Neuroprotection and antioxidants
2016-12-02MariaLalkoviovVieraDanielisov
Maria Lalkovičová, Viera Danielisová
Institute of Neurobiology, Slovak Academy of Sciences, Kosice, Slovakia
INVITED REVIEW
Neuroprotection and antioxidants
Maria Lalkovičová*, Viera Danielisová
Institute of Neurobiology, Slovak Academy of Sciences, Kosice, Slovakia
Ischemia as a serious neurodegenerative disorder causes together with reperfusion injury many changes in nervous tissue. Most of the neuronal damage is caused by complex of biochemical reactions and substantial processes, such as protein agregation, reactions of free radicals, insufficient blood supply, glutamate excitotoxicity, and oxidative stress. The result of these processes can be apoptotic or necrotic cell death and it can lead to an irreversible damage. Therefore, neuroprotection and prevention of the neurodegeneration are highly important topics to study. There are several approaches to prevent the ischemic damage. Use of many modern therapeutical methods and the incorporation of several substances into the diet of patients is possible to stimulate the endogenous protective mechanisms and improve the life quality.
neuroprotection; ischemia; oxidative stress; antioxidants; cerebral damage; nutrition; vitamins; omega-3 fatty acids, flavonoids; polyphenols; statins
orcid: 0000-0002-9645-5645 (Mária Lalkovičová)
Introduction
Neuroprotection is a widely studied treatment option for various central nervous system (CNS) disorders including neurodegenerative diseases, stroke and trauma. Cerebral ischemia with a notable cessation of blood supply to the brain tissues leads to necrosis and apoptosis (Lee et al., 2001). It is the third most common cause of mortality and the leading cause of adult neurological disability (Carter et al., 2007). The most important pathological mechanisms in the process of ischemia-induced brain damage include inflammatory reaction, blood-brain barrier (BBB) disruption, oxidative stress, and neuronal apoptosis, all of which have been widely considered to be the four major therapeutic targets for acute ischemic stroke (Rodrigo et al., 2013). Degeneration takes place from a period of hours to days, which offers a window for therapeutic interventions. Antioxidants have a propensity to block or delay apoptosis. Many reports have suggested therapeutic effects of various natural antioxidants against cerebral ischemic damage (Mukherjee et al., 2007; Yousuf et al., 2007). Insufficient levels of antioxidants, or inhibition of antioxidant enzymes, cause oxidative stress which results in damage to cell structure and function and chronic excessive inflammation. Neuroprotective methods often target oxidative stress and excitotoxicity, which together with other factors lead to neuronal death and aggravate neurodegeneration. Therefore, glutamate antagonists and antioxidants are successfully used in research and therapy.
Oxidative Stress and Endogenous Antioxidants
Oxidative stress can be described as an imbalance between free radical production and opposing antioxidant defenses. Oxidative stress can be traced primarily to the formation of superoxide and nitric oxide. Both molecules have important roles in health, serving as regulators of blood flow and neurotransmission (Li et al., 2014). Perturbations in the production and/or metabolism of either molecule can have pathologic consequences. Oxidation reactions in organism can produce peroxides and free radicals which trigger chain reactions in cells. This can lead to damage or cell death. Several endogenous and exogenous antioxidants are used to neutralize and protect the body from free radicals by maintaining redox balance. The brain seems to be more susceptible than other organs to peroxidation, this is probably due to its high oxygen requirements, using up to 20% of the total oxygen intake while weighing only 2% of the total body weight (Bigos et al., 2015). Neuronal cells are considered the most susceptible cells to oxidative damage due to their low antioxidant activity in comparison to other tissues', as well as the high content of methyl ions in certain brain regions (Floyd and Carney, 1992). Damage to the brain due to ischemia is caused by injury that results from the interruption of blood flow, i.e., lack of oxygenation, and a subsequent reoxygenation of the brain (ischemia/reperfusion = I/R). Aerobic tissue suffers damage once it undergoes an I/R insult. Ischemic brain stroke is caused due to the partial or complete lack of blood flow into the brain tissue. The extent of damage usually depends on duration, severity, and location of ischemia. Immediately after the trauma the primary brain damage occurs. The biochemical processes known as ischemic casade are initiated. They progress to local depletion of oxygen or glucose, causing failure of production of high energy phosphate compounds, like adenine triphosphate (ATP). This can together with other interrelated events lead to an irreversible injury and cell death (Deb et al., 2010). During the reperfusion phase most of the damage occurs and it is due to oxygen free-radical-mediated oxidative events. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are implicated in secondary brain damage as they damage proteins, DNA, and membrane lipids (Chan, 2001). The major sources of ROS are leakage from malfunctioning mitochondria,arachidonic acid metabolism, and activated neutrophils. The brain is also exposed to ROS from exogenous sources such as irradiation, pollutants that can reach the brain via blood carriers, as well as xenobiotics and drugs. The concentration of ROS or RNS in organisms is determined by the balance between the rate of production of reactive species and the elimination rate of these compounds by the action of enzymes and antioxidants. Thus, under conditions of physiological homeostasis, a balance exists between the cellular processes that contribute to the production of ROS/ RNS, and those factors such as superoxide dismutase (SOD), catalase (Cat) and glutathione peroxidase (GPx), which aid in their elimination (Martinez et al., 2010). Two classes of endogenous mechanisms neutralize ROS: antioxidant enzymes (e.g., SOD) and low molecular weight antioxidants (LMWA) (e.g., ascorbic acid, uric acid (UA), vitamin E, Ames et al., 1993). Enzymatic antioxidants regulate superoxide concentration by dismutation of superoxide to hydrogen peroxide (SOD; Fridovich, 1995), which is then converted to water (peroxidases such as glutathione peroxidase and peroxiredoxin) or dismuted to water and oxygen (catalase). These enzymatic levels (amounts of enzymes) have been observed to vary for different brain regions and species. The activity of SOD (quantity of active enzyme; moles of substrate converted per unit time) is rather high compared to that of catalase, the former dismutting superoxide radicals to hydrogen peroxyde while the latter results in the degradation of hydrogen peroxide to water and oxygen (Sinet et al., 1980; Reiter et al., 1995). There are three major endogenous superoxide dismutases. Cu, Zn-SOD (SOD1) is mainly found in the cytosolic and lysosomal fractions, but is also in the mitochondrial intermembrane space (Okado-Matsumo and Fridovich, 2001). MnSOD (SOD2) is found in the mitochondrial matrix. Both Cu, Zn-SOD and MnSOD are abundant in neural tissue. Ni-SOD (SOD3) is primarily found in prokaryotic organisms (Shearer, 2014). The exogenous SOD (present in the extracellular cell space) has limited effect on ischemia as it cannot penetrate the cells. Therefore, polyethylene glycol-conjugated SOD was prepared and used in humans with head injuries (Muizelaar, 1994) and experimental animals (Hamm et al., 1996). These studies showed that neutralization of superoxide radicals has a beneficial effect on functional outcome and cerebral blood flow after traumatic injury, implicating that these radicals play a role in the pathophysiology of brain trauma. LMWA donate electron(s) to ROS and readily penetrate cells providing site-specific protection against oxidative stress (Kohen and Nyska, 2002). During ischemia ATP levels fall, xanthine accumulates, and calcium influx triggers activation of the protease calpain to cleave a peptide bond in xanthine dehydrogenase to form xanthine oxidase which, when oxygen is restored, catalyzes the oxidation of xanthine to UA and produces superoxide in the process. Several studies provide evidence that the xanthine oxidase mechanism may contribute to the tissue injury that occurs as a result of the I/R insult (Granger er al., 1986). The NADPH-oxidase complex utilizes electrons to produce superoxide radicals from oxygen molecules. NADPH oxidase may be measured by chemiluminescence (Yamazaki et al., 2011) or electrochemical methods, among others. ROS are produced in the ischemic area and may account for the consumption of brain low molecular weight antioxidants (LMWA) at 5 minutes of reperfusion. A significant recovery in brain LMWA occurs at 30 and 60 minutes after reperfusion, with an increase above basal 4 hours later. Oxidation of arachidonic acid leads to ROS production after ischemia (Kontosh, 2002). The LMWA group of molecules can be further classified into indirect-acting (e.g., chelating agents) and direct-acting antioxidants (e.g., scavengers and chainbreaking antioxidants). The latter antioxidants are extremely important in combating against oxidative stress. The source of the LMWA increase in the brain is not known. Bloodbrain barrier (BBB) disruption may allow circulating LMWA to reach the brain. UA was shown to penetrate compromised BBB and block toxic products of ROS (Hooper et al., 2000). Due to the complexity of the ischaemic cascade, numerous molecular targets have been tackled in order to achieve neuroprotection. The endogenous attempt of the injured brain to repair the structural and functional damage in the days and weeks following acute ischemic stroke might provide the opportunity for neurorepair possibilities. In the last decade, many promising pharmacological and non-pharmacological neuroprotectants have been under investigation with positive outcomes for prevention and treatment of brain ischemia (Figure 1).
Nutritional Antioxidants
There is growing interest in diet and lifestyle, considering diet as the main source of natural antioxidants due to its ability to provide a variety of molecules that activate or enhance the action of several endogenous antioxidants (Sun et al., 2008). Consumption of nutritional substances such as berries, nuts or fish oil can have a dramatic impact on the aging brain, possibly leading to improved motor and cognitive skills (James et al., 1999). Some classifications suggest also a group of nutritional antioxidants, since diet is the major source of substances with antioxidant properties or elements for the synthesis of antioxidant enzymes. Several metals (copper, zinc, selenium, manganese, iron) are involved as components or cofactors of numerous enzymes antioxidants, and certain vitamins (ascorbic acid, α-tocopherol and β-carotene, folic acid) act as a sequestrant of ROS (Perez et al., 2008).
Resveratrol
Resveratrol (trans-3,4′,5-trihydroxystilbene) is a natural phytoalexin found in grape skin. It is a potent antioxidant, has anticancer and cardioprotective properties (Hao et al., 2004). Various reports have suggested different mechanisms of action of neuroprotective effect of resveratrol against cerebral ischemia. Modulation of matrix metalloprotease-9 activity by resveratrol preconditioning has been suggested to ameliorate BBB disruption, edema formation, neuronal cell death, cerebral angiogenesis and brain regeneration during acute or delayed phases after stroke (Dong et al., 2008). Whenadult rats were systemically administered resveratrol, they demonstrated suppression of reactive oxygen species accumulation and BBB instability, accompanied with increased density of neurons, and reductions in the density of degenerative neurons after intracortical microelectrode-mediated neurodegeneration (Potter et al., 2013). Modulation of nitric oxide expression in ischemic brain by resveratrol (Tsai et al., 2007) or inhibition of postsynaptic glutamate receptors by trans-resveratrol have also been suggested to ameliorate ischemic brain injury (Gao et al., 2006) (Figure 2).
Ascorbic acid and UA
Ascorbic acid is a major antioxidant in biological systems (Rice, 2000). It is transported from the blood into the brain extracellular fluid, stored in neurons in concentrations approximately 10-fold higher than in plasma, and its extracellular levels increase in response to pharmacological and physiological stimulation (Miele et al., 1994). In addition, ascorbic acid increases after brain trauma (Moor et al., 2001) and ischemia-reperfusion injury (Hillered et al., 1998; Yousa, 2000). UA, a waste product of purine degradation protects against excitotoxicity and Fe2+insults in cell culture and against damage from focal ischemia in vivo (Yu et al., 1998). The exogenous administration of UA exerts robust neuroprotective properties in experimental models of CNS disease, including brain ischemia, spinal cord injury, meningitis, and experimental allergic encephalitis. In experimental brain ischemia, exogenous UA and the thrombolytic agent alteplase exert additive neuroprotective effects when administered in combination. UA prevents the increase in the circulating levels of the lipid peroxidation marker malondialdehyde and of active matrix metalloproteinase (MMP) 9, a marker of BBB disruption (Llull et al., 2016). The antioxidant activities of ascorbic acid and UA are interrelated: UA reacts with hydroxyl radicals producing a stable radical that is regenerated by ascorbic acid to its prior state (Sevanian et al., 1991).
Vitamin E
Vitamin E (α-tocopherol) is a monophenolic lipophilic compound and acts as a direct/antioxidant. Biochemically, the hydroxyl group in the vitamin E molecule - as in other phenols - may donate a proton to saturate and detoxify the unpaired electron, e.g., of the highly reactive hydroxyl radical. Vitamin E prevents the oxidation of the nonsaturated carbohydrate side chains of the membrane lipids and blocks the development of a chain reaction of lipid oxidation. It has been used in clinical trials for the treatment of Alzheimer's disease (AD). In patients with moderately severe impairment from AD, treatment with selegiline or alpha-tocopherol slows the progression of disease (Sano et al., 1997). Other research showed that various types of tocopherols have shown to increase amyloid-β (Aβ) level by enhancing the Aβ production and decreasing the Aβ degradation. Aβ production is enhanced by an elevated activity of the involved enzymes, the β- and γ-secretase. These results suggest that beside the beneficial antioxidative effects of vitamin E, tocopherol has in respect to AD also a potency to increase the amyloid-β level, which differ for the analyzed tocopherols (Grimm et al., 2015). Administration of vitamin E exerts protective effects against ischemic events, such as cerebral infarction. Its protective effect has been widely believed to be due to its anti-oxidant activity. During cerebral ischemia and subsequent reperfusion large amounts and a variety of radicals are generated. Vitamin E as a radical scavenger may reduce oxidative damage. When vitamin E is exposed to nitric oxide (NO) in vitro, it converts NO to nitrite ester, which is less toxic to neurons (D'Ischia and Novellino, 2007). In addition, dietary vitamin E reduces the generation and availability of NO andin brain tissue (Chow and Hong, 2002).
Selenium
Selenium is an essential biological trace element that plays a significant role in maintaining physiological functions. Selenium demonstrates protective properties in neoplastic and cardiovascular diseases (Loef et al., 2001; Steinbrennen and Sies, 2013), and its insufficiency in humans has been associated with an increased risk of Alzheimer's and Parkinson's disease (Chen and Berry, 2003). In addition, selenium supplementation has been shown to have a neuroprotective role in trauma induced oxidative injuries (Senol et al., 2014). Previous studies have shown that selenium is an important component of antioxidant enzymes such as glutathione peroxidase (GPx) and thioredoxin reductase (TrRx) (Kieliszek and Blazejak, 2013). Thus, selenium may play roles in antioxidative function and against oxidative stress initiated by an excess of ROS.
Coenzyme Q10
Much research in recent years has focused on mitochondrial dysfunction in ischemic neuronal injury. Mitochondria are well known to be a major source of ROS production. Potential agents such as coenzyme Q10 (CoQ10), creatine, and Schiller peptide (SS-31) were proven to improve mitochondrial dysfunction. Coenzyme Q10 (CoQ10), an essential cofactor of the electron transport chain, acts by maintaining the mitochondrial membrane potential, supporting ATP synthesis and inhibiting ROS generation for protecting neuronal cells against oxidative stress in neurodegenerative diseases (Beal and Shults, 2003; McCarthy et al., 2004). It is also a potent free radical scavenger in lipid and mitochondrial membranes. A number of studies suggest that it has protective and antioxidant effects in many neurodegenerative diseases (Beal, 1999; Shultz et al., 2002; Spindler et al., 2009). It has also been demonstrated that CoQ10 ameliorates most of the biochemical changes induced by cerebral I/R in irradiated rat brain and serum and reduces brain lactate accumulation, protects against ATP depletion and improves cellular respiratory activity in the endothelin in a rat model of cerebral ischemia. Thus, its ability to preserve mitochondrial function and facilitate neuronal ATP synthesis can lead to the reduction of anaerobic lactate production and brain lactate dehydrogenase (LDH) activity. It may also regulate cytosolic Ca2+homeostasis via modulation of energy generation (Ostrowski,2000; Almaas et al., 2002). Coenzyme Q10 therapy involves resistance against oxidative stress which can improve the brain bioenergetics, when supplemented during reperfusion after ischemic brain injury (Horecky et al., 2011). During past years, it has been in clinical trials for many neurodegenerative diseases such as Parkinson's disease (PD) and Huntington's disease (HD, Yang et al., 2009). In a recent study, its administration (100 mg/kg) led to a significant decrease in neuronal loss caused by I/R. The mentioned dose showed also a positive effect on the diameter of cells in comparison with that in ischemia group. It seems that the neurotrophic effect of CoQ10 is able to reduce the severity of lesions in the hippocampus following transient global I/R (Hashemzadeh et al., 2014).
Albumin and omega-3 fatty acids
Serum albumin concentrations are inversely associated with increased stroke risk. In experimental studies on ischaemic stroke in rodents, human intravenous albumin was a promising neuroprotectant. It decreased infarction volume and improved neurological score (Belayev et al., 2001), and enhanced cerebral blood flow both in the ischaemic core and in the penumbra, an effect which was related to the extent of collateral flow (DeFazio et al., 2012). High-dose human albumin (Alb) therapy is strongly neuroprotective in animal models of focal cerebral ischemia (Belayev et al., 2001), as well as in global cerebral ischemia (Belayev et al., 1999) and traumatic brain injury (Belayev et al., 2009). The neuroprotective efficacy of albumin is attributed to its multifunctional properties, which include antioxidant action, hemodilution and oncotic effects, binding of copper ions, fatty-acid transport, preservation of endothelial integrity, platelet anti-aggregatory effects and decreased red blood cell sedimentation under low-flow conditions (Belayev et al., 2002). Nutrition provides the proper building blocks for the brain to create and maintain connections, which is critical for improved cognition and academic performance. Dietary factors have a broad and positive action on neuronal function and plasticity. For example, the omega-3 fatty acids provide building material to the brain. They are essential for supporting intercellular signaling events, and therefore positively influence synaptic function. However, diets rich in sugar, saturated fats, or high in calories are considered deleterious for neural function, as they act to elevate levels of oxidative stress and reduce synaptic plasticity and cognitive functions (Gomez-Pinilla, 2002). Recent studies have established that omega-3 fatty acids reduce inflammation and may help lower risk of chronic diseases such as heart disease, cancer, and arthritis. Docosahexaenoic acid (DHA; 22:6, n - 3), a member of omega-3 fatty acid family, is highly concentrated in the brain and appears to be important for cognitive (brain memory and performance) and behavioral function. DHA therapy in low (3.5 mg/kg) and medium (5 mg/kg) doses improves neurological and histological outcome following focal cerebral ischemia in rats (Belayev et al., 1999). Docosahexaenoic acid is an essential omega-3 fatty acid that must be consumed from the diet. DHA can be found in fish (salmon,
tuna, mackerel, herring, sardines, halibut), other seafood (algae, krill) and some nuts and plants. It is well established that DHA and other omega-3 fatty acids have an important role in heart health. Omega-3 fatty acids also play a critical role in brain function, as well as normal growth and development (Bazan et al., 2007). DHA is a crucial component of the body's endogenous mechanism to protect the brain after injury. When albumin is complexed with DHA, it is possible to achieve neuroprotection at lower albumin doses. This significantly improves neurological outcomes, reduces infarct volumes, and promotes cellular survival, but it also has these effects in an aged rat model. DHA—Alb complex therapy given to aged animals after middle cerebral artery occlusion results in high-grade neuroprotection equaling or exceeding that afforded by native albumin, improves behavioral outcome even when treatment is delayed up to 3 hours after stroke onset, and reduces subcortical and total infarct volumes (Tiffany et al., 2014) (Figure 3).
Polyphenols
There has recently been growing interest, supported by a number of epidemiological and experimental studies, on the possible beneficial effects of polyphenols on brain health. Polyphenols are abundant micronutrients in plant-derived foods and are powerful antioxidants. Fruits and beverages such as tea, red wine, cocoa, and coffee are major dietary sources of polyphenols. Polyphenols have been reported to exert their neuroprotective actions through the potential to protect neurons against injury induced by neurotoxins, an ability to suppress neuroinflammation, and the potential to promote memory, learning, and cognitive function (Shukitt-Hale et al., 2008). Recent evidence suggests that their beneficial effects involve decreases in oxidative/inflammatory stress signaling, increases in protective signaling and neurohormetic effects, leading to the expression of genes that encode antioxidant enzymes, neurotrophic factors, and cytoprotective protein (Vauzour, 2012). The largest group of polyphenols is the flavonoids. The non-flavonoid group of polyphenols may be separated into two different classes: the phenolic acids, including the hydroxybenzoicacids (C1—C3 skeleton) and hydroxycinnamic acids (C3—C6 skeleton), and the stilbenes (C6—C2—C6 skeleton). Resveratrol, the main stilbene, can be found in grapes, wine, and peanuts. Of particular interest is the ability of flavonoids to activate the extracellular signal-regulated kinase and the Akt signaling pathways, leading to the activation of the cyclic adenosine monophosphate response element binding protein, a transcription factor that increases the expression of a number of neurotrophins important in LTP and long-term memory. One such neurotrophin is BDNF, which is known to be crucial in controlling synapse growth, promoting increase in dendritic spine density, and enhancing synaptic receptor density (Spencer, 2008). Other potent polyphenol is curcumin, antioxidant enriched in tumeric. It has been shown to elicit a variety of biological effects through its antioxidant and antiinflammatory properties. Curcumin (7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is the major yellowpigment extracted from turmeric, which is isolated from the powdered dry rhizome of the herb Curcuma longa. Accumulating evidences have shown that curcumin possesses a wide range of properties including anti-inflammation, antioxidation, anticancer, antiparasite, and antimalaria (Gupta et al., 2012). Recent studies have demonstrated the neuroprotective effect of curcumin on cerebral ischemic injury in rodent animals (Jiang et al., 2000; Zhao et al., 2000; Wu et al., 2013). It also attenuates lipid peroxidation and scavenges free radicals (Giordano et al., 2013). During ischemia, caspases are the proteases involved in apoptosis like cell death. Programmed cell death (PCD) is an active process resulting in cell suicide. Apoptosis, the classical form of eukaryotic PCD, is specifically characterized by DNA fragmentation and membrane depolarization. Apoptotis like cell-death show some similar hallmarks with apoptotis, however it is triggered by severe DNA damage and operated by a novel death pathway (Erental et al., 2012). Neuronal death involving caspases is more extensive after permanent than transient focal brain ischemia and may contribute to the delayed loss of neurons in the penumbral region of infarcts in rat brain. Administration of caspase inhibitors including carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]- fluoromethylketone (z-VAD.FMK), an interleukin-1β-converting enzyme inhibitor (z-VAD-DCB), and N-benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone (z-DEVD.FMK) reduces neuronal apoptosis after occlusion of the middle cerebral artery in rats (Loddick et al., 1996). Similar effects in diminishing the effects of caspases have also been demonstrated with the administration of polyphenols. Protection was seen even in the brain of pups, whose mothers had been fed pomegranate juice, after undergoing cerebral ischemia (Loren et al., 2005).
Flavonoids and essential oils
Ginger root contains flavonoids, such as quercetin, epicatechin, catechin, kaempferol, fisetin, morin, and a considerable amount of methoxyphenols with various substituents, which are called gingerols and gingerones (Feng et al., 2011). Many substances found in the extract of ginger root are known as efficient natural antioxidants. Several studies have found that baicalein, a flavonoid derived from Scutellaria baicalensis, reduced brain infarction following focal brain ischemia (Jin et al., 2008; Pallast et al., 2010). Recently, it has been shown that baicalein is also able to attenuate the neuronal damage in the hippocampus of mice subjected to transient global brain ischemia. It was recently hypothesized that the protective role of baicalein against global ischemia is due, at least in part, to inhibition of MMP-9 activity (Lee and Lee, 2012). It is also very effective in treatment with some essential oils. Treatment with essential oils substantially improves the antioxidant state of the body. It has been shown, that essential oils decreased the lipid peroxidation intensity in erythrocyte membranes, leading to the improvement of their resistance, a decrease in microviscosity, and the maintenance of their structural integrity and functional activity. Clove essential oil was mostly an efficient bioantioxidant. It decreased the contents of products of lipid peroxidation in the liver and the brains of animals and in addition enhanced the resistance of lipids of these organs to autooxidation. A mixture of lemon essential oil and a ginger extract exhibited the maximum efficiency in this process. The intake of the essential oil induced the activation of the enzymatic antioxidant system in mice, significantly decreased the LPO intensity in the liver and the brain of mice and increased the activity of antioxidant enzymes in the liver. Thereby it increased the antioxidant status of the body and its resistance to oxidative stress (Misharina et al., 2014).
Statins Antioxidant Properties
Statins, the competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, are the most activecholesterol-lowering drugs and reduce the risk of carotid atherosclerotic plaque. At high doses they also exert pleiotropic effects that are potentially neuroprotective in ischemic stroke: improvement of endothelial function, vasodilatation, antioxidant, anti-thrombotic, anti-inflammatory and immuno-modulating actions were observed (Cimino et al., 2007; Goldstein, 2009). Several studies indicate that therapy with statins may reduce lipoprotein oxidation and ameliorate free radical injury. As well as having favorable antioxidant effects, such as increased lag time of copper-induced LDL oxidation and reduced leukocyte-induced LDL oxidation, statins may have broader antioxidant effects. Statin therapy may modulate central nervous system cytokine production. The clinical benefit of statins is also supported by the observation that statin treatment reduces progression of carotid intima-media thickening and also reduces ischemic stroke (Crouse et al., 1995). Most studies have explored the antioxidant properties of statins in relation to LDL; however, statins may exert broader antioxidant effects through preservation of superoxide dismutase activity (Chen et al., 1997; Hussein et al., 1997). There has been mentioned the first evidence that atorvastatin acutely administered immediately after an atherosclerotic ischemic stroke in patients exerts a lowering effect on immune-inflammatory activation of the acute phase of stroke. Patients with the daily administration of atorvastatin (80 mg) showed significantly lower plasma levels of tumor necrosis factor-α, interleukin (IL)-6, and vascular cell adhesion molecule-1. Its early use is associated to a better functional and prognostic profile (Tuttolomondo et al., 2016).
Melatonin
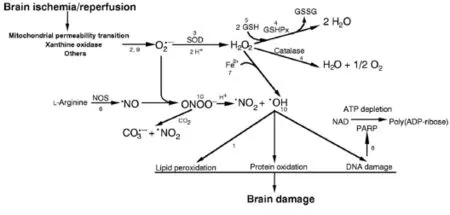
Figure 1 Biochemical reactions of ischemia/reperfusion brain injury.
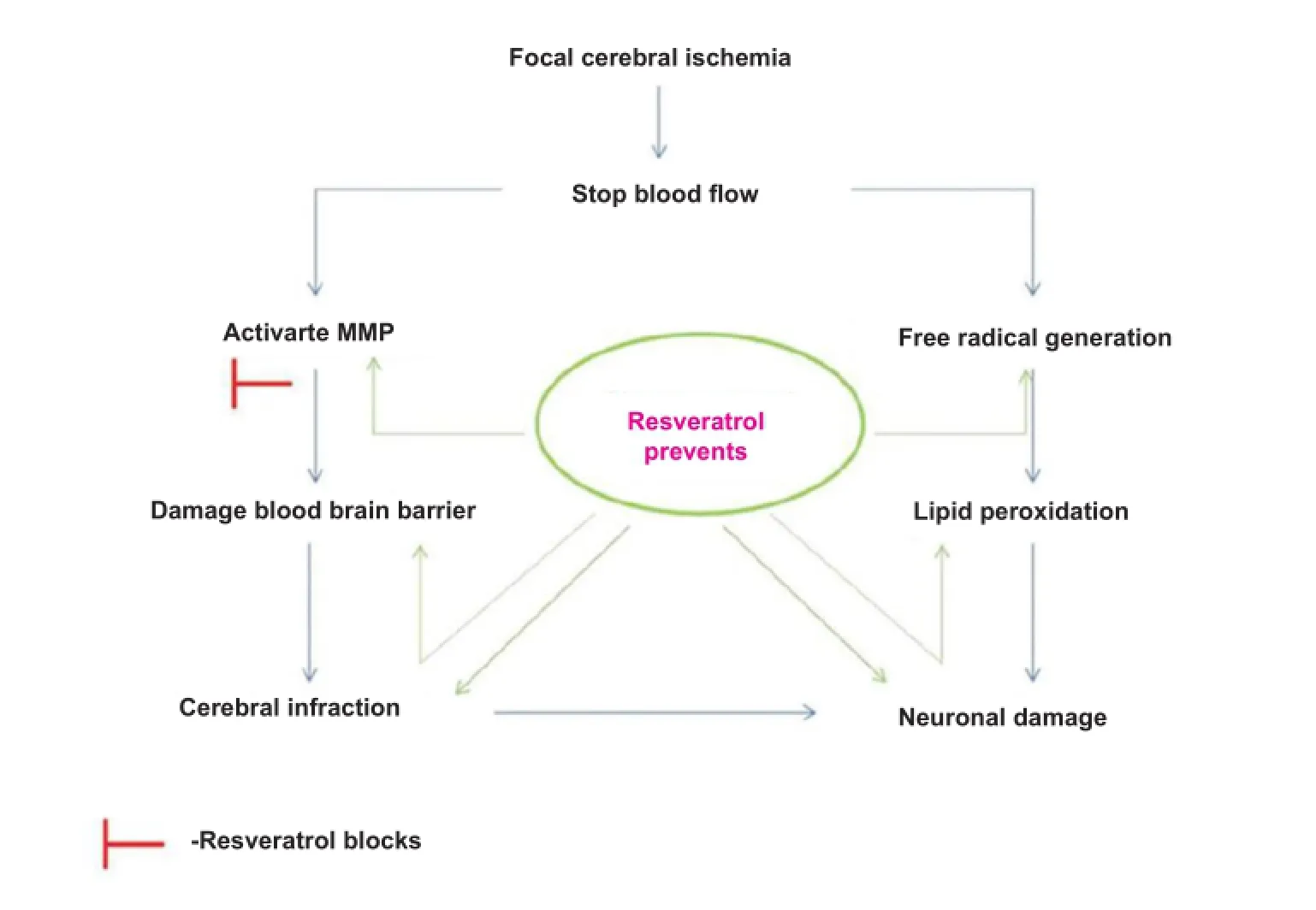
Figure 2 Possible pathway of matrix metalloproteinase-targeted neuroprotection of resveratrol in cerebral ischemia.
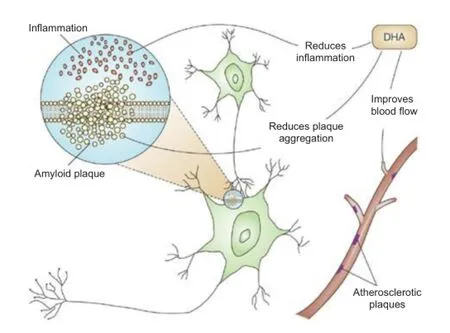
Figure 3 Proposed neuroprotective properties of DHA.
In the last decade, melatonin has emerged as a very powerful free radical scavenger and antioxidant. A number of studies have reported the important role of melatonin on neuroprotection in animal models of stroke. Melatonin administration after experimental stroke reduces infarction volume (Pei et al., 2003). It also reduces inflammatory response (Lee et al., 2007), and BBB permeability (Chen et al., 2006). In mice, melatonin significantly ameliorated secondary brain injury induced by traumatic brain injury (TBI). It enhanced autophagy, which inhibits mitochondrial apoptotic pathway, thus protecting mice from secondary brain injury after TBI (Ding et al., 2015). Melatonin is a potent free radical scavenger and antioxidant and has protective effects against ischemic damage. Melatonin protected the brain against damage induced by ischemic insults and prevented the ischemia-related neuronal death in the CA1 after I/R. In addition, melatonin treatment attenuated the activation of astrocytes and microglia and reduced the lipid peroxidation in the hippocampus after transient cerebral ischemia. The activation of MT2 melatonin receptor in the CA1 after melatonin treatment may be involved in the neuroprotective effect associated with melatonin after ischemic injury (Choong et al., 2010). Functionally, melatonin administration improves grip strength and motor coordination, and attenuates hyperactivity and anxiety (Kilic et al., 2008). Recently, the novel melatonin MT1/MT2 receptor agonist Neu-P11 has been studied. This melatonine derivate proved to be a good antioxidant, to protect against glutamate-induced excitotoxicity and oxygen and glucose deprivation in hippocampal slices, and to reduce infarct volume in an in vivo stroke model. Neu-P11 affordsneuroprotection against brain ischemia in in vitro and in vivo models by activating a pro-survival signaling pathway that involves melatonin receptors (Buendia et al., 2015).
Glial Cell Properties
The attempts of endogenous repair also focus on increasing comprehension of the neurobiology of neuroplasticity, synaptogenesis and neurogenesis. Neuroblasts are produced in the subventricular zone of the lateral ventricle and the subgranular layer of the dentate gyrus. Following acute ischemic stroke, a few of these cells proliferate and migrate towards the ischaemic lesion along blood vessels. The potentiation of endogenous post-stroke neurogenesis, axonal growth and angiogenesis could be relevant for functional recovery. Glial cells are actively involved in homeostasis and brain repair after injury. In the developing central nervous system, astrocytes have been shown to correctly guide migration and proliferation of neurons, whereas in the adult, astrocytes have been implicated in the maintenance of neuronal homeostasis and synaptic plasticity (Vernadakis, 1996). Astrocytes have been shown to release growth factors under normal conditions or in response to brain injury (Lehrmann et al., 1998). Additional properties of astrocytes include their ability to control water balance, and to reduce glutamate toxicity (Hansson and Ronnback, 1995). Astrocytes siphon excess extracellular water and potassium ions, which are then redistributed to their networks or excreted into the blood vessels. They also transport glutamate into soma and simultaneously detoxicate glutamine by converting toxic OH2into less harmful H2O2. These findings suggest that glial cells can exert protective effects and increase neuronal survival through their trophic, siphoning, and detoxicating actions.
Synthetic Antioxidants
There is a need for new stroke therapies and finding other possible approaches towards neuroprotection. New antioxidant therapies using synthetic antioxidants have shown some potential to be neuroprotective. The synthetic polyphenolic antioxidant di-tert-butyl-bisphenol (BP) was designed as a superior radical scavenging agent following structure and function analysis of more than 60 natural and synthetic (poly)phenols. BP supplementation significantly limits ROS generation, modulates acute phase gene responses, enhances cellular energetics, and improves cell viability following hypoxia/re-oxygenation injury in cultured human neuronal cells (Rayner et al., 2006; Duong et al., 2008). Dietary supplementation with BP before ischemic injury decreases infarction and vascular complications in experimental stroke in an animal model. Pre-treatment with BP significantly lowered lipid, protein and thiol-oxidation and decreased infarct size in animals subjected to middle cerebral artery occlusion and reperfusion injury. It ameliorated oxidative damage and preserves cerebral tissue during focal ischemic insult, inhibited of oxidative stress through BP scavenging free radicals in vivo and thereby contributed significantly to neuroprotection (Duong et al., 2014). In the medical application of nanoparticles, nanomedicine comes also with other neuroprotective agents. Chemical element cerium is found in a number of minerals and applications of cerium and its oxides are numerous. Cerium oxide nanoparticles (nanoceria) are widely used as catalysts in industrial applications because of their potent free radical-scavenging properties. Ceria nanoparticles reduced ischemic cell death in mouse by approximately 50%. The neuroprotective effects of nanoceria were due to a modest reduction in reactive oxygen species, in general, and partial reductions in the concentrations of superoxide and nitric oxide, specifically. Nanoceria may be useful as a therapeutic intervention to reduce oxidative and nitrosative damage after a stroke (Estevez et al., 2011). Uniform 3 nm-sized ceria nanoparticles can protect against ischemic stroke by scavenging ROS and reducing apoptosis. Optimal doses of ceria nanoparticles reduced infarct volumes and the rate of ischemic cell death in vivo (Kim et al., 2012). Synthetic melanin-like nanoparticles can also be used in biomedicine. Melanin-like nanoparticles that are <100 nm showed excellent dispersion stability in water as well as biological media and good biocompatibility to HeLa cells after the appropriate surface modification with thiol-terminated methoxy-poly(ethylene glycol) (mPEGSH). Furthermore, the demonstrated ability of melanin-like nanoparticles to reduce 2,2-diphenyl-1-picrylhydrazyl (DPPH) suggests free radical scavenging activity of the material (Ju et al., 2011). Synthetized dopamine-containing poly(β-amino ester) (DPAE) DPAE has excellent scavenging properties towards 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radicals in a dose dependent manner and could even reduce the dissolved oxygen content of physiological solution. The concentrations required for radical scavenging were shown to be non-toxic towards dopaminergic SH-SY5Y cells as well as primary astrocytes and primary embryonic rat ventral midbrain cultures (Newland et al., 2016).
In last years there has been deep research to find an effective strategy of exogenous delivery of the native form of SOD to neutralize the deleterious effect of ROS. One synthetized type of SOD encapsulated in sustained-release biodegradable poly(D,L-lactide co-glycolide) nanoparticles (SOD-NPs) showed its protective effect in reducing cerebral injury and promoting neurological recovery in a rat cerebral ischemia-reperfusion model. The protective effect of SODNPs could be due to the direct antioxidant effect of the active enzyme released from the NPs localized in the brain. SOD-NPs maintained BBB integrity, thereby preventing edema, reduced the level of ROS formed following reperfusion, and protected neurons from undergoing apoptosis. Animals treated with SOD-NPs demonstrated greater survival than those with saline control and later regained most vital neurological functions (Reddy and Lebhasetwar, 2009).
Conclusions
The brain is vulnerable to oxidative stress as a result of ischemia-reperfusion damage, since it consumes a large quantity of oxygen. In the processes of ischemic cascades, oxidative stress becomes a critical factor that leads to cell death. Oxidative stress can be traced primarily to formationof superoxide and nitric oxide. Both molecules serve as regulators of blood flow and neurotransmission. Perturbations in the production and/or metabolism of either molecule can have pathologic consequences. The formation of highly reactive products, including peroxynitrite and hydroxyl radical, results in lipid, protein and DNA damage. The brain has potent defenses against these processes including endogenous and enzymatic antioxidants. Recent studies show multiple effective approaches to the study of ROS/RNS in ischemic brain and many possible therapeutic interventions. A better understanding of endogenous defense mechanisms and brain biochemistry in collaboration with diet changes, new therapeutic methods and the use of pharmacological antioxidants target specific mechanisms of oxidative damage. However, long-term studies are critical in predicting clinical efficacy. Therefore, prevention and treatment of ischemic brain injury requires multiple interventions and further study for an effective outcome.
Author contributions: ML researched the latest knowledge and publications in the field, and wrote the article. VD designed, supervised and corrected the article. Both of these two authors approved the final version of this article for publication.
Conflicts of interest: None declared.
References
Almaas R, Saugstad OD, Pleasure D, Rootwelt T (2002) Neuronal formation of free radicals plays a minor role in hypoxic cell death in human NT2-N neurons. Pediatr Res 51:136-143.
Ames BN, Shigenaga MT, Hagen M (1993) Oxidants, antioxidants and the degenerative and pharmacological evidence for the involvement of oxygen radicals and lipid peroxidation. Free Radical Biol Med 6:303-313.
Bazan NG (2007) Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care 10:136-141.
Beal MF (1999) Coenzyme Q10 administration and its potential for treatment of neurodegenerative diseases. Biofactors 9:261-266.
Beal MF, Shults CW (2003) Effects of Coenzyme Q10 in Huntington's disease and early Parkinson's disease. Biofactors 18:153-161.
Belayev L, Alonso OF, Huh PW, Zhao W, Busto R, Ginsberg MD (1999) Posttreatment with high-dose albumin reduces histopathological damage and improves neurological deficit following fluid percussion brain injury in rats. J Neurotrauma 16:445-453.
Belayev L, Khoutorova L, Atkins KD, Bazan NG (2009) Robust docosahexaenoic acidmediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke 40:3121-3126.
Belayev L, Pinard E, Nallet H, Seylaz J, Liu Y, Riyamongkol P, Zhao W, Busto R Ginsberg MD (2002) Albumin therapy of transient focal cerebral ischemia: in vivo analysis of dynamic microvascular responses. Stroke 33:1077-1084.
Belayev L, Saul I, Huh PW, Finotti N, Zhao W, Busto R, Ginsberg MD (1999) Neuroprotective effect of high-dose albumin therapy against global ischemic brain injury in rats. Brain Res 845:107-111.
Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD (2001) Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke 32:553-560.
Bigos K, Hariri AR, Weinberger D (2015) Neuroimaging genetics: Principles and practices. Oxford University Press. p157.
Buendia I, Gómez-Rangel V, González-Lafuente L, Parada E, León R, Gameiro I, Michalska P, Laudon M, Egea J, López MG (2015) Neuroprotective mechanism of the novel melatonin derivative Neu-P11 in brain ischemia related models. Neuropharmacology 99:187-195.
Carter AM, Catto AJ, Mansfield MW, Bamford JM, Grant PJ (2007) Predictive variables for mortality after acute ischemic stroke. Stroke 38:1873-1880.
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21:2-14.
Chen J, Berry MJ (2003) Selenium and selenoproteins in the brain and brain diseases. J Neurochem 86:1-12.
Chen L, Haught WH, Yang B, Saldeen TG, Parthasarathy S, Mehta JL (1997) Preservation of endogenous antioxidant activity and inhibition of lipid peroxidation as common mechanisms of antiatherosclerotic effects of vitamin E, lovastatin and amlodipine. J Am Coll Cardiol 30:569-575.
Chen TY, Lee MY, Chen HY, Kuo YL, Lin SC, Wu TS, Lee EJ (2006) Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J Pineal Res 40:242-250.
Choong HL, Yoo KY, Choi JH, Park OK, Hwang IK, Kwon YG, KimYM, Won MW (2010) Melatonin's protective action againstischemic neuronal damage is associated with up-regulation of the MT2 melatonin receptor. J Neurosci Res 88:2630-2640.
Chow CK, Hong CB (2002) Dietary vitamin E and selenium and toxicity of nitrite and nitrate Toxicology 180:195-207.
Cimino M, Gelosa P, Gianella A, Nobili E, Tremoli E, Sironi L (2007) Statins: multiple mechanisms of actions in the ischemic brain. Neuroscientist 13:208-213.
Crouse JR, Byington RP, Bond MG, Espeland MA, Craven TE, Sprinkle JW, McGovern ME, Furberg CD (1995) Pravastatin, Lipids, and Atherosclerosis in the Carotid arteries (PLAC-II). Am J Cardiol 75:455-459.
Deb P, Sharma S, Hassan KM (2010) Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology 17:197-218.
DeFazio RA, Zhao W, Deng X, Obenaus A, Ginsberg MD (2012) Albumin therapy enhances collateral perfusion after laser-induced middle cerebral artery branch occlusion: a laser speckle contrast flowstudy. J Cereb Blood Flow Metab 32:2012-2022.
Ding K, Xu J, Wang H, Zhang L, Wu Y, Li T (2015) Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem Int 91: 46-54.
D'Ischia M, Novellino L (2007) Nitric oxide-induced oxidation of α-tocopherol. Bioorg Med Chem 4:1747-1753.
Dong W, Gao D, Lin H, Zhang X, Li N, Li F (2008) New insights into mechanism for the effect of resveratrol preconditioning against cerebral ischemic stroke: possible role of matrix metalloprotease-9. Med Hypotheses 70:52-55.
Duong TT, Antao S, Ellis NA, Myers SJ, Witting PK (2008) Supplementation with a synthetic polyphenol limits oxidative stress and enhances neuronal cell viability in response to hypoxiare-oxygenation injury. Brain Res 1219:8-18.
Duong TT, Chami B, McMahon AC, Fong GM, Dennis JM, Freedman SB, Witting PK (2014) Pre-treatment with the synthetic antioxidant T-butyl bisphenol protects cerebral tissues from experimental ischemia reperfusion injury. J Neurochem 130:733-747.
Erental A, Sharon I, Engelberg-Kulka H (2012) Two programmed cell death systems in Escherichia coli: an apoptotic-like death is inhibited by the mazEF mediated death pathway. PLoS Biol 10:e1001281.
Estevez AY, Pritchard S, Harper K, Aston JW, Lynch A, Lucky JJ, Andreescu S (2011) Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic Biol Med 51:1155-1163.
Floyd RA, Carney JM (1992) Free radical damage to protein and DNA: Mechanism involved and relevant observations on brain undergoing oxidative stress. Ann Neurol 32:22-27.
Fotuhi M, Mohassel P, Yaffe K (2009) Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat Clin Pract Neurol 5:140-152.
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97-112.
Gao ZB, Chen XQ, Hu GY (2006) Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res 1111:41-47.
Giordano S, Darley-Usmar V, Zhang J (2013) Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biology 2:82-90.
Goldstein LB (2009) Statins and ischemic stroke severity: cytoprotection. Curr Atheroscler Repn 11:296-300.
Gomez-Pinilla F (2011) The combined effects of exercise and foods in preventing neurological and cognitive disorders. Prev Med 52:75-80.
Granger DN, McCord JM, Parks DA, Hollwarth ME (1986) Xanthine oxidase inhibitors attenuate ischemia induced vascular permeability changes in the cat intestine. Gastroenterology 90:80-84.
Grimm MO, Stahlmann CP, Mett J, Haupenthal VJ, Zimmer VC, Lehmann J, Hartmann T (2015) Vitamin E: Curse or benefit in Alzheimer's disease? A systematic investigation of the impact of α-, γ- and δ-tocopherol on Aβ generation and degradation in neuroblastoma cells. J Nutr Health Aging 19: 646-654.
Gupta SC, Patchva S, Koh W, Aggarwal BB (2012) Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol 39:283-299.
Hamm RJ, Temple MD, Pike BR, Ellis EF (1996) The effect of postinjury administration of polyethylene glycol-conjugated superoxide dismutase (pegorgotein, dismutec) or lidocaine on behavioral function following tluid percussion braininjury in rats. J Neurotrauma 13:325-332.
Hansson E, Ronnback L (1995) Astrocytes in glutamate neurotransmission. FASEB J 9:343-350.
Hao HD, He LR (2004) Mechanisms of cardiovascular protection by resveratrol. J Med Food 7:290-298.
Hashemzadeh E, Movassaghi S, Shafaroodi H, Barmchi A, Sharif Z (2014) Effect of Coenzyme Q10 (ubiquinone) on hippocampal CA1 pyramidal cells following transient global ischemia/reperfusion in male wistar rat. J Pharm Heal Sci 3:21-28.
Hillered L, Persson L, Bolander HG, Hallstrom A, Ungerstedt U (1998) Increased extracellular levels of ascorbic acid in the striatum after middle cerebral artery occlusion in the rat monitored by intracerebral microdialysis. Neurosci Lett 95:1-3.
Hooper DC, Scott GS, Zborek A, Mikheeva T, Kean RB, Koprowski H, Spitsin SV (2000) Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J 14:691-698.
Horecký J, Gvozdjáková A, Kucharská J, Obrenovich ME, Palacios HH, Li Y, Vančová O, Aliev G (2011) Effects of coenzyme Q and creatine supplementation on brain energy metabolism in rats exposed to chronic cerebral hypoperfusion. Curr Alzheimer Res 8:868-875.
Hussein O, Schlezinger S, Rosenblat M, Keidar S, Aviram M (1997) Reduced susceptibility of low density lipoprotein (LDL) to lipid peroxidation after fluvastatin therapy is associated with the hypocholesterolemic effect of the drug and its binding to LDL. Atherosclerosis 128:11-18.
James JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC (1999) Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci 9:8114 -8121.
Jiang J, Wang W, Sun YJ, Hu M, Li F, Zhu DY (2007) Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur J Pharmacol 561:54-62.
Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, van Leyen K (2008) Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke 39:2538-2543.
Ju KY, Lee Y, Lee S, Park SB, Lee JK (2011) Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules 12:625-632.
Kieliszek M, Blazejak M (2013) Selenium: significance, and outlook for supplementation. Nutrition 29:713-718.
Kilic E, Kilic U, Bacigaluppi M, Guo Z, Abdallah B, Wolfer DP, Reiter RJ, Hermann DM, Bassetti CL (2008) Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery, and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J Pineal Res 45:142-148.
Kim CK, Kim T, Choi IY, Soh M, Kim D, Kim YJ, Park SP (2012) Ceria nanoparticles that can protect against ischemic stroke. Angew Chem Int Ed Engl 51:11039-11043.
Kohen R, Nyska A (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620-650.
Kontos HA (2002) Oxygen radicals in cerebral ischemia. Stroke 11:2712-2716.
Lee JH, Lee SR (2012) The effect of Baicalein on hippocampal neuronal damage and metalloproteinase activity following transient global cerebral ischaemia. Phytother Res 26:1614-1619.
Lee MY, Kuan YH, Chen HY, Chen TY, Chen ST, Huang CC, Yang IP, Hsu YS, Wu TS, Lee EJ (2007) Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J Pineal Res 42:297-309.
Lee SH, Kim M, Yoon BW, Kim YJ, Ma SJ, Roh JK, Lee JS, Seo JS (2001) Targeted hsp701 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke 32:2905-2912.
Lehrmann E, Kiefer R, Christensen T, Toyka KV, Zimmer J, Diemer NH, Hartung HP, Finsen B (1998) Microglia and macrophages are major sources of locally produced transforming growth factor beta (1) after transient middle cerebral artery occlusion in rats. Glia 24:437-448.
Li H, Horke S, Förstermann U (2014) Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 237: 208-219.
Loddick SA, MacKenzie A, Rothwell NJ (1996) An ICE inhibitor, zVADDCB attenuates ischaemic brain damage in the rat. Neuroreport 7:1465-1468.
Loef M, Schrauzer GN, Walach H (2001) Selenium and Alzheimer's disease: a systematic review. J Alzheimers Dis 26:81-104.
Loren DJ, Seeram NP, Schulman RN, Holtzman DM (2005) Maternal dietary supplementation with pomegranate juice is neuroprotective in an animal model of neonatal hypoxic-ischemic brain injury. Pediatr Res 57:858-864.
Llull L, Amaro S, Chamorro Á (2016) Administration of uric acid in the emergency treatment of acute ischemic stroke. Curr Neurol Neurosci Rep 16:1-11.
Martínez J, Boll-Woehrlen C, Hernández A, Rubio M, Sánchez M, Ríos C, Pérez F (2010) Radicales libres y estrés oxidativo en las enfermedades neurodegenerativas. Mensaje Bioquímico 34:43-59.
McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S (2004) Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol 201:21-31.
Miele M, Boutelle MG, Fillenz M (1994) The physiologically induced release of ascorbic acid in rat brain is dependent on impulse traffic, calcium influx and glutamate uptake. Neuroscience 62:87-91.
Misharina TA, Fatkullina LD, Alinkina S, Kozachenko AI, Nagler LG, Medvedeva IB, Goloshchapov AN, Burlakova EB (2014) Effects of low doses of essential oils on the antioxidant status of the erythrocytes, liver and the brain of mice. Prikladnaya Biokhimiya i Mikrobiologiya 50:101-107.
Moor E, Kohen R, Reiter RJ, Shohami E (2001) Closed head injury increases extracellular levels of antioxidants in rat hippocampus in vivo: an adaptive mechanism? Neurosci Lett 316:169-172.
Muir JK, Tynan M, Cadwell R, Ellis EF (1995) Superoxide dismutase improves post-traumatic cortical blood flow in rats. J Neurotrauma 12:179-188.
Muizelaar JP (1994) Clinical trials with Dismutec (pegorgotein, polyethyleneglycol- conjugated superoxide dismutase, PEG-SOD) in the treatment of severe closed head injury. Adv Exp Med Bio 366:389-400.
Mukherjee PK, Ahamed KF, Kumar V, Mukherjee K, Houghton PJ (2007). Protective effect of biflavones from Araucaria bidwillii Hook in rat cerebral ischemia/reperfusion induced oxidative stress. Behav Brain Res 178:221-228.
Newland B, Wolff P, Zhou D, Wang W, Zhang H, Rosser A, Werner C (2016) Synthesis of ROS scavenging microspheres from a dopamine containing poly (β-amino ester) for applications for neurodegenerative disorders. Biomater Sci 4:400-404
Okado-Matsumoto A, Fridovich I (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver. J Biol Chem 276:38388-38393.
Ostrowski RP (2000) Effect of coenzyme Q on biochemical and morphological changes in experimental ischemia in the rat brain. Brain Res 53:399-407.
Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X, Lo EH, van Leyen K (2010) Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. J Cereb Blood Flow Metab 30:1157-1167.
Pandey AK, Bhattacharya P, Shukla SC, Paul S, Patnaik R (2015) Resveratrol inhibits matrix metalloproteinases to attenuate neuronal damage in cerebral ischemia: a molecular docking study exploring possible neuroprotection. Neural Regen Res 10:568-575.
Pei Z, Pang SF, Cheung RT (2003) Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke 34:770-775.
Pérez A, Abilés J, Castańo J (2008) Estrés oxidativo y su implicación en distintas patologías. Nutrición clínica en Medicina 2:45-64.
Potter A, Buck AC, Self WK, Callanan ME, Sunil S, Capadona JR (2013) The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microelectrodes. Biomaterials 34:7001-7015.
Rayner BS, Duong TT, Myers SJ, Witting PK (2006) Protective effect of a synthetic anti-oxidant on neuronal cell apoptosis resulting from experimental hypoxia re-oxygenation injury. J Neurochem 97:211-221.
Reddy MK, Labhasetwar V (2009) Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB J 23:1384-1395.
Reiter RJ (1995) Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J 9:526-533.
Rice ME (2000) Ascorbic acid regulation and its neuroprotective role in the brain. Trends Neurosci 23:209-216.
Rodrigo R, Fernandez-Gajardo R, Gutierrez R, Manuel Matamala J, Carrasco R, Miranda-Merchak A, Feuerhake W (2013) Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord Drug Targets 12:698-714.
Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ (1997) A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. N Engl J Med 336:1216-1222.
Senol N, Naziroglu M, Yuruker V (2014) N-acetylcysteine and selenium modulate oxidative stress, antioxidant vitamin and cytokine values in traumatic brain injury-induced rats. Neurochem Res 39:685-692.
Sevanian A, Davies KL, Hochstein P (1991) Serum uric acid as an antioxidant for ascorbic acid. Am J Clin Nutr 54:1129-1134.
Shearer J (2014) Insight into the structure and mechanism of nickel-containing superoxide dismutase derived from peptide-based mimics. Acc Chem Res 47:2332-2341.
Shukitt-Hale B, Lau FC, Carey AN, Galli RL, Spangler EL, Ingram DK, Joseph JA (2008) Blueberry polyphenols attenuate kainic acid-induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutr Neurosci 11:172-182.
Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J, Kompoliti K, Perlmutter JS, Reich S, Stern M, Watts RL, Kurlan R, Molho E, Harrison M (2002) Lew Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol 59:1541-1550.
Sinet PM, Heikkila RE, Cohen G (1980) Hydrogen peroxide production in rat brain in vivo. J Neurochem 34:1421-1428.
Spencer J (2008) Food for thought: the role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc Nutr Soc 67:238-252.
Spindler M, Beal MF, Henchcliffe C (2009) Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr Dis Treat 5:597-610.
Steinbrenner H, Sies H (2013) Selenium homeostasis and antioxidant selenoproteins in brain: implications for disorders in the central nervous system. Arch Biochem Biophys 536:152-157.
Sun A, Wang Q, Simonyi A, Sun G (2008) Botanical phenolics and brain health. Neuromolecular Med 4:259-274.
Tiffany N, Khoutorova L, Obenaus A, Mohd-Yusof A, Bazan NG, Belayev L (2014) Docosahexaenoic acid complexed to albumin provides neuroprotection after experimental stroke in aged rats. Neurobiol Dis 62:1-7.
Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW, Liu HY, Zhang FB, Huang SS (2007) Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg 46:346-353.
Tuttolomondo A, Di Raimondo D, Pecoraro R, Maida C, Arnao V, Della Corte V, Iacopino DG (2016) Early high-dosage atorvastatin treatment improved serum immune-inflammatory markers and functional outcome in acute ischemic strokes classified as large artery atherosclerotic stroke: a randomized trial. Medicine 95:e3186.
Vauzour D (2012) Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev 914273pp.
Vernadakis A (1996) Glia-neuron intercommunications and synaptic plasticity. Prog Neurobiol 49:185-214.
Warner DS, Sheng H, Batinić-Haberle I (2004) Oxidants, antioxidants and the ischemic brain. J Exp Biol 207:3221-3231.
Wu J, Li Q, Wang X, Yu S, Li L, Wu X, Chen Y, Zhao J, Zhao Y (2013) Neuroprotection by curcumin in ischemic brain injury involves the akt/nrf2 pathway. PLoS One 8:e59843.
Yamazaki T, Kawai C, Yamauchi A, Kuribayashi F (2011) A highly sensitive chemiluminescence assay for superoxide detection and chronic granulomatous disease diagnosis. Trop Med Health 39:41-45.
Yang L, Calingasan NY, Wille EJ, Cormier K, Smith K, Ferrante R, Beal MF (2009) Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson's and Huntington's diseases. J Neurochem 109:1427-1439.
Yousuf S, Atif F, Ahmad M, Hoda MN, Khan MB, Ishrat T, Islam F (2007) Selenium plays a modulatory role against cerebral ischemia-induced neuronal damage in rat hippocampus. Brain Res 1147:218-225.
Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP (1998) Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res 53:613-625.
Yusa T (2000) Continuous real-time measurement of extracellular ascorbic acid release in the rat striatum in vivo during ischemia—reperfusion. Neurosci Lett 293:123-126.
Zhao J, Yu S, Zheng W, Feng G, Luo G, Wang L, Zhao Y (2000) Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res 35:374-379.
10.4103/1673-5374.184447 Accepted: 2016-05-16
How to cite this article: Lalkovičová M, Danielisová V (2016) Neuroprotection and antioxidants. Neural Regen Res 11(6)∶865-874.
*Correspondence to: Mária Lalkovičová, lalkovicova@saske.sk.
杂志排行
中国神经再生研究(英文版)的其它文章
- Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies
- Role of myelin auto-antigens in pain: a female connection
- Endogenous bioelectric fields: a putative regulator of wound repair and regeneration in the central nervous system
- The intricacies of neurotrophic factor therapy for retinal ganglion cell rescue in glaucoma: a case for gene therapy
- Discovery of nigral dopaminergic neurogenesis in adult mice
- Methylprednisolone for acute spinal cord injury: an increasingly philosophical debate
