Time dependent differences in gray matter volume post mild traumatic brain injury
2016-12-02PrabhjyotSingh,WilliamD.S.Killgore
PERSPECTIVE
Time dependent differences in gray matter volume post mild traumatic brain injury
When the brain is subjected to excessive physical forces, including blunt impact, high-speed rotation, or blast overpressure waves, its tissue structure and function can be compromised, leading to traumatic brain injury (TBI). Based on the level of structural and functional damage, these injuries can be classified as mild, moderate, or severe, with mild TBI (mTBI) being by far the most common. Also known as concussion, mTBI frequently occurs in a wide variety of activities, including accidental falls, sports injuries, moving vehicle accidents, military training, and combat related events such as blast exposure. mTBI can lead to various cognitive, sensory and motor complaints like reduced memory, attention, and information processing speed, and emotional dysregulation (Carroll et al., 2004). Most individuals with mTBI will recover from these symptoms within 90 days post injury (Karr et al., 2014), but for some individuals, the symptoms may be protracted, persisting up to a year or longer (Satz et al., 1999). For a small minority of individuals, these cognitive and emotional symptoms are severe enough to significantly affect social and occupational functioning.
In contrast to moderate and severe injuries, one of the defining features of an mTBI is the absence of detectible structural lesions on a standard clinical imaging scan. While individual lesions may not be present, there is emerging evidence that, as a group, patients with mTBI may actually be differentiated from non-injured controls based on brain volume data. For instance, previous studies have shown decreased gray matter volume (GMV) post mTBI, suggesting a loss of cortical neurons (List et al., 2015). Very few studies, however, have explored differences in GMV at different time intervals post mTBI and their relationship with neuropsychological performance. Such research is crucial to understanding the recovery process because the brain is not static and neuroplastic remodeling may continue for some time after an injury. Understanding this relationship can facilitate better-targeted intervention strategies to aid in rehabilitation following mTBI.
We recently reported findings suggesting that mTBI may not simply be associated with reduced cortical volume, but instead may show specific increases in gray matter volume (GMV) as well (Killgore et al., 2016). In that project we studied the cortical volume changes and their association with neuropsychological task performance at various time intervals up to a year following injury. We used a 3.0 Tesla magnetic resonance imaging scanner (Siemens Trim Trio, Erlangen, Germany) with a 32-channel head coil for our study. A T1 weighted 3D MPRAGE sequence (TR/TE/flip angle = 2.1 s, 2.3 ms, 12°) was used to acquire 176 sagittal slices (256 × 256 matrix) with a 1-mm slice thickness, yielding a voxel size of 1 × 1 × 1 mm3. The VBM8 toolbox in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/ spm8/) was used to process the T-1 weighted structural images. All images were spatially realigned to the anterior-posterior commissure axis and then segmented into GM, WM, and CSF using VBM8. A custom DARTEL template was created using the segmented images and then the images were normalized to Montreal Neurological institute (MNI) space. Images were then smoothed with a 10 mm full width at half maximum (FWHM) isotropic Gaussian kernel.
The study participants included 26 right-handed adults (age range 20—45 years, mean age 23.38 ± 5.23, 11 males, 15 females), with English as their primary language. All participants had a history of sports-related mTBI experienced within the 12 months prior to participation this study (2 weeks [n = 2], 1 month [n = 6], 3 months [n = 5], 6 months [n = 10], 1 year [n = 3]). All of these participants sustained mTBI while engaging in sports activities such as rugby (n = 7), basketball (n = 3), softball (n = 1), ultimate frisbee (n = 1), soccer (n = 1), ice hockey (n = 2), lacrosse (n = 1), martial arts (n = 2), weight lifting/ gym (n = 4) and track and field (n = 4). The participants were initially screened over the telephone for the details of their head injury, medical and psychiatric history. Participants were ruled out for any serious chronic medical, neurological or psychiatric condition like hypertension, diabetes, epilepsy, bipolar disorder, attention deficit hyperactivity disorder etc. The only exception was depression and anxiety developing after the concussion. Also, they were required to provide official documentation of head injury signed by an impartial but professionally responsible witness to the head injury or its immediate consequences (e.g., physician, nurse, ambulance driver, medical records, neuropsychologist). Additionally, 12 healthy control participants (age range 20—43 years, mean age 25.00 ± 6.55, 4 males, 8 females), with no history of head injury or loss of consciousness were recruited as a comparison group. On the day of visit, the healthy and mTBI individuals underwent same series of neuropsychological assessments and MRI sequences.
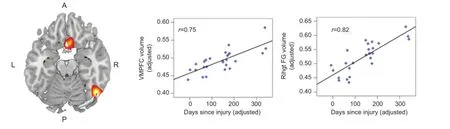
Figure 1 Regions where larger gray matter volume was significantly correlated with time since injury, including the ventromedial prefrontal cortex and the right fusiform gyrus.
Remarkably, in contrast to the general finding of reduced GMV following mTBI found in other studies, our results did not show such reductions, but instead showed that longer time since injury (TSI) was associated with increased GMV in two brain regions (see Figure 1), including the cortex of the right fusiform gyrus (RFG) and bilateral ventromedial prefrontal cortex (VMPFC). In other words, the cortex of these regions appeared to be larger among those whose injuries were mostdistal in time. Moreover, larger GMV was associated with better performance for visual motor, visual attention, and emotional functioning tasks, suggesting that greater cortical volume in specific regions was associated with better functional outcome. We speculate that these data point toward significant cortical remodeling occurring in the months following injury. To further evaluate that possibility, we divided our sample roughly in half so that we could compare those in the post-acute stage (0—99 days post-injury) to those in the chronic stage (100—365 days post-injury), and further compared them to a separate sample of healthy individuals with no reported history of head injury. Consistent with our hypothesis, the chronic group showed significantly greater GMV in both regions compared to the post-acute group, confirming that gray matter was increased with longer TSI. Moreover, the chronic group also showed significantly greater GMV compared to the healthy controls, suggesting that not only was the GMV returning to normal with greater TSI, it was actually exceeding the volume seen in healthy normals. Thus, for these individuals, the later stages of recovery were associated with exaggerated GMV in specific regions that are involved in regulating emotion as well as sustaining visual attention and information processing speed.
We interpreted these findings as evidence of experience dependent cortical plasticity. In other words, we propose that for many individuals, mTBI leads to a host of subtle core cognitive impairments and emotional regulation deficits post-injury, which over time, lead the injured individual to draw upon these other cortical regions to compensate. For example, reduced frustration tolerance and emotional dysregulation are common experiences after mTBI and are not specific to a particular lesion site (Ryan and Warden, 2003). It is conceivable that individuals with these emotional difficulties may more routinely activate the ventromedial prefrontal cortical regions, which play an important role in emotional and visceromotor regulation, in an attempt to maintain emotional control. Similarly, many people experience slowed processing speed and attentional difficulties following a concussion (Levin et al., 1987). This may cause such individuals to draw more heavily upon regions such as the fusiform gyrus and other visual attention regions in order to compensate. With sustained and exaggerated use, it is conceivable that these highly exercised regions may begin to develop larger cortical volume through more extensive dendritic arborization. It is well established that repeated practice with certain motor or cognitive skills can lead to an increase in specific cortical regions supporting that skill (Quallo et al., 2009). The preliminary findings from our study are encouraging, suggesting that mTBI is not uniformly defined by decreased cortical volumes. On the contrary, regional increases in volume are possible within this population and these volume changes are associated with improved cognitive and emotional functioning. The fact that we identified specific regions of volume increases is remarkable given the fact that mTBI is an extremely heterogeneous injury, with multiple potential causes and diffuse locations of damage (Bigler, 2008). The fact that these areas of increased volume were consistent and focal suggests that they are likely independent of lesion location—rather they likely reflect common pathways for compensation that are relatively independent of the site of impact or location of damage.
Previous studies have shown that behavioral experience interacts with regenerative and degenerative changes in the brain to induce structural and motor plasticity (Kerr et al., 2011). Compensatory remodeling is one of the ways neuroplasticity works and may undergird the mechanisms behind rehabilitative training, which forms one the mainstays of treatment post mTBI. On the basis of our findings we suggest that rehabilitative training might be even more beneficial if it can capitalize on this aspect of neuroplasticity. Perhaps by focusing rehabilitation efforts toward exercising existing compensatory skills that draw upon these regions (e.g., emotional regulation; regulating attention from distraction), patients can further develop the cortical volume of those regions and, over time, gain greater functional capacity. This would be encouraging and suggest that there is more that could be done for patients recovering from concussions than merely to “wait and see.” Clearly this is speculative at this point, but further research should examine whether the cortical volume, structural and functional connectivity, and functional capacity of these same regions can be voluntarily enhanced in patients recovering from mTBI via focused training. Finally, it will be important for future work to focus efforts toward using functional neuroimaging. This will enable linkage among the cognitive tasks and identified deficits caused by an injury and the regions of increased gray matter volume identified in our study.
The present study was supported by a USAMRAA grant to WDSK (W81XWH-12-1-0386).
Prabhjyot Singh, William D. S. Killgore*
Department of Psychiatry, University of Arizona, Tucson, AZ, USA
施工质量会直接影响到路面的压实度,需有效控制好路面碾压及摊铺工程中的工艺。工作人员需确定出各有效指标并满足制定形式的要求。除此之外,碾压的速度和力度更应严格控制。另外,碾压工作涉及众多因素,工作人员需从根本上分析影响因素并确定好碾压工作程序,最终保证在施工中,压实度可符合其具体要求。在实际工作中,对摊铺机的力度也有要求,所以为了能够保证碾压程序有效化,需制定出有效的处理方式,以当前规范形式为基准,加强重视起碾压工艺。依据已有的规范标准,保证碾压工作的有序性,及时控制好各项干预程序[5]。
*Correspondence to: William D. S. Killgore, Ph.D. Killgore@psychiatry.arizona.edu.
Accepted: 2016-05-17
orcid: 0000-0002-5328-0208 (William D. S. Killgore)
How to cite this article: Singh P, Killgore WDS (2016) Time dependent differences in gray matter volume post mild traumatic brain injury. Neural Regen Res 11(6):920-921.
References
Bigler ED (2008) Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc 14:1-22.
Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, Paniak C, Pepin M, Injury WHOCCTFoMTB (2004) Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med:84-105.
Karr JE, Areshenkoff CN, Garcia-Barrera MA (2014) The neuropsychological outcomes of concussion: a systematic review of meta-analyses on the cognitive sequelae of mild traumatic brain injury. Neuropsychology 28:321-336.
Kerr AL, Cheng SY, Jones TA (2011) Experience-dependent neural plasticity in the adult damaged brain. J Commun Disord 44:538-548.
Killgore WD, Singh P, Kipman M, Pisner D, Fridman A, Weber M (2016) Gray matter volume and executive functioning correlate with time since injury following mild traumatic brain injury. Neurosci Lett 612:238-244.
Levin HS, Mattis S, Ruff RM, Eisenberg HM, Marshall LF, Tabaddor K, High WM, Jr., Frankowski RF (1987) Neurobehavioral outcome following minor head injury: a three-center study. J Neurosurg 66:234-243.
List J, Ott S, Bukowski M, Lindenberg R, Floel A (2015) Cognitive function and brain structure after recurrent mild traumatic brain injuries in youngto-middle-aged adults. Front Human Neurosci 9:228.
Quallo MM, Price CJ, Ueno K, Asamizuya T, Cheng K, Lemon RN, Iriki A (2009) Gray and white matter changes associated with tool-use learning in macaque monkeys. Proc Natl Acad Sci U S A 106:18379-18384.
Ryan LM, Warden DL (2003) Post concussion syndrome. Int Rev Psychiatry 15:310-316.
Satz PS, Alfano MS, Light RF, Morgenstern HF, Zaucha KF, Asarnow RF, Newton S (1999) Persistent post-concussive syndrome: a proposed methodology and literature review to determine the effects, if any, of mild head and other bodily injury. J Clin Exp Neuropsychol 21:620-628.
10.4103/1673-5374.184487
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies
- Oligodendrocyte ablation as a tool to study demyelinating diseases
- Endogenous bioelectric fields: a putative regulator of wound repair and regeneration in the central nervous system
- Neuroprotection and antioxidants
- The intricacies of neurotrophic factor therapy for retinal ganglion cell rescue in glaucoma: a case for gene therapy
- Discovery of nigral dopaminergic neurogenesis in adult mice
