A Colorimetric H2O2Sensor Based on the CdS-SiO2Nanocomposite as a Peroxidase-like Mimic
2017-03-28DINGYanyuanGAOYanXUZheLIUQingyun
DING Yanyuan, GAO Yan, XU Zhe, LIU Qingyun
(College of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao,Shandong 266590, China)
A Colorimetric H2O2Sensor Based on the CdS-SiO2Nanocomposite as a Peroxidase-like Mimic
DING Yanyuan, GAO Yan, XU Zhe, LIU Qingyun
(College of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao,Shandong 266590, China)
CdS-SiO2nanocomposites were synthesized on a glass substrate and characterized by X-ray diffraction (XRD) and transmission electron microscopy (TEM), respectively. Similar to nature enzyme, CdS-SiO2nanocomposites can catalyze the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of H2O2to produce a blue product which can be seen by the naked eye, demonstrating that the CdS-SiO2nanocomposites possess the peroxidase-like activity. Moreover, CdS-SiO2nanocomposites exhibited a high activity and stability in HAc-NaAc buffer (pH=4.0) at 60 ℃. Furthermore, the catalysis mechanism was studied using a probe, and proved to be from hydroxyl radicals. Based on the peroxidase-like activity of the CdS-SiO2nanocomposites, a novel colorimetric sensor was designed to detect quantitatively H2O2in the range of 5 to 40 μM (R2= 0.994) with a low detection limit of 4.2 μM.
CdS-SiO2nanocomposites; peroxidase-like mimic; colorimetric H2O2sensor
Hydrogen peroxide (H2O2) is not only the final or the intermediate product of many biochemical reactions which are closely related with biological processes, but also an essential material used in pharmaceutical, biological, and environmental analyses[1]. For example, H2O2is one of the main products of the glucose oxidase (GOx)-catalyzed reaction. Hence, the level of blood glucose can be monitored by detecting the produced H2O2in the process of glucose oxidation in order to control some diseases such as diabetes mellitus. Colorimetric biosensing been applied in the various research fields, e.g., biomedical diagnosis, environmental monitoring and food safety analysis[2-7], due to its several important advantages, such as simplicity, practicality, low cost. In this method, color changes can be distinguished by the naked eye instead of discriminated by sophisticated instrumentation and can be applied to field analysis and point-of-care diagnosis[8-10]. Several methods and strategies have been developed for this purpose, including aggregation based colorimetric immunoassay[11], lateral-flow colorimetric immunoassay[12-13]and enzyme-mediated colorimetric immunoassay[14-15]. However, the sensitivity of the methods described above is relative low because of the lack of a signal amplification step. Considering the sensitivity and selectivity of the colorimetric sensor, the use of catalysts or enzyme mimics is necessary and important. Hence, scientists have paid more attention to find peroxidase mimics instead of natural peroxidase.
Recently, with the wide development of nanoscience and technology, a lot of smart materials were found to possess intrinsic enzyme activity and shown much potential application for colorimetric biosensing on account of their unique physical or chemical properties. According to the previous reports, there are various nanomaterials have been studied, such as carbon nanotubes[16-17], nanodots[18], graphene oxide (GO) and GO composites[19], metal oxides[20], and bimetallic nanostructures[21-23], etc. These nanomaterials have been found to possess the peroxidase-like catalytic activity and could serve as promising candidates for natural enzymes in colorimetric biosensing. On the other hand, functional organic molecules modified inorganic nanomaterials have been found to possess an enhanced peroxidase mimetic activity, such as H2TCPP-CdS nanocomposites[24], H2TCPP-Fe3O4nanocomposites[25], H2TCPP-NiO nanoparticles[26], H2TCPP-γ-Fe2O3nanoparticles[27], H2TCPP-Co3O4nanoparticles[28]and H2TCPP-CeO2nanorods[29], etc. Furthermore, some montmorillonites (MMT) supported nanocomposites, including ZnS-MMT[30]and Ag2S-MMT[31], can catalytically oxidize peroxidase substrate TMB in the presence of H2O2to produce a blue color reaction that can be easily observed by naked eye. However, to the best of our knowledge, there is no report on CdS-SiO2nanocomposites as a peroxidase mimic to detect H2O2.
Herein, CdS-SiO2nanocomposites were synthesized on a glass substrate by a facile method under mild conditions at room temperature, as illustrated in Fig. 1. As expected, the CdS-SiO2nanocomposites exhibited the intrinsic peroxidase-like activity and catalyzed the oxidation of the substrate TMB in the presence of H2O2to produce a blue color reaction. Thus, the CdS-SiO2nanocomposites were used as peroxidase mimic to design a H2O2colorimetric sensor. In addition, fluorescent results demonstrated that the peroxidase-like activity of CdS-SiO2nanocomposites originated from the generation of·OH radicals. Furthermore, the colorimetric method developed here showed a much wider linear detection range than that of H2TCPP-CdS nanocomposites as catalyst (4-14 μM) in the previous publication[24].
1 Experimental
1.1 Chemicals
Ethyl silicate (C8H20O4Si), ammonia (25%, NH3·H2O), ethanol (CH3CH2OH), cadmium acetate (Cd(Ac)2·2H2O), thioacetamide (C2H5NS), hydrogen peroxide (30%, H2O2), acetic acid (HAc), sodium acetate (NaAc), 3,3′,5,5′-tetramethylbiphenyl dihydrochloride (TMB·2HCl) was purchased from Solarbio (Beijing, China). All the regents above were of analytical reagent grade and used without further purification.
1.2 Characterization
Structural analysis of the synthesized samples was carried out using powder the X-ray diffraction (XRD) over the 2θ range of 10°~80°and the scan rate was 8°·min-1with graphite monochromatized Cu Kα radiation (D/Max2500PC, Rigaku). The morphology of the nanocomposites was studied by using a transmission electron microscope at an accelerating voltage of 200 kV (TEM JEM-2100, JEOL, Japan). The elements composition of the CdS-SiO2nanocomposites was determined by energy dispersive X-ray spectroscopy (EDX, Hitachi, S4800). Ultraviolet spectra were recorded on a MAPADA UV-3200PC spectrophotometer (Shanghai, China). The photoluminescence spectras were obtained using an F-4600 FLSPECTOROPHOTOMET spectrofluorophotometer (Hitachi High-Tech Science Corporation, Tokyo, Japan).

Fig.1 The synthesis process of CdS-SiO2
1.3 Preparation of CdS-SiO2
As shown in Fig.1,20 mg as-prepared SiO2powder was dissolved into 2 mL deionized water and the obtained solution was dealt under ultrasonic condition for 5 min to form a homogeneous suspension. Then, the suspension was laid on a hydrophilic glass substrate. Subsequently, the glass substrate was transferred to an oven and maintained at 50℃ for 3 h. Cd(Ac)2·2H2O was dissolved into some deionized water to form an aqueous solution (0.4 mol/L), which was spread on the surface of the glass substrates loaded with SiO2nanoparticles. Simultaneously, thioacetamide was dissolved in HCl solution(0.5 mol/L). Then, the substrates and thioacetamide solution were placed into the same container for 24 h at room temperature. Finally, the faint yellow CdS-SiO2nanocomposites were obtained for the subsequent experiments.
1.4 Test procedure
In order to investigate the peroxidase-like catalytic activity of the CdS-SiO2nanocomposites, the oxidation of TMB was examined in HAc-NaAc buffer (pH=4.0) containing CdS-SiO2samples (2 mg/mL), H2O2(25 mM) and TMB (0.1 mM) at room temperature. The tests were monitored in wavelength-scan mode after reacted for 30 min or time-course mode at 652 nm. While keeping the TMB and H2O2concentration constant, kinetic analysis was performed by varying pH of the buffer (2.2-9) and temperature (20-75 ℃). Steady-state kinetic assays were carried out in HAc-NaAc buffer (pH=4.0) containing CdS-SiO2, H2O2(0.01-0.08 mM) and TMB (0.08-0.2 mM) by recording the absorption spectras at 652 nm in scanning kinetics mode.
To illuminate the catalytic mechanism of CdS-SiO2as a peroxidase mimic for the oxidation of TMB, a probe method was used. The detailed method is as follows, 25 mM H2O2, 0.5 mM terephthalic acid, and CdS-SiO2with different concentration(0-1.4 mg·mL-1) were incubated in 1.4 mL HAc-NaAc buffer (pH=4.0) at 60 ℃ for 1 h. After that, the incubated solutions were used for the fluorometric measurement.

Fig.2 XRD patterns of pure CdS nanoparticles (a),

Fig.3 TEM image of as-synthesized CdS-SiO2
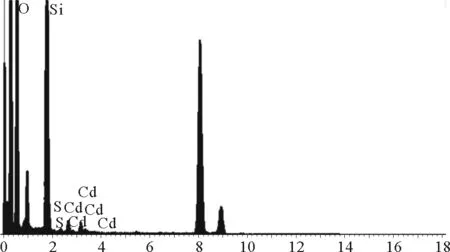
Fig.4 EDX spectrum of CdS-SiO2
2 Results and discussion
2.1 Characterization of CdS-SiO2
Fig.2 is the XRD patterns of pure CdS, pure SiO2and the CdS-SiO2nanocomposites, respectively. As shown in Fig. 2, comparing curve b with curve a and curve c, it was easily to find that some diffraction peaks (marked with*) in curve b can be assigned to the (100), (002), (102), (110) crystal planes of the CdS nanoparticles in curve a. This result confirmed that the CdS nanoparticles was successful deposited on SiO2nanospheres and gave the CdS-SiO2nanocomposites.
The shape, size, and structure of the as-synthesized CdS-SiO2nanocomposites were investigated by TEM, shown in Fig. 3. From Fig. 3, it can be seen that the nanocomposites are irregular particles with the diameter in the range of 50-150 nm.
In order to confirm the successful deposition of CdS nanoparticles on the surface of SiO2nanospheres, the composition of the CdS-SiO2nanocomposites was characterized by EDX, shown in Fig. 4. The signals of elements, including Si, O, Cd and S can be found in the EDX spectrum, revealing the formation of CdS-SiO2nanocomposites.
2.2 Peroxidase activity of the CdS-SiO2
In this study, we evaluated the catalytic activity of the CdS-SiO2nanocomposites using a peroxidase-like catalytic reaction involving the oxidation of TMB in the presence of H2O2. Control experiments suggested that CdS-SiO2nanocomposites demonstrate the peroxidase-like activity. It can be seen that the absorbance (652 nm) of reaction systems of CdS-SiO2+TMB, as well as H2O2+ TMB, is relative weak, shown in Fig. 5, curve a and c. Nevertheless, the absorbance (652 nm) of reaction systems of CdS-SiO2+ H2O2+ TMB is stronger than that of two others described above at the identical conditions, shown in Fig. 5, curve d. Furthermore, the similar results can be also seen from the inset of corresponding photograph. In experiments, it can be found that the blue color of system d is deeper than that of system a, b and c, indicating that both H2O2and CdS-SiO2nanocomposites are required for the catalytic reactions. According to the previous report, as shown in Fig. 5, curve d, strong adsorption at 652 nm appeared for the CdS-SiO2nanocomposites+TMB+H2O2system, are ascribed to the charge-transfer complexes derived from the one-electron oxidation of TMB[32], similar to the phenomena observed for the commonly used horse radish peroxidase (HRP) enzyme[33], a natural enzyme. This result revealed that CdS-SiO2nanocomposites could catalyze the oxidation of TMB in the presence of H2O2, demonstrating the peroxidase-like activity of CdS-SiO2nanocomposites.
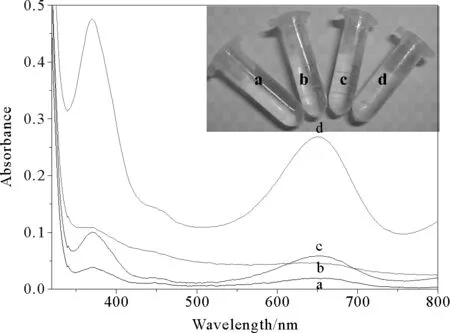
Fig.5 UV-Vis spectra of different samples: TMB solution (a),
The peroxidase-like catalytic activity of CdS-SiO2was further investigated using steady-state kinetics with TMB and H2O2as substrates, respectively. The details of the peroxidase-like activity of CdS-SiO2were fitted with the classical Michaelis-Menten model[34]. Within a certain range of TMB and H2O2concentration, typical Michaelis-Menten curves can be obtained, shown in Fig. 6 (a) and (b), respectively. The double reciprocal plots were obtained according to the calculated series of the initial reaction rates by the Michaelis-Menten equation,ν=Vmax× [S]/(Km+ [S]), whereνis the initial velocity,Vmaxis the maximal reaction velocity, [S] is the concentration of the substrate andKmis the Michaelis constant. In Fig. 6 (c) and (d), the lines were obtained by changing reciprocal initial velocity with reciprocal TMB and H2O2. TheKmvalue andVmaxvalue were obtained from Lineweaver-Burk plots, shown in Table 1. As we all known,Kmis an important parameter to measure binding affinity of the enzyme to the substrate, and can be applied similarly here to study the interaction between CdS-SiO2nanocomposites with H2O2and TMB. SmallerKmvalue indicates a higher affinity between enzyme and substrate. As can be seen in Table 1, theKmvalues of CdS-SiO2with TMB and H2O2were all smaller than that of HRP, demonstrating that the CdS-SiO2has higher affinity to TMB and H2O2, compared with that of HRP[33].
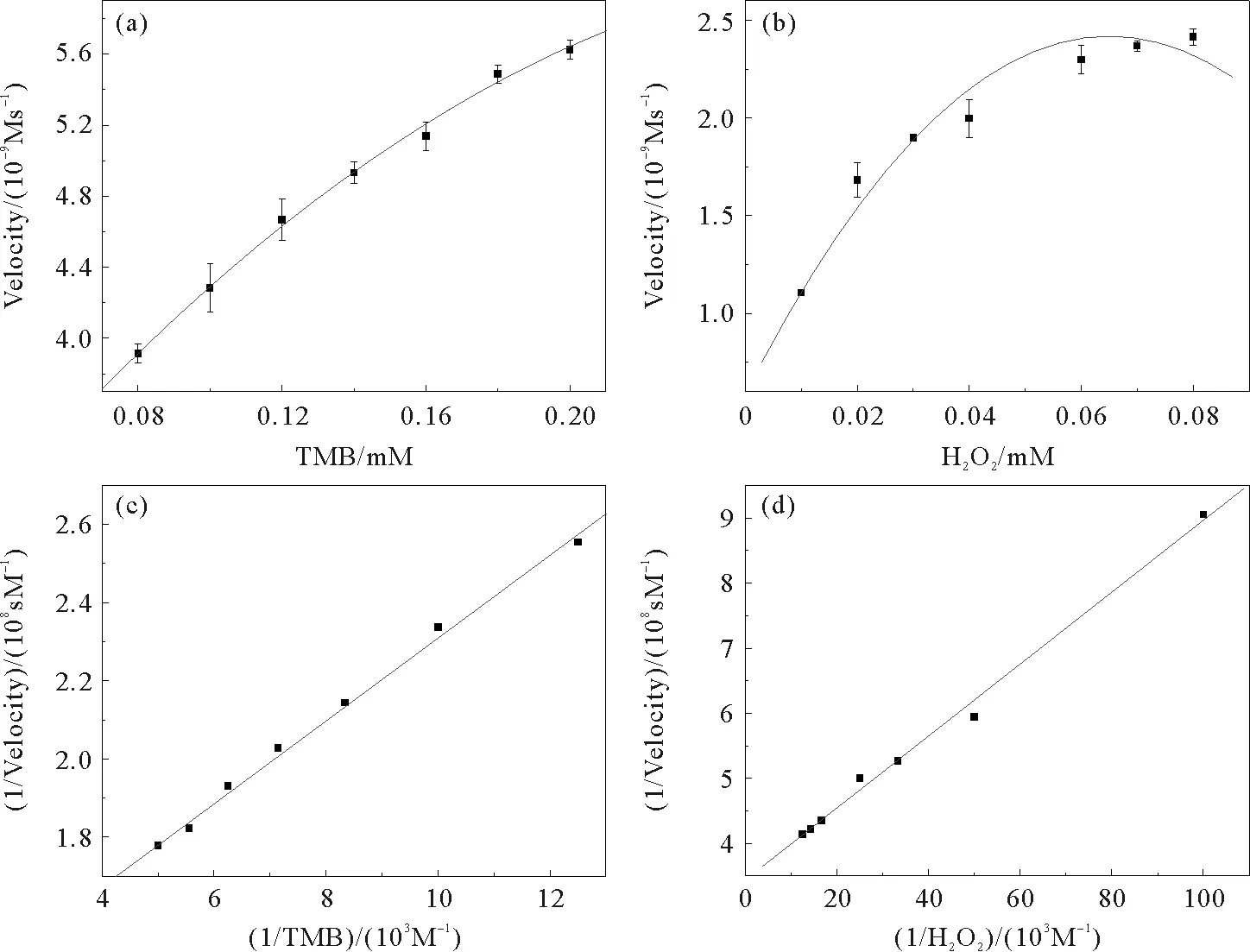
Fig.6 Steady-state kinetic analysis using the Michaelis Menten model (a and b) and Lineweaver Burk model (c and d) for CdS-SiO2. The concentration of TMB was 0.1 mM and H2O2concentration was varied (b and d), the concentration H2O2of was 25 mM and TMB concentration was varied (a and c).
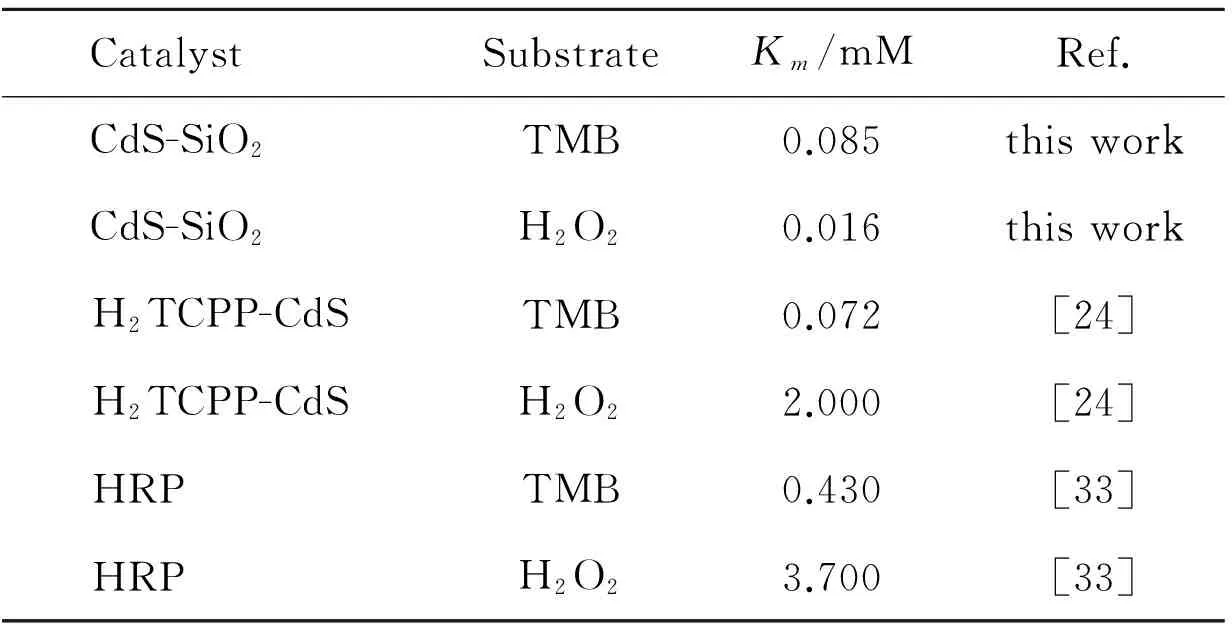
CatalystSubstrateKm/mMRef.CdS-SiO2TMB0.085thisworkCdS-SiO2H2O20.016thisworkH2TCPP-CdSTMB0.072[24]H2TCPP-CdSH2O22.000[24]HRPTMB0.430[33]HRPH2O23.700[33]
2.3 Optimization of experimental conditions
Any catalytic reaction depends on experimental parameters to obtain the maximum activity. Efforts were made to explore the catalytic activity of CdS-SiO2under various conditions such as pH and temperature. The catalytic activity of CdS-SiO2nanocomposites was tested by varying pH values from 2.2 to 9.0 or by changing reaction temperature from 20 to 75 ℃, while keeping the other substrate constant. From the experimental results (Fig. 7 (a) and (b)), the optimized pH and temperature were found as 4.0 and 60 ℃, respectively. Therefore, 4.0 was selected as the optimum pH as well as 60 ℃ was regarded as the optimum incubated temperature.

Fig.7 The effect of physicochemical conditions on peroxidase-like activity of CdS-SiO2: (a) pH,(b) temperature. The relative activity was defined as the ration of catalytic response at a given condition to
2.4 Mechanism analysis
In order to explore the catalytic mechanism of the CdS-SiO2nanocomposites, a fluorescent probe (terephthalic acid) was chosen to evaluate the effects of CdS-SiO2nanocomposites on generation of hydroxyl radicals[35]. From Fig. 8, it clearly showed that there was no fluorescence signal in the absence of H2O2(curve a), while the fluorescence intensity gradually increased with the increase of the amount of the catalyst, CdS-SiO2nanocomposites (curves b-g, Fig. 8). This result indicated that the catalytic activity of CdS-SiO2nanocomposites was attributed to the decomposition of H2O2into hydroxyl radicals, as shown in Scheme 1.

Fig.8 Influences of the CdS-SiO2nanocomposites on

Scheme 1 The catalytic mechanism of the CdS-SiO2nanocomposites toward the oxidation of TMB

Fig. 9 A dose-response curve for H2O2detection using CdS-SiO2nanocomposites(Inset: Plot of calibration curve for H2O2concentration (5-40 μM). The error bars
2.5 Detection of H2O2
Because the absorbance of oxTMB is H2O2concentration-dependent in the presence of the CdS-SiO2nanocomposites, the system described above could be used to detect H2O2. Fig. 9 shows a typical H2O2concentration response curve under the optimal conditions. There is a good linear relationship between the absorbance at 652 nm and the concentration of H2O2in the range of 5-40 μM with a detection limit of 4.2 μM. The detection method based on CdS-SiO2nanocomposites gave a much wider linear detection range than that of H2TCPP-CdS NCs as catalyst (4-14 μM) in the previous publication[24].
3 Conclusions
In summary, the CdS-SiO2nanocomposites were found to possess intrinsic peroxidase-like activity and could catalytically oxidize the substrate TMB by H2O2to produce a typical color reaction. Moreover, for the CdS-SiO2nanocomposites, the kinetic parameter (Km) is significantly smaller than that of HRP indicating a stronger affinity between H2O2and TMB. Furthermore, fluorescent results suggest that the nature of the peroxidase-like activity of CdS-SiO2nanocomposites may originate from their catalytic ability to H2O2decomposition into hydroxyl radicals. In addition, based on this finding, we provide a simple, highly sensitive visual and colorimetric method for detection of quantitative detection of H2O2. This colorimetric assay demonstrates not only higher sensitivity but also a wider responding range to H2O2, suggesting promising applications in biochemical analysis and environmental monitoring.
[1]YIN F, SHIN H K, KWON Y S. A hydrogen peroxide biosensor based on Langmuir-Blodgett technique: Direct electron transfer of hemoglobin in octadecylamine layer[J]. Talanta, 2005, 67(1):221-226.
[2]ARAGAY G, PINO F, MERKOCI A. Nanomaterials for sensing and destroying pesticides[J]. Chemical Reviews, 2012, 112(10):5317-5338.
[3]SONG Y J, WEI W L, QU X G. Colorimetric biosensing using smart materials[J]. Advanced Materials, 2011, 23(37):4215-4236.
[4]LEI J P, JU H X. Signal amplification using functional nanomaterials for biosensing[J]. Chemical Society Reviews, 2012, 41(6):2122-2134.
[5]MARZO A, PONS J, BLAKE D, et al. All-integrated and highly sensitive paper based device with sample treatment platform for Cd2+immunodetection in drinking/tap waters[J]. Analytical Chemistry, 2013, 85(7):3532-3538.
[6]PERFEZOU M, TURNER A, Merkoci A. Cancer detection using nanoparticle-based sensors[J]. Chemical Society Reviews, 2012, 41(7):2606-2622.
[7]SWIERCZEWSKA M, LIU G, LEE S, et al. High-sensitivity nanosensors for biomarker detection[J]. Chemical Society Reviews, 2012, 41(7):2641-2655.
[8]KONG B, ZHU A W, LUO Y P, et al. Sensitive and selective colorimetric visualization of cerebral dopamine based on double molecular recognition[J]. Angewandte Chemie International Edition, 2011, 50(8):1837-1840.
[9]MIRANDA O R, LI X N, GARCIA-GONZALEZ L, et al. Colorimetric bacteria sensing using a supramolecular enzyme-nanoparticle biosensor[J]. Journal of the American Chemical Society, 2011, 133(25):9650-9653.
[10]JIANG Y, ZHAO H, LIN Y Q, et al. Colorimetric detection of glucose in rat brain using gold nanoparticles[J].Angewandte Chemie International Edition, 2010, 49(28):4800-4804.
[11]RAGAVAN K V, SELVAKUMAR L S, THARKUR M S. Functionalized aptamers as nano-bioprobes for ultrasensitive detection of bisphenol-A[J]. Chemical Communications, 2013, 49(53):5960-5962.
[12]LI Z H, WANG Y, WANG J, et al. Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip[J]. Analytical Chemistry, 2010, 82(16):7008-7014.
[13]YANG D, MA J Z, ZHANG Q L, et al. Polyelectrolyte-coated gold magnetic nanoparticles for immunoassay development: Toward point of care diagnostics for syphilis screening[J]. Analytical Chemistry, 2013, 85(14):6688-6695.
[14]ALKASIR R S J, ORNATSKA M, ANDREESCU S. Colorimetric paper bioassay for the detection of phenolic compounds[J]. Analytical Chemistry, 2012, 84(22):9729-9737.
[15]ORNATSKA M, SHARPE E, ANDREESCU D, et al. Paper bioassay based on ceria nanoparticles as colorimetric probes[J]. Analytical Chemistry, 2011, 83(11):4273-4280.
[16]SONG Y J, WANG X H, ZHAO C, et al. Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity[J]. Chemistry-A European Journal, 2010, 16(12):3617-3621.
[17]CUI R J, HAN Z D, ZHU J J. Helical carbon nanotubes: Intrinsic peroxidase catalytic activity and its application for biocatalysis and biosensing[J]. Chemistry-A European Journal, 2011, 17(34):9377-9384.
[18]SHI W B, WANG Q L, LONG Y J, et al. Carbon nanodots as peroxidase mimetics and their applications toglucose detection[J]. Chemical Communications, 2011, 47(23):6695-6697.
[19](a) ZHANG L N, DENG H H, LIN F L, et al. In situ growth of porous platinum nanoparticles on graphene oxide for colorimetric detection of cancer cells[J]. Analytical Chemistry,2014,86(5): 2711-2718; (b)TAO Y, LIN Y H, HUANG Z Z, et al. Incorporating graphene oxide and gold nanoclusters: A synergistic catalyst with surprisingly high peroxidase-like activity over a broad pH range and its application for cancer cell detection[J]. Advanced Materials, 2013, 25(18):2594-2599; (c) SONG Y J, CHEN Y, FENG L Y, et al. Selective and quantitative cancer cell detection using target-directed functionalized graphene and its synergetic peroxidase-like activity[J]. Chemical Communications, 2011, 47(15):4436-4438; (d) SONG Y J, QU K G, ZHAO C, et al. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection[J]. Advanced Materials, 2010, 22(19):2206-2210.
[20](a) ANDRE R, NATA L F, HUMANES M, et al. V2O5nanowires with an intrinsic peroxidase-like activity[J]. Advanced Functional Materials, 2011, 21(3):501-509; (b) WANG Y X, ZHANG X, LUO Z M, et al. Liquid-phase growth of platinum nanoparticles on molybdenum trioxide nanosheets: An enhanced catalyst with intrinsic peroxidase-like catalytic activity[J]. Nanoscale, 2014, 6(21):12340-12344.
[21]HE W W, LIU Y, YUAN J S, et al. Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays[J]. Biomaterials, 2011, 32(4):1139-1147.
[22]HE W W, WU X C, LIU J B, et al. Design of AgM bimetallic alloy nanostructures (M = Au, Pd, Pt) with tunable morphology and peroxidase-like activity[J]. Chemistry of Materials, 2010, 22(9):2988-2994.
[23](a) GE S G, LIU F, LIU W Y, et al. Colorimetric assay of K-562 cells based on folic acid-conjugated porous bimetallic Pd@Au nanoparticles for point-of-care testing[J]. Chemical Communications, 2014, 50(4):475-477; (b) LI X R, XU M C, CHEN H Y, et al. Bimetallic Au@Pt@Au core-shell nanoparticles on graphene oxide nanosheets for high-performance H2O2bi-directional sensing[J]. Journal of Materials Chemistry B, 2015, 3(21):4355-4362.
[24]LIU Q Y, JIA Q Y, ZHU R R, et al. 5,10,15,20-tetrakis(4-carboxyl phenyl)porphyrin-CdS nanocomposites with intrinsic peroxidase-like activity for glucose colorimetric detection[J]. Materials Science and Engineering C, 2014, 42:177-184.
[25]LIU Q Y, LI H, ZHAO Q R, et al. Glucose-sensitive colorimetric sensor based on peroxidase mimics activity of porphyrin-Fe3O4nanocomposites[J]. Materials Science and Engineering C, 2014, 41:142-151.
[26]LIU Q Y, YANG Y T, LI H, et al. NiO nanoparticles modified with 5,10,15,20-tetrakis(4-carboxyl pheyl)-porphyrin: Promising peroxidase mimetics for H2O2and glucose detection[J]. Biosensors and Bioelectronics, 2015, 64:147-153.
[27]LIU Q Y, ZHANG L Y, LI H, et al. One-pot synthesis of porphyrin functionalized γ-Fe2O3nanocomposites as peroxidase mimics for H2O2and glucose detection[J]. Materials Science and Engineering C, 2015, 55:193-200.
[28]LIU Q Y, ZHU R R, JIANG Y L, et al. A facile one-pot synthesis of higher yield porphyrin functionalized Co3O4nanoparticles[J]. Materials Science and Engineering B, 2015, 198: 57-61.
[29]LIU Q Y, DING Y Y, YANG Y T, et al. Enhanced peroxidase-like activity of porphyrin functionalized ceria nanorods for sensitive and selective colorimetric detection of glucose[J]. Materials Science and Engineering C, 2016, 59:445-453.
[30]LIU Q Y, JIANG Y L, ZANG L Y, et al. The catalytic activity of Ag2S-montmorillonites as peroxidase mimetic toward colorimetric detection of H2O2[J]. Materials Science and Engineering C, 2016, 65:109-115.
[31]DING Y Y, SUN L F, JIANG Y L, et al. Facile strategy for the preparation of ZnS nanoparticles deposited on montmorillonite and their higher catalytic activity for rapidly colorimetric detection of H2O2[J]. Materials Science and Engineering C, 2016, 67:188-194.
[32]SUN X L, GUO S J, CHUNG C S, et al. A sensitive H2O2assay based on dumbbell-like PtPd-Fe3O4nanoparticles[J]. Advanced Materials, 2013, 25(1):132-136.
[33]GAO L Z, ZHUANG J, NIE L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles[J]. Nature Nanotechnology, 2007(2): 577-583.
[34]SWITALA J, LOEWEN P C. Diversity of properties among catalases[J]. Archives of Biochemistry and Biophysics, 2002, 401(2):145-154.
[35]MU J S, WANG Y, ZHAO M, et al. Intrinsic peroxidase-like activity and catalase-like activity of Co3O4nanoparticles[J]. Chemical Communications, 2012, 48(19): 2540-2542.
(责任编辑:吕海亮)
2017-01-05
国家自然科学基金项目(21271119)
丁艳园(1990—),女,山东聊城人,硕士研究生,主要从事纳米复合材料的制备及比色传感器研究. 刘青云(1970—),女,山东高密人,教授,博士生导师,主要从事纳米复合材料的制备及模拟酶性质的应用研究,本文通信作者.E-mail: qyliu@sdust.edu.cn
基于CdS-SiO2纳米复合材料的过氧化物模拟酶H2O2比色传感器
丁艳园,高 燕,许 哲,刘青云
(山东科技大学 化学与环境工程学院,山东 青岛 266590)
在玻璃基板上合成CdS-SiO2纳米复合材料,并分别通过X射线衍射(XRD)和透射电子显微镜(TEM)进行表征。 与自然酶辣根酶类似,CdS-SiO2纳米复合材料可以催化H2O2氧化3,3′,5,5′-四甲基联苯胺(TMB),产生可以通过肉眼观察到的蓝色产物,证明CdS-SiO2纳米复合材料具有过氧化物酶样活性。 此外,CdS-SiO2纳米复合材料在60℃下于HAc-NaAc缓冲溶液(pH=4.0)中表现出较高的活性和稳定性。 另外,利用荧光探针的方法研究了催化机理,结果证明该催化机理来自于产生的羟基自由基。基于CdS-SiO2纳米复合材料的过氧化物酶活性,设计了一种新奇的H2O2比色传感器,检测H2O2的线性范围为5~40 μM(R2= 0.994),检测限为4.2 μM。
CdS-SiO2纳米复合材料;过氧化氢模拟酶;H2O2比色传感器
TB383; O657
A
1672-3767(2017)02-0048-09
