. Fig.6 shows the ARC curves of AN/10%KCl (solution mixing method), the onset temperature was 205.93 ℃, and the initial self-heating rate was 0.071 ℃·min-1. Like the modified AN that prepared by mechanical mixing method, the pressure has a sharp increase, and the maximum reaction pressure was 2.566 MPa.

a. 1#AN (0.196 g)

b. 2#AN (0.372 g)
Fig.4 ARC curves of samples of AN

a. temperature and pressure vs. time

b. rise rates of pressure and temperature vs. time
Fig.5 ARC curves of samples of AN/KCl (mechanical mixing)

a. temperature and pressure vs. time
b. rise rates of pressure and temperature vs. time
Fig.6 ARC curves of samples of AN/KCl (solution mixing)
In order to observe temperature and pressure changes more intuitively, the self-heating rate (dT/dt) and pressure rising rate (dp/dt) were drawn as shown in Table 2. According to those figures, it was found that the dT/dtvalues of AN/10%KCl were large than AN, which means that the AN decomposed violently in this stage. In general, the ARC results consisted with DSC results. KCl can accelerate the decomposition of AN.
Table 2 Summary of ARC results

parameter2#ANAN/KCl(mechanicalmixing)AN/KCl(solutionmixing)samplemass/g0.3720.5000.410Tonset/℃205.33 215.48204.36205.93onsettemp.rate/℃·min-10.037 0.0330.0600.071Tf/℃210.85 223.54271.56252.41pf/MPa0.944 1.8813.9482.449max.temp.rate/℃·min-10.038 0.0397.7515.67max.pres.rate/℃·min-10.037 0.0535.939.87
3.2 Explosive Testing
3.2.1 Results of the Koenen Test
As shown in Fig.7, the AN/25%KCl mixture (solution mixing method) showed a violent reaction, as after just over 62 s, the flame began to rise. The height of the flame rose rapidly in the next 16 s. However, the flames began to decrease in the next few seconds, and then rose rapidly again. The sample just lasted only 98 s before exploding. The reaction rate produced more heat energy, and thus a vicious cycle led to thermal runaway. The pressure in the tube must have exceeded the critical value due to the violent reaction. The explosion was caused by the excessive heat energy and pressure. Fig.9a show that the seamless steel tube was completely torn apart and the test result is “+”, the mixtures have explosive effect.
Fig.8 shows the flame of AN/30% KCl mixture. The flame began to show an upward trend after 76 s. In the next 49 s, there appear spray flame, but the flame remained relatively stable. That means the reaction of AN/30% KCl mixture was relatively mild, and the pressure inside the test tube kept relative balance. The mixture did not explode after sustained heating for 5 mins, as shown in the Fig.9b, and the test result is “-”. The test results were consistent for 2 parallel experiments (Table 3). As shown in Fig.9c-9d, for the mechanical mixing method, the critical value is 35%.
When the temperature rises to 290 ℃, the conceivable reaction mechanism is shown in reaction (12)—(15). KCl can react with OH· radicals in the process of reaction, and the activity of OH· radicals was reduced as the reaction proceeds[4, 27]. Meanwhile KCl has a high thermal capacity that can absorb large amounts of heat energy in the detonation process, so KCl can decrease thermal sensitivity of AN with increasing the content. Meanwhile the results show that solution mixing method is better than the mechanical mixture to reduces the temperature-sensitivity of AN. For the solution mixing method, KCl and AN mixed more uniform due to re-crystallization. On the other hand, the ion exchange between the KCl and AN will inhibit the oxidation-reduction reaction of AN, so the results of AN that prepared by different mixing method show a different result. While the mixing methods is not significantly affected the thermal sensitivity of AN.

Fig.7 The flame of AN/25% KCl(solution mixing)
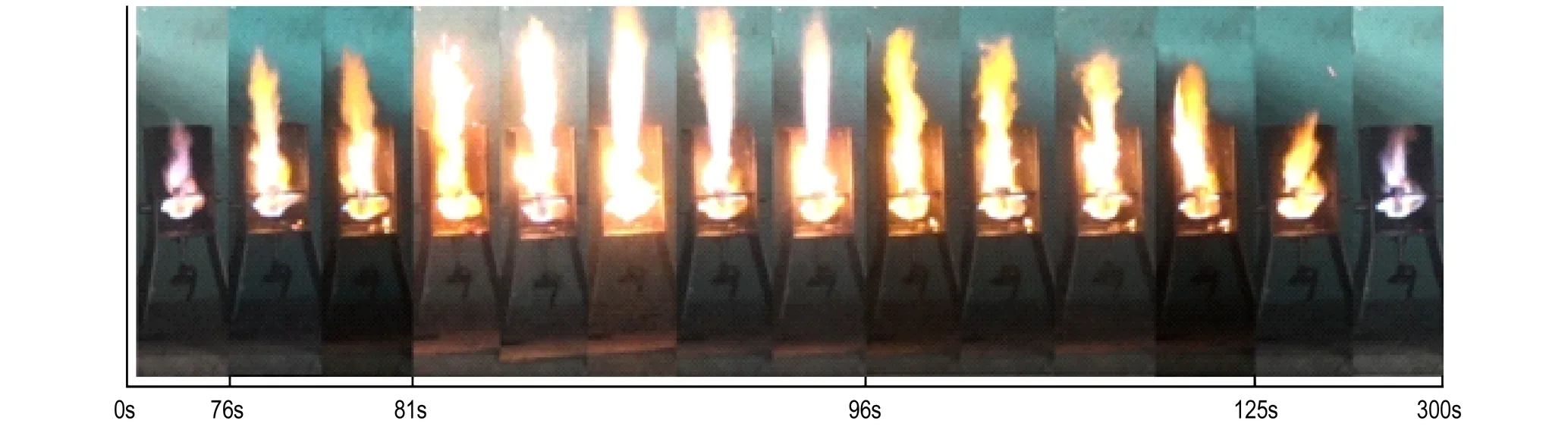
Fig.8 The flame of AN/30% KCl(solution mixing)

a. AN/25% KCl b. AN/30% KCl
(solution mixing) (solution mixing)

c. AN/30% KCld. AN/35% KCl
(mechanical mixing) (mechanical mixing)
Fig.9 Physical map of Koenen test
Table 3 The Koenen test results of AN/KCl mixtures

mixingmethodKClcontent/%mass/gdensity/g·cm-3t/sresultsolution1022.400.8382+solution1523.100.8588+solution2023.800.8795+solution2524.500.9098+solution3024.620.91300-solution3024.840.92300-mechanical1022.400.8373+mechanical1522.20.8277+mechanical2023.20.8579+mechanical2524.10.8681+mechanical3025.640.9584+mechanical3526.000.95300-mechanical3525.610.95300-
Note: “+” indicates that the sample is sensitive to hyperthermia under experimental conditions. “-” indicates that the sample is not sensitive to hyperthermia under experimental conditions.

(12)

(13)

(14)

(15)
3.2.2 Results of the UN Gap Test
The AN-KCl mixtures were made into ammonium nitrate fuel oil (ANFO) in accordance with industry standards (94% AN-KCl mixture: 6% diesel). Table 4 shows the results of the UN gap test. For the 94% (75% AN/25% KCl)-6% diesel mixture (solution mixing method), the seamless steel tubes were completely torn apart. In addition, the witness plates were wholly broken down by shock waves. The diameter of the dent was 9.9 cm in Fig.10a, and the result was “+”. When KCl was increased to 30%, as shown in Fig.10b, the witness plates were not breakdown, and the seamless steel tubes were not tear apart completely. The test result was consistent for 2 times parallel experiments, and the test result was "- ". For the mechanical mixing method, even when the KCl was increased to 45%, as shown in Fig.10c, the witness plates were still wholly broken down, and the result was “+”. It can be concluded that solution mixing method is better than the mechanical mixture to suppress the detonation of AN.
Table 4 The UN gap test results of ANFO/KCl mixtures

content/%mixingmethodmass/gdensity/g·cm-3result25solution4440.89+30solution4380.87-30solution4220.84-40solution4530.90-40solution4730.94-40mechanical4570.91+45mechanical4490.89+50mechanical4520.90-50mechanical4620.92-
Note: “+” means that the sample can stably propagate the detonation under experimental conditions. “-” means that the material cannot stably propagate the detonation under experimental conditions.

a. ANFO/45% KCl b. ANFO/50% KCl
(mechanical mixing) (mechanical mixing)

c. ANFO/25% KCl d. ANFO/30% KCl
(solution mixing) (solution mixing)
Fig.10 Physical map of the UN gap test for ANFO/KCl mixtures
Hot spots played an important role in the detonation of AN. During the detonation process, the shock wave deforms and compresses AN, which then engulfs the area occupied by the void. What remains are hot spots-small isolated regions of explosive at a much higher temperature than the surrounding material. These hot spots are where ignition of the explosive begins[4, 27]. It is known that physical structure has a significant influence on the formation of hot spots. The SEM images of the AN/KCl mixtures at different magnification are shown in Fig.11, and the surface of AN/KCl mixtures exhibited different shape. For the solution mixing, the growth of a given face is governed by the crystal structure and defects, environmental conditions, growth rates, etc. Fig.11a. shows the surface and edges of AN/10% KCl mixtures (solution mixing method). The surface and edges were structured, smooth and fewer gaps. The surface and edges of mixtures (mechanical mixing method) mainly depend on mechanical strength. In the process of mechanical mixing, the crystal has a certain degree of broken under the action of external stress and friction. Fig.11b shows that the AN/10% KCl (mechanical mixing method) mixtures have irregular shapes and rough surfaces. Irregular shapes and rough surfaces are more favorable for forming these hot spots[4, 28-29].

a. solution mixing

b. mechanical mixing
Fig.11 SEM images of AN/10% KCl mixtures
On the other hand,AN has five polymorphic forms under ambient pressure. The phase Ⅳ to phase Ⅲ near the temperature (32 ℃) can cause variation in density and 3.8% volume change, this lead to porous structure and cracked crystals with poor mechanical strength[30-31]. These structures increase the possibility of formation of hot spots. Fig.12 shows the phase transition of AN, the endothermic peak at 38.5 ℃ is the Ⅳ to Ⅲ phase change, and the endothermic peak of AN/10% KCl mixture (solution mixing method) is disappeared. It can be concluded that KCl can inhibit Ⅳ to Ⅲ phase change effectively and reduce the possibility of the formation of hot spots. For the mechanical mixing method, there is no ions exchange between AN and KCl, so the AN exhibited sequential Ⅳ↔Ⅲ↔Ⅱ↔Ⅰ transitions as temperatures rise.

Fig.12 DSC curves of AN and AN/10% KCl mixtures
According to the quaternary phase diagram of KCl-NH4NO3-KNO3-NH4Cl, the crystallization area of KCl and NH4NO3did not connect to each other. KCl and NH4NO3was unstable salt pair in the process of re-crystallization. On the contrary, KNO3/NH4Cl were stable salt pair, this provides conditions for the metathesis reaction[32]. In the process of detonation, KNO3and NH4Cl will interact with each other and generate NH4NO3and KCl under the action of high temperature and high pressure. The ion exchange will generate the molecular level of KCl[4, 27], and the multi-step reaction will delay the explosion reaction and reduced the intensity of explosion. Meanwhile in the process of re-crystallization, KCl and AN mixed more uniform, so solution mixing method is better than the mechanical mixture to suppress the detonation of AN.
4 Conclusion
To explore the explosion suppression mechanism of AN, thethermal stability and the explosive performance of AN/KCl mixtures have been investigated. The following conclusions can be drawn:

(2) The onset temperature of AN/20%KCl mixture (solution mixing method) was increased from 204.33 ℃ to 235.15 ℃, and the AN/20%KCl mixture (mechanical mixing method) was increased to 244.50 ℃, KCl can increase the onset temperature of AN decomposition.
(3) The AN/30%KCl(solution mixing method) and AN/35%KCl(mechanical mixing method) mixtures did not explode after sustained heating for 5 minutes, showing that KCl can reduce the thermal sensitivity of the AN. In addition, the mixing methods is not significantly affected the thermal sensitivity of AN.
(4) The AN/30%KCl(solution mixing method) can reduce the ability to propagate a detonation, while AN can still produce a steady detonation, even when containing 45% KCl(mechanical mixing method). Solution mixing method is better than the mechanical mixture to suppress the detonation of AN, and mixing method is an important parameter affecting the detonation characteristics of AN.
(5) KCl can accelerate the decomposition of AN, while it still can reduce the thermal sensitivity and the ability to propagate a detonation of AN. Whether additives can reduce or increase the explosivity of AN needs to be confirmed by test, rather than just judge by whether the additives can increase the thermal stability of AN or not.
[1] Kirk-Othmer. Encyclopedia of Chemical Technology[M]. New York: Wiley, 1992.
[2] Ullmann. Encyclopedia of Industrial Chemistry[M]. Germany: VCH, 1989.
[3] Paszula J, Trzciński W A, Sprzątczak K. Detonation performance of aluminium-ammonium nitrate explosives[J].CentralEuropeanJournalofEnergeticMaterials, 2008, 5: 3-11.
[4] Lu C X, Liu Z l, Ni O Q. Theory of industrial explosives. Beijing: Arms Industry Press, 1994.
[5] Oommen C,Jain S R. Phase modification of ammonium nitrate by potassium salts[J].JThermAnalCalorim, 1998, 55: 903-918.
[6] Klimova I, Kaljuvee T, Turn L, et al. Interactions of ammonium nitrate with different additives[J].JThermAnalCalorim, 2011, 105: 13-26.
[7] Kazuomi K, Izato Y, Atsumi M. Thermal characteristics of ammonium nitrate, carbon, and copper(II) oxide mixtures[J].JThermAnalCalorim, 2013, 113(3): 1475-1480.
[8] Buczkowski D, Zygmunt B. Detonation properties of mixtures of ammonium nitrate based fertilizers and fuels[J].CentralEuropeanJournalofEnergeticMaterials, 2011, 8(2): 99-106.
[9] Richard G, Zhang D K. Thermal stability and kinetics of decomposition of ammonium nitrate in the presence of pyrite[J].JournalofHazardousMaterials, 2009, 165: 751-758.
[10] Atsumi M, Keiya T. Influence of physical properties of ammonium nitrate on thedetonation behaviour of ANFO[J].JournalofLossPreventionintheProcessIndustries, 2001, 14: 533-538.
[11] AtsumiM, Hidefumi K. Detonation characteristics of ammonium nitrate andactivated carbon mixtures[J].JournalofLossPreventionintheProcessIndustries, 2007, 20: 584-588.
12] Vytenis B. Explosions of ammonium nitrate fertilizer in storage or transportation are preventable accidents[J].JournalofHazardousMaterials, 2016, 304: 134-149.
[13] Anuj A V, Mijia S J. Effect of copper Oxide, titanium dioxide, and lithium fluoride on the thermal behavior and decomposition kinetics of ammonium nitrate[J].JournalofEnergeticMaterials, 2014, 32: 146-161.
[14] Tang S L, Liu Z L, Zhu G J. and Lu C X. Effect of additives on detonation safety and heat stability of ammonium nitrate[J].ChemicalFertilizerIndustry, 2003, 30: 28-29.
[15] Bernard J W, Henry W. Acid catalysis in the thermal decomposition of ammonium nitrate[J].JournalofChemicalPhysics, 1955, 23(4): 693-696.
[16] Jimmie C O, James L S, Evan R et al. Ammonium nitrate: thermal stability and explosivity modifiers[J].ThermochimicaActa, 2002, 384: 23-45.
[17] Zhe H, Sonny S, Maria I P, et al. Ammonium nitrate thermal decomposition with additives[J].JournalofLossPreventionintheProcessIndustries, 2015, 35: 307-315.
[18] Rubtsov Y I, Strizhevskii I I, Kazakov A I. Kinetics of the influence of Cl on thermal decomposition of AN[J].ZhurnalPrikladnoiKhimii, 1989, 62 (11): 2417-2422.
[19] LI X R, KOSEKI H. Study on the contamination of chlorides in ammonium nitrate[J].ProcessSafetyandEnvironmentalProtection, 2005, 83(B1): 31-37.
[20] United Nations. Recommendations on the transport of Dangerous Goods-model regulations (16thed)[M]. New York and Geneva: United Nations Publications, 2009.
[21] United Nations. Recommendations on the Transport of Dangerous Goods-model of Tests and Criteria(16th ed)[M]. New York and Geneva: United Nations Publications, 2009.
[22] Fu Z M, Li X R, Koseki H, et al. Evaluation on thermal hazard of methyl ethyl ketone peroxide by using adiabatic method[J].JournalofLossPreventionintheProcessIndustries, 2003, 16: 389-393.
[23] Sun J H, Li Y F, Hasegawa K. A study of self-accelerating decomposition temperature(SADT) using reaction calorimetry[J].JournalofLossPreventionintheProcessIndustries, 2001,14:331-336.
[24] Townsend D I, Tou J C. Thermal hazard evaluation by an accelerating rate calorimeter[J].ThermochimicaActa, 1980, 37: 1-30.
[25] Sun J H, Sun Z H. Catalytic effects of inorganic acids on the decomposition of ammonium nitrate[J].JournalofHazardousMaterials, 2005, 127: 204-210.
[26] Medard L A. AN and its thermal decomposition in accidental Explosions, Vol.2, Types of Explosive Sub-stances, New York: Wiley,1989.
[27] Lu C X. A study on crystalline characteristics of expanded ammonium nitrate[J].ActaArmamentarii, 2003, 23(3): 316-319.
[28] Joseph H M, Zhang H T, Polly B, et al. Catalytic decomposition of ammonium nitrate in superheated aqueous solution[J].JAmChemSoc, 1997, 119: 9738-9744.
[29] Sun Y. Study on the flame inhibitors used to improve the safety of coalmine permissible emulsion explosive[J].CoalMineBlasting, 2011, 95(4): 15-18.
[30] Michael J H, Walter E. Phase transitions and lattice dynamics of ammonium nitrate[J].Propellants,Explosives,Pyrotechnics, 1997, 22: 143-147.
[31] Anthony J L, Sergey V. Phase and thermal stabilization of ammonium nitrate in theform of PVP-AN glass[J].MaterialsLetters, 2008, 62: 1757-1760.
[32] Li T W, Zhang T L. Progress on study of phase equilibria of solid solution containing salt water systems[J].Sea-LakeSaltandChemicalIndustry, 2001, 30: 7-11.
