基于苯并噻唑Zn2+荧光探针及其细胞造影应用
2016-12-20张长丽田佳津邵阳徐鉴
张长丽 田佳津 邵阳 徐鉴
(1南京晓庄学院环境科学学院,南京211171)(2南京大学配位化学国家重点实验室,南京210023)
基于苯并噻唑Zn2+荧光探针及其细胞造影应用
张长丽*,1,2田佳津1邵阳1徐鉴1
(1南京晓庄学院环境科学学院,南京211171)
(2南京大学配位化学国家重点实验室,南京210023)
设计合成基于苯并噻唑Zn2+荧光增强型探针BHP,在HEPES缓冲液中测其对Zn2+识别性能。实验结果表明,BHP对Zn2+有较高的选择性,对其他金属离子如Cd2+,Fe2+,Ni2+,Pb2+,Hg2+,Al3+,Mn2+,Ag+,Cu2+,Co2+,Na+,K+,Mg2+和Ca2+无明显荧光增强响应。BHP与Zn2+按1:1计量比配位,在生理条件下荧光强度不受pH值影响。在HeLa细胞中对Zn2+的造影表明BHP可用于生物体Zn2+检测。
锌离子;镉离子;荧光探针;造影
Mobile Zn2+is an important transmitter in neural signal transmission,and is also proposed to regulate the pathophysiology of many severe neurological diseases[1-3].Mobile Zn2+is also involved in the signal transduction process[4-5].Fluorescent Zn2+imaging with Zn2+sensors has demonstrated great success in providing temporal-spatial information regarding the mobile Zn2+-involved physiological process[6-8].Herein,many Zn2+selective fluorescent probes have been developed[9-10].These classicalZn2+fluorescence sensorsexhibit the distinct Zn2+-triggered“turn-on”fluorescence,cell permeability and favorable pH-independence emission[11].However,itis stilla challenge fordetecting Zn2+selectively without interference of other transition metal ions,especially Cd2+,because being group IIB elements ofthe periodic table Zn2+and Cd2+show cross-reactivity and record similar spectral changes while coordinated with fluorescent sensors[12-13].Therefore, there is a great demand for developing Zn2+selective sensors that can distinguish Zn2+from Cd2+[14-15].
Those compounds with an unbridged C=N structure are poorly fluorescence,mostly due to the isomerization of the C=N double bond in the excited state[16].The C=N isomerization may be inhibited upon the coordination with certain metal ions[17-18].Therefore, the stable chelation of sensor with zinc ions makes the C=N isomerization of sensor inhibited,resulting in fluorescence enhancement.In this study,we design a CHEF type Schiffbase sensornamed as 2-((2-(benzo[d] thiazol-2-yl)hydrazono)methyl)-5-(diethylamino)phenol, BHP,having hydroxyl and imine groups to facilitate the chelation of Zn2+.After coordinating with Zn2+,the C=N isomerization of imine group in BHP may be inhibited,and a distinct emission enhancement is expected.BHP was synthesized by the addition reaction between 4-(diethylamino)-2-hydroxybenzaldehyde and 2-hydrazinylbenzo thiazole(Scheme 1).
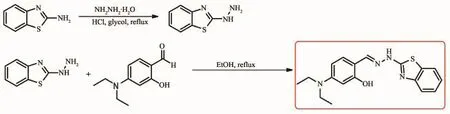
Scheme 1 Synthesis of BHP
1 Experimental
1.1Materials and general methods
Reagents and solvents for synthesis were of analytic grade.The solutions of different metal ions were prepared by dissolving NaCl,CaCl2,KCl,MgCl2· 6H2O,FeSO4·7H2O,Zn(NO3)2·6H2O,Al(NO3)3·9H2O, Hg(NO3)2·H2O,NiCl2·6H2O,CuCl2,CdCl2,MnCl2·4H2O, CoCl2·6H2O,AgNO3and Pb(NO3)2in double distilled water(all salts are of analytical grade).For the spectroscopic study,all solvents are of spectrum grade, and water is the double distilled water.All pH value measurements were recorded by HI-8014 meter.1H NMR and13C NMR spectra were recorded on Bruker DRX-400 spectrometer with TMS as internal standard. Fluorescence spectra were determined by an AMINCO Bowman series 2 for both excitation and emission. Absorption spectra were measured on a Shimadzu UV-3100 or an UV-VIS-NIR spectrophotometer.
1.2Synthesis of BHP
1.2.12-hydrazinylbenzothiazole[19]
To a suspension of 2-aminobenzothiazole(7.5 g) in ethylene glycol(40 mL),hydrazine hydrate(85%, 10 mL)and concentrated hydrochloric acid(10 mL) was added at 5~6℃.The reaction mixture was refluxed for 3 h and cooled to room temperature.The reaction mixture was filtered and resulting precipitate was washed with distilled water.The resulting crude was crystallized from ethanol to obtained brownish black crystalline product.
1.2.22-((2-(benzo[d]thiazol-2-yl)hydrazono)methyl)-5-(dieth-ylamino)phenol(BHP)
To a solution of 2-hydrazinybenzothiazole(165 mg,1 mmol)in ethanol(5 mL),4-(diethylamino)-2-hydroxybenzaldehyde(193 mg,1 mmol)was added at room temperature.The reaction mixture was refluxed for 5 h and coolded to room temperature.The resluting precipitation was collected by filtering to give BHP as yellow solid.Yield:98%.1H NMR(400 MHz, CDCl3)δ10.77(s,1H),8.18(s,1H),7.55(d,J=7.8 Hz,1H),7.30(d,J=3.9 Hz,2H),7.11(m,1H),6.98(d, J=8.6 Hz,1H),6.27(d,J=2.4 Hz,2H),6.25(dd,J= 8.6,2.4 Hz,1H),5.05(s,1H),3.39(q,J=7.1 Hz,4H), 1.20(t,J=7.1 Hz,6H)(Fig.S1).13C NMR(100 MHz, CDCl3)δ166.11,160.19,152.02,150.50,143.84, 132.29,126.57,126.34,122.03,122.00,114.20,106.86,104.02,98.22,44.65,12.67(Fig.S2).
1.3Absorption and emission spectroscopic study
For this study,sample solution was added into the quartz cuvette(3.0 mL,10μmol·L-1).All measurements were conducted at least in triplicate.
UV-Vis and fluorescence titration of BHP were carried outin HEPES buffer(VCH3CN:VHEPES=1:1;HEPES 50 mmol·L-1,pH 7.2;0.1 mol·L-1KNO3).The titration was finished by adding aliquots of 2.5μL of Zn(NO3)2aqueous solution(1.2 mmol·L-1)to BHP solution.The spectra were all recorded immediately after the solution was completely mixed.
The fluorescent pH titration experiments of BHP (10μmol·L-1)were determined in solution(VCH3CN:VH2O=1:1).The pH value of each solution was adjusted by NaOH(5 mol·L-1)or HNO3(5 mol·L-1)aqueoussolution.
1.4Cell culture methods and confocalimaging
HeLa cells were cultured in glass bottom dishes. The culture medium was Dulbecco′s Modified Eagle Medium(DMEM)supplemented with 10%fetal bovine serum,100 U·mL-1penicillin,100μg·mL-1streptomycin and 3.7 mg·mL-1NaHCO3.
HeLa cells were removed the incubation media and rinsed three times with 1×PBS.Then the cells were incubated in 10μmol·L-1BHP solution for 30 min at room temperature.After removing the solution, the dish was washed three times with 1×PBS.The confocal images of the cells were obtained using a Zeiss LSM 710 confocal microscope equipped with a 63×oil-immersion objective under irradiation at 405 nm.A band path from 420 to 510 nm was adopted for imaging.After removing the media,the exogenous Zn2+was introduced by incubating the cells with 10μmol· L-1ZnSO4+2-mercaptopyridine-N-oxide(1:1,n/n,20 min)solution.Then,the cells were dyed with the BHP solution in a similar procedure described above and imaged.At last the cells were treated with 100μmol· L-1TPEN solution to remove the exogenous Zn2+.After washing with metal free 1×PBS,the cells were imaged by confocal microscope again.A Zeiss LSM710 microscope equipped with a 63×oil-immersion objective was used for confocalimaging.The excitation wavelength for BHP in co-staining experiments was 405 nm,and the band path was 420~510 nm.
2 Results and discussion
2.1Fluorescence of BHP and its pH dependence
BHP can be readily dissolved in HEPES buffer (VCH3CN:VHEPES=1:1;HEPES 50 mmol·L-1,pH 7.2;0.1 mol·L-1KNO3),and its fluorescence spectroscopic study was carried out in HEPES buffer except for the fluorescent pH-dependence determination.BHP fluoresces weakly in neutral solution(pH 7.2)withλexandλemat 368 and 448 nm,respectively(Fig.S5).The Stokes shift of 80 nm is larger than the reported values of many visible light sensors derived from xanthenone analogues(their Stokes shifts are normally~30 nm),which satisfies the requirement for a practical sensor providing high quality imaging.Its emission intensity does not change significantly at pH 5.0~8.0.However,a distinct emission enhancement due to ICT blockage can be observed when reducing pH from 4.5 to 3.0(Fig.S6).The stable fluorescence of BHP at around pH 7.0 is favorable for its in vivo application.
2.2Zn2+binding behavior of BHP
The metal-binding behavior of BHP has been determined by UV-Vis and fluorescence spectroscopic studies.BHP exhibits an intensive absorption band centered at366 nm(ε=6.04×104L·mol-1·cm-1),which can be assigned to theπ-π*transition band(Fig.1a). Zn2+titration demonstrates the decrease of this band and increase of a new band centered at 388 nm.The stable spectra thereafter and the clear isosbestic point at 377 nm suggest that only one Zn2+complex species is formed.The stoichiometry of the Zn2+/BHP complex has also been confirmed by mass spectroscopic determination.The electrospray ionization mass spectrum of this complex displays a signalof m/z 403.17,which can be assigned as the signal for[M+Zn-H]+.BHP is most likely to chelate with metal ions via their benzothiazole N,imide N and phenolic O atoms(Fig.S4)[20].
Free BHP exhibits very weak fluorescence in HEPES buffer with quantum yield of 0.002.The enhancement of fluorescence intensity at 448 nm was not significant before the addition of 0.5 equiv.ofZn2+,but became obvious with Zn2+from 0.6 to 1.0 equiv,and still kept a small amount with Zn2+from 1.1 to 2.0 equiv.Moreover,the Job plot showed 1:1 binding stoichiometry for Zn2+and BHP.Following a Benesi-Hildebrand-type analysis[21],the dissociation constant Kdwas determined to be 1.1 nmol·L-1(Fig. S7).The quantum yield of Zn2+/BHP complex is determined 0.061 with quinine sulfate solution(Φ= 0.546,0.5 mol·L-1H2SO4)as reference.The limit of detection(LOD)for Zn2+is~67.3 nmol·L-1[22](3σ/slope, Fig.S8).The remarkable emission enhancement,1:1 binding stoichiometry and low LOD made BHP a good“turn-on”sensor for Zn2+sensing and imaging.

Fig.1(a)Absorption spectra and(b)Emission spectra of BHP(10μmol·L-1)in HEPES buffer(VCH3CN:VHEPES=1:1; HEPES 50 mmol·L-1,pH 7.2;0.1 mol·L-1KNO3)in a 1 cm quarts cuvette when titrated by Zn2+(1.2 mmol·L-1)
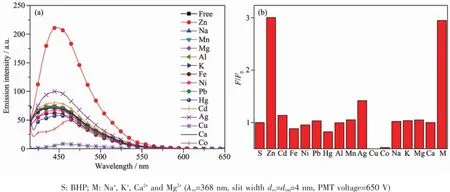
Fig.2(a)Emission spectra and(b)histogram of the emission enhancement factor(F/F0at 448 nm)of BHP(10μmol·L-1)in HEPES buffer(VCH3CN:VHEPES=1:1;HEPES 50 mmol·L-1,pH=7.2;0.1 mol·L-1KNO3)induced by 1 equiv Zn2+,Cd2+, Fe2+,Ni2+,Pb2+,Hg2+,Al3+,Mn2+,Ag+,Cu2+,Co2+,Na+,K+,Mg2+,Ca2+and 1 000 equiv alkaline/alkaline earth metal cations
2.3Zn2+Fluorescent Response of BHP
Cation-induced changes in emission enhancement factor F/F0at 448 nm were investigated to determine the specific Zn2+sensing ability of BHP.Treatmentwith Zn2+(1.0 eq.)resulted in 3-fold increase of the emission. In contrast,treatment with other relevant metal ions such as Cd2+,Fe2+,Ni2+,Pb2+,Hg2+,Al3+,Mn2+,Ag+, Na+,K+,Mg2+and Ca2+,do not lead to any obvious emission enhancement(Fig.2),except for Co2+and Cu2+which quench the fluorescence.The quenching effect ofCo2+and Cu2+was observed foralmostallthe reported Zn2+sensors,which doesnotinterfere with Zn2+detection distinctly.It is worth mentioning that BHP candistinguish Zn2+from Cd2+,whereas the discrimination of Zn2+from Cd2+was not reflected in other reported Zn2+sensors.Moreover,the presence of 1 000 equiv of Na+,K+,Ca2+or Mg2+,which are abundant in cells, does not affect the Zn2+sensing behavior of BHP.All these suggest that BHP is a suitable candidate for intracellular Zn2+-staining.
2.4Intracellular Zn2+Imaging with BHP as Imaging Agent
The intracellular Zn2+imaging ability of BHP has been verified on HeLa cells by confocal imaging(Fig. 3).After incubation with BHP solution(10μmol·L-1in PBS,0.1%DMSO)at 25℃for 30 min,the HeLa cells displayed very faint intracellular fluorescence (Fig.3b).However,HeLa cells exhibited intensive fluorescence when exogenous Zn2+was introduced into the cells via incubation with ZnSO4/pyrithione solution (Fig.3c).Moreover,the intensive fluorescence was deeply depressed by scavenging Zn2+from the cells with the cell permeable metal chelator,N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine(TPEN)(Fig. 3d).According to the random selected regions,the average fluorescence intensity of cells before the introduction of exogenous Zn2+is~4.80,while that for cells with exogenous Zn2+is~15.21.The followed Zn2+deprival by TPEN treatment makes the fluorescence intensity of the cells reduce to~2.87,even lower than that before Zn2+introduction.This result indicates BHP is an effective intracellular Zn2+imaging agent.

Fig.3 Confocal microscopic images of HeLa cells stained by BHP(10μmol·L-1,1×PBS,30 min of incubation)
3 Conclusions
In conclusion,a new benzoimidazole based Zn2+fluorescence sensor BHP has been prepared.The sensor displays a rapid response to Zn2+and the 1:1 Zn2+binding stoichiometry in aqueous media.The presence of other metal ions especially Cd2+does not interfere with its Zn2+response.The pH-independent fluorescence in physiological condition and its cellmembrane permeability make this sensor an effective intracellular Zn2+imaging agent.
Supporting information is available athttp://www.wjhxxb.cn
References:
[1]Finney L A,O′Halloran T V,Science,2003,300:931-936
[2]WAN Dan-Dan(万丹丹),SU Guang-Yu(苏光余),XU Zi-Hua (许子华),et al.Chinese J.Inorg.Chem.(无机化学学报), 2008,24:1253-1260
[3]Choi D W,Koh J Y.Ann.Rev.Neurosci.,1998,21:347-375
[4]Nies D H.Science,2007,317:1695-1696
[5]Lu M,Fu D.Science,2007,317:1746-1748
[6]Zhang C L,Liu Z P,Li Y L,et al.Chem.Commun.,2013, 49:11430-11432
[7]Liu Z P,Zhang C L,Chen Y C,et al.Chem.Commun.,2014, 50:1253-1255
[8]Qian F,Zhang C L,Zhang Y M,et al.J.Am.Chem.Soc., 2009,131:1460-1468
[9]Liu Z P,He W J,Guo Z J.Chem.Soc.Rev.,2013,42:1568-1600
[10]Chen Y,Bai Y,Han Z,et al.Chem.Soc.Rev.2015,44:4517-4546
[11]Zhang C L,Zhang Y M,Chen Y C,et al.Inorg.Chem. Commun.,2011,14:304-307
[12]Kim J H,Noh J Y,Hwang I H,et al.Tetrahedron Lett., 2013,54:2415-2418
[13]Jana A,Sukul P K,Mandal S K,et al.Analyst,2014,39: 495-504
[14]WANG Wei-Na(王维娜),ZHENG Yuan-Mei(郑元梅),CHEN Xue-Mei(陈雪梅).Chinese J.Inorg.Chem.(无机化学学报), 2014,30:872-878
[15]Li P,Zhou X,Huang R,et al.Dalton.Trans.,2014,43:706-713
[16]Wu J,Liu W,Zhuang X,et al.Org.Lett.,2007,9:33-36
[17]Liu Z P,Zhang C L,Li Y L,et al.Org.Lett.,2009,11:795-798
[18]Na Y J,Hwang I H,Jo H Y,et al.Inorg.Chem.Commun., 2013,35:342-345
[19]Sharma V,Sharma K V.E-J.Chem.,2009,6:348-356
[20]Li M M,Wang F W,Wang X Y,et al.Anal.Chim.Acta, 2014,826:77-83
[21]Yuan M J,Zhou W D,Liu X F,et al.J.Org.Chem.,2008, 73:5008-5014
[22]Joshi B P,Park J,Lee W I,et al.Talanta,2009,78:903-909
Benzothiazole-Based Zn2+-Specific Fluorescent Probe and Its Cell Imaging Application
ZHANG Chang-Li*,1,2TIAN Jia-Jin1SHAO Yang1XU Jian1
(1School of Environmental Science,Nanjing Xiaozhuang College,Nanjing 210017,China) (2State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing 210023,China)
A fluorescent probe 2-((2-(benzo[d]thiazol-2-yl)hydrazono)methyl)-5-(diethylamino)phenol(BHP)was designed and synthesized as a Zn2+selective fluorescent chemosensor.It demonstrates Zn2+-specific emission enhancement,while many other ions such as Cd2+,Fe2+,Ni2+,Pb2+,Hg2+,Al3+,Mn2+,Ag+,Cu2+,Co2+,Na+,K+,Mg2+and Ca2+,have no response.Notably,this chemosensor can distinguish Zn2+from Cd2+.Its binding stoichiometry with Zn2+is 1:1 ratio.Its pH-independent Zn2+-induced emission enhancement in physiological condition and cell permeability make itan effective intracellular Zn2+imaging agent.
zinc ion;cadmium ion;fluorescent sensor;imaging
O614.24+1
A
1001-4861(2016)12-2069-06
10.11862/CJIC.2016.274
2016-04-11。收修改稿日期:2016-10-01。
国家自然科学基金(No.21401106)、江苏省自然科学青年基金(No.BK20140090)和南京晓庄学院校级科研项目(No.2013NXY03,2015XSKY078)资助。
*通信联系人。E-mail:carbon314@163.com
