Synthesis of sulfone-containing polyimide and preparation of hollow fiber membranes
2017-02-21GanCongjunJiangXueweiDongJieZhaoXinZhangQinghua
Gan Congjun, Jiang Xuewei, Dong Jie, Zhao Xin, Zhang Qinghua
(State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai, 201620)
Synthesis of sulfone-containing polyimide and preparation of hollow fiber membranes
Gan Congjun, Jiang Xuewei, Dong Jie, Zhao Xin, Zhang Qinghua*
(State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai, 201620)
A 4,4′-(hexafluoroisopropylidene)diphthalic anhydride- bis[4-(4-aminophenoxy)phenyl] sulfone (6FDA-BAPS) polyamic acid (PAA) was synthesized by using 6FDA and BAPS as reactive monomers andN-methyl-2-pyrrolidone(NMP)asasolvent.ThePAAsolutionwaspreparedintomembranesbycastingtechnologyandwasalsopreparedintoPAAhollowfibermembranesbydrywetspinningprocess.ThePAAmembranesandPAAhollowfibermembraneswereexposedtothehigh-temperaturethermalcyclizationatabout300 ℃toproducea6FDA-BAPSpolyimide(PI)membranesandhollowfibermembranes.Thestructureandpropertiesof6FDA-BAPSPIproductswerestudied.Theresultsshowedthattheobtained6FDA-BAPSPIwasthetargetproductwhichwasoffairlygoodsolubilityinNMP,dimethylacetamideandtetrahydrofuran;the6FDA-BAPSPIpossessedthedenseouterlayerandthelooseandporoussupportlayer;thehollowfibermembraneshadthe5%weightlosstemperatureof511 ℃andbreakingstrength26.5MPa,indicatingrelativelyhighthermalandmechanicalproperties.
polyamic acid; polyimide; hollow fiber membrane; structure; properties
Polyimide (PI) is one of a high performance polymers, exhibiting outstanding properties such as electric insulation, radiation resistance, thermostability and good permeability, and the polymer has been extensively used in micro-electronics, aerospace, high temperature filtration, membrane separation and other fields[1-5].The PI hollow fiber membranes possess enormous utilization potentiality in gas absorption, membrane distillation and membrane bioreactor due to their high thermostability and large specific surface area[6-7].
Here we presents a new polymer containing bulky trifluoromethyl moieties (—C(CF3)2—) in dianhydride and polar group —O— and —SO2— in diamine to weaken the packing density of the molecular chain, and reduce the intermolecular forces as well as the solubility. Meanwhile, the introduction of the trifluoromethyl moieties can increase the free volume of the polymer and accelerate the gases diffusion within the membrane. The polar group —O— and —SO2— in diamine can increase the solubility coefficient of the CO2and H2S. This new PI is expected to improve permeance and separation performance of the PI hollow fiber membranes and has broad application prospects in the field of gas separation membrane.
1 Experimental
1.1 Materials and Measurements
4,4′-(hexafluoroisopropylidene) diphthalicanhydride (6FDA): purity 99%, purchased from Sunlight Pharmaceutical Co., Ltd..Bis[4-(4-aminophenoxy) pheny-l] sulfone (BAPS):purity 98%, purchased from J&K Chemical Ltd.N-methyl-2-pyrrolidone(NMP)andtetrahydrofuran(THF):analyticalpurity,purchasedfromSinopharmChemicalReagentCo..Nicolet8700spectrometer:ThermoElectronCo.,USA.BrukerAvance400nuclearmagnetcresonancespectrometer:BrukerCo.,Switzerland.DiscoveryTGAQ5000IRthermalanalyzer:TAInstrumentCo.,USA.HitachiSU8010scanningelectronmicroscope:HitachiLtd.,Japan.Instron5380tensilestrengthtester:InstronCo.,USA.
1.2 Experimantal method
1.2.1 Preparation of 6FDA-BAPS PI
To synthesize 6FDA-BAPS PI, BAPS (13.040 g) was dissolved in a 250 mL three-neck round-bottomed flask filled with NMP (40 mL) and THF (30 mL). After stirring for 20 min under nitrogen atmosphere, 6FDA (13.394 g) and NMP (80 mL) were slowly added into the solution in twice. The viscosity of the solution increased apparently after stirring 8 h in an ice bath of 3.5 ℃. After 5 h, a colourless sulfone-con-taining poly(amic acid) (PAA) yielded. The intermediate PAA solution was degassed at room temperature, cast onto a glass plate or spin by dry-jet-wet spinning to prepare film or hollow fiber, and then thermally treated to complete the thermal ring-closure reaction up to 300 ℃ in an oven.
1.2.2 Preparation of 6FDA-BAPS PI membranes
PAA membranes were produced by casting 3% PAA/NMP/THF solution by mass fraction onto a 2 cm×5 cm glass slide, and then stored in a vacuum oven at 60 ℃ for 24 h to ensure that the solvent was completely evaporated. Subsequently, the thermally treated at 100,200 and 300 ℃ for each 1 h to obtain 6FDA-BAPS PI membranes.
1.2.3 Fabrication of 6FDA-BAPS PI hollow fiber membranes
Asymmetric 6FDA-BAPS PI hollow fiber membranes were fabricated by the dry-jet-wet spinning process. PAA/NMP/THF spinning solution was filtered and degassed before spinning using a homemade hollow fiber spinning device. The polymer solution was pressurized through a spinneret by N2at 0.3 MPa and the internal coagulation bath directly flows into the hollow part of the spinneret. Then the polymer solution went through a certain air gap and immersed in the external coagulant bath. These fibers were taken up by a roller at a free falling velocity and stored in a water bath for at least 20 h to remove residual solvent. The membranes were dried under vacuum and then underwent thermal imidization.
2 Results and discussion
2.1 Structure of PI
As shown in Fig.1, all the vibrational bands can be assigned according to the results reported previously[8].
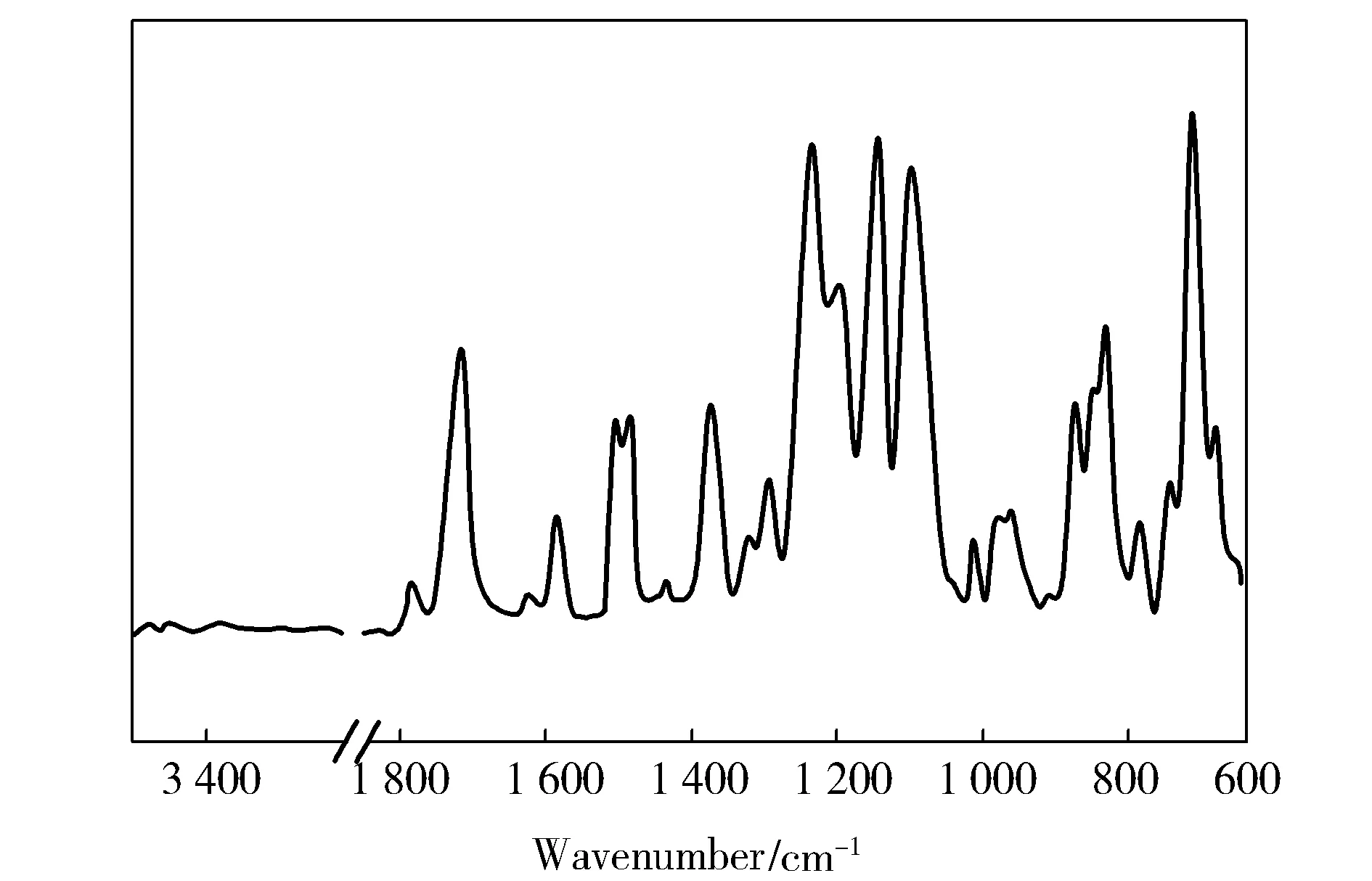
Fig.1 FTIR spectra of 6FDA-BAPS PI
The characteristic bands of 6FDA-BAPS PI at 1 781,1 719,1 371 and 718 cm-1are assigned to CO asymmetric and symmetric stretch peaks, C—N stretch peak and bending vibrations of the imide ring respectively. No characteristic peak of PAA at 3 300-3 400 cm-1indicates completely thermal imidization. Besides 1 301, 1 146 and 1 100 cm-1are attributed to the absorption peak of OSO, C—F and C—O,respectively.
Assignments of each proton are also given in Fig.3 and agree well with the proposed molecular structure in Fig.2.

Fig.2 Structure formula of 6FDA-BAPS PI
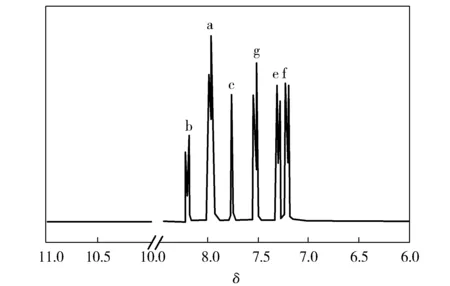
Fig.3 1H-NMR spectra of PI
The chemical shift (δ)ofthepeaksatb,c,d,e,fandginFig.3correspondstothehydrogenpositiononthefunctionalgroups.Inaddition,NMRspectraofPAAshowthelinearunit—COOHabsorptionpeakatδof10.57and—NHCO—absorptionpeaksatδof6.55[9].NoprotonpeakofPAAisfoundinthe1H-NMRspectra,indicatingthatPAAcompletelytransformstoPIbythermalimidization.
2.2 Solubility of 6FDA-BAPS PI
The solubility properties of 6FDA-BAPS PI at room temperature and heating conditions are shown in Tab.1.

Tab.1 Solubility of 6FDA-BAPS membranes
Note:++ soluble at room temperature; + soluble on heating.
Attributing to the bulky trifluoromethyl moieties (—C(CF3)2—) in dianhydride and polar group —O— and —SO2— in diamine, the 6FDA-BAPS PI is soluble in NMP,N,N-dimethylacetamide(DMAc)andTHFatroomtemperatureandevensolubleindimethylsulfoxide(DMSO),dimethylformamide(DMF)atheatingconditions.
2.3 Thermal properties of PI
As shown in Fig.4, TGA measurement reveals 5% and 10% weight loss temperature in nitrogen atmosphere at 511 ℃ and 531 ℃, respectively, and the residual rate at 800 ℃ is 57%. The above data indicate that the obtained PI exhibits the high thermal stability and overcomes the shortcoming of the thermal property loss accompanied by the solubility improvement of traditional soluble PI.
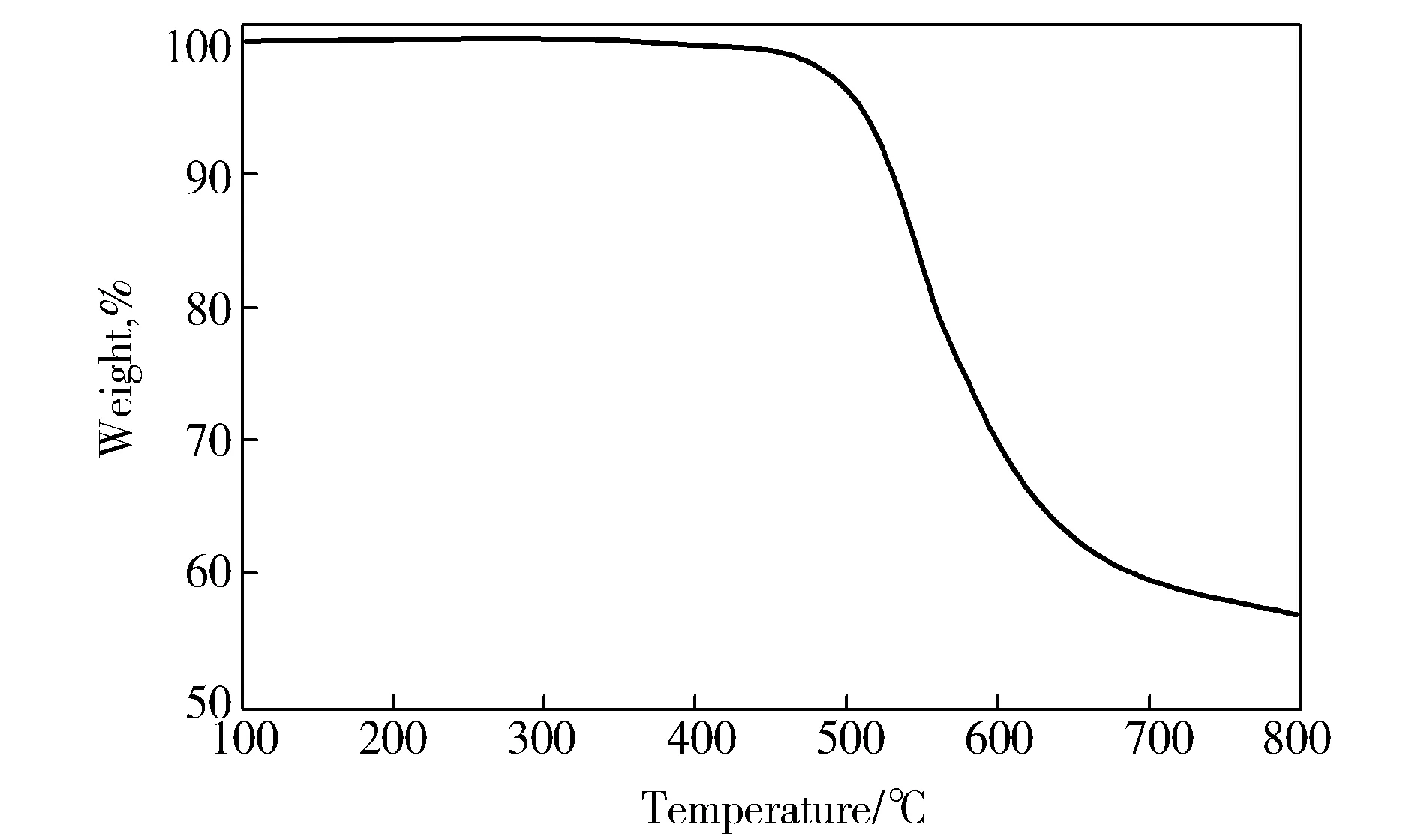
Fig.4 Thermogravimetric curve of 6FDA-BAPS PI
2.4 Mechanical properties of film and hollow fiber membrane
As shown in Fig.5, the tensile strength of the dense film is up to 51.7 MPa, while the value of the hollow fiber membrane is 26.5 MPa.

Fig.5 Mechanical properties of 6FDA-BAPS PI1—PI film;2—PI hollow fiber
2.5 Morphology of PI hollow fiber membranes
The cross sections of the hollow fiber membranes are porous spongy shape, as shown in Fig.6a. This is attributed to the fact that the internal coagulant involves high content NMP with low solidification capacity, which causes the delayed phase separation. Furthermore, the external coagulant is of high content pure water with high solidification capacity which causes the instantaneous phase separation. All of these results in the needle-shaped pore structure in the cross sections of the hollow fiber membranes. Fig.6b are the SEM images of the inner surface which shows lacunosis porous structure. Fig.6c are the SEM images of the outer surface which are dense layers with defect partly.
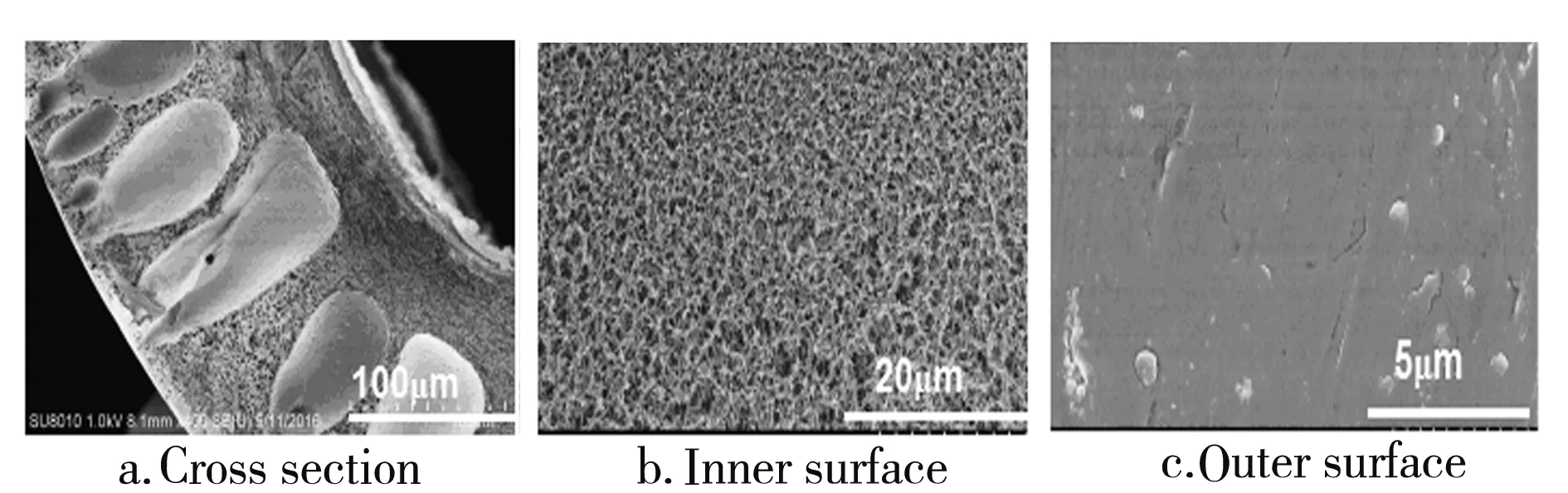
Fig.6 SEM images of 6FDA-BAPS PI hollow fiber membranes
3 Conclusions
6FDA-BAPS PI containing trifluoromethyl, polyether and sulfonyl groups was prepared. The PI was soluble in polar aprotic solvents such as NMP, DMAc and THF, and was of high thermostability at 511 ℃ in nitrogen atmosphere. Hollow fiber membranes with dense skin layer and the loose and porous support layer were prepared by dry-jet-wet spinning process, and the tensile strength is 26.5 MPa.
[1] Wang Lina,Cao Yiming, Zhou Meiqing, et al. Novel coPI membranes for gas separation[J]. J Membr Sci, 2007, 305(1): 338-346.
[2] Sazanov Y N. Applied significance of PIs [J]. Russ J Appl Chem, 2001, 74(8): 1253-1269.
[3] Zhang Zixin,Fan Yangyang,Yin Chaoqing,et al.Wet spinning formation of partially imidized polyamic acid[J].Chin Syn Fiber Ind, 2015, 38(4):21-23.
[4] Baker R W. Future directions of membrane gas separation technology [J]. Ind Eng Chem Res, 2002, 41(6): 1393-1411.
[5] Xiao Youchang,Low B T,Hosseini S S,et al. The strategies of molecular architecture and modification of PI-based membranes for CO2removal from natural gas: A review [J]. Prog Polym Sci, 2009, 34(6): 561-580.
[6] Wang Yan,Deng Jianmian,Lin Jindun,et al.Research advance of preparation technology of hollow fiber membrane[J]. Ind Saf Environ Prot, 2012, 38(6):17-19.
[7] Jeon Y W, Lee D H. Gas membrane for CO2/CH4(biogas) separation: A review [J]. Environ Eng Sci, 2015, 32(2): 71-85.
[8] Seo H, Chae B,Ji H I,et al. Imidization induced structural changes of 6FDA-ODA poly (amic acid) by two-dimensional (2D) infrared correlation spectroscopy [J]. J Mol Struc, 2014, 1069(1): 196-199.
[9] Qi Xiuxiu,Lu Jianmei,Zhang Zhengbiao,et al.Copolycondensation synthesis of polyamic acid and polyimide by microwave irradiation[J].Polym Mater Sci Eng, 2004, 20(4):210-213.
含砜基聚酰亚胺的合成及其中空纤维膜的制备甘从军,江雪微,董 杰,赵 昕,张清华(东华大学材料科学与工程学院纤维材料改性国家重点实验室,上海 201620)摘要:以4,4′-(六氟异丙烯)二酞酸酐(6FDA)与4,4′-双(4-氨基苯氧基)二苯砜(BAPS)为反应单体,以N-甲基-2-吡咯烷酮(NMP)为溶剂,合成了聚酰胺酸(PAA),将PAA溶液采用流延成膜的方法制备成薄膜;另外,将PAA溶液采用干-湿法纺丝工艺制得PAA中空纤维膜,再将PAA薄膜及其中空纤维膜在300 ℃左右的高温热环化制得6FDA-BAPS型聚酰亚胺(PI)膜。研究了6FDA-BAPS型PI及其中空纤维膜的结构与性能。结果表明:所合成的6FDA-BAPS型PI为目标产物,其在NNP、N,N-二甲基乙酰胺、四氢呋喃中具有良好的溶解性能。6FDA-BAPS型PI中空纤维膜外皮层致密、支撑层疏松多孔,该中空纤维膜具有较高的热学性能和力学性能,在氮气氛围中热失重5%的温度为511 ℃,断裂强度为26.5MPa。 关键词:聚酰胺酸 聚酰亚胺 中空纤维膜 结构 性能
TQ342+.731 Document code:A Article ID: 1001- 0041(2017)01- 0050- 04
