High-frequency electroacupuncture evidently reinforces hippocampal synaptic transmission in Alzheimer's disease rats
2016-12-02WeiLiLihongKongHuiWangFengShenYawenWangHuaZhouGuojieSun
Wei Li, Li-hong Kong, Hui Wang, Feng Shen Ya-wen Wang Hua Zhou Guo-jie Sun
1 Hubei University of Chinese Medicine, Wuhan, Hubei Province, China
2 School of Acupuncture Orthopedics, Hubei University of Chinese Medicine, Wuhan, Hubei Province, China
RESEARCH ARTICLE
High-frequency electroacupuncture evidently reinforces hippocampal synaptic transmission in Alzheimer's disease rats
Wei Li1, Li-hong Kong2,*, Hui Wang1, Feng Shen2, Ya-wen Wang2, Hua Zhou2, Guo-jie Sun2
1 Hubei University of Chinese Medicine, Wuhan, Hubei Province, China
2 School of Acupuncture Orthopedics, Hubei University of Chinese Medicine, Wuhan, Hubei Province, China
Graphical Abstract
orcid: 0000-0002-4287-0536 (Li-hong Kong)
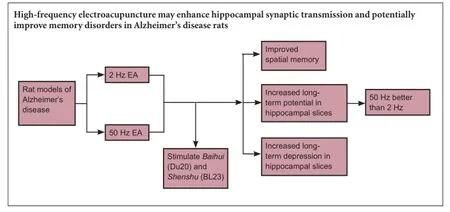
The frequency range of electroacupuncture in treatment of Alzheimer's disease in rats is commonly 2—5 Hz (low frequency) and 50—100 Hz (high frequency). We established a rat model of Alzheimer's disease by injecting β-amyloid 1—42 (Aβ1—42) into the bilateral hippocampal dentate gyrus to verify which frequency may be better suited in treatment. Electroacupuncture at 2 Hz or 50 Hz was used to stimulate Baihui (DU20) and Shenshu (BL23) acupoints. The water maze test and electrophysiological studies demonstrated that spatial memory ability was apparently improved, and the ranges of long-term potentiation and long-term depression were increased in Alzheimer's disease rats after electroacupuncture treatment. Moreover, the effects of electroacupuncture at 50 Hz were better than that at 2 Hz. These findings suggest that high-frequency electroacupuncture may enhance hippocampal synaptic transmission and potentially improve memory disorders in Alzheimer's disease rats.
nerve regeneration; Alzheimer's disease; frequency; electroacupuncture; long-term potentiation; long-term depression; learning and memory; Baihui (DU20); Shenshu (BL23); neural regeneration
Introduction
Alzheimer's disease (AD) is a chronic neurodegenerative disease characterized by progressive cognitive impairment associated with memory impairment. The major pathological changes are neurofibrillary tangles, senile plaques and neuronal loss (Lister and Barnes, 2009). The prevention and treatment of AD are very complicated. Modern medicine has studied AD at the genetic and molecular levels, but cannot reveal its exact etiology and pathogenesis. Learning and memory deficits are major clinical manifestations of AD, but synaptic plasticity is believed to be an important cellular basis for learning and memory (Tang et al., 2012). Synaptic plasticity mainly includes structural and functional plasticity. Han (2005) considered that synaptic plasticity has direct effects on neural activity. Long-term potentiation and long-term depression are two indicators and forms of synaptic transmission (Peng and Guo, 2008; Collingridge et al., 2010). Long-term potentiation as an experimental model of synaptic plasticity has been used in the study of learning and memory by neuroscientists (Bliss, 1999; Amici et al., 2009).Generally, long-term potentiation and long-term depression are factors that affect neuronal synaptic plasticity and some learning and memory activities. Long-term potentiation is associated with memory formation and storage. Long-term depression is associated with memory integration, forgetting, and recovery of long-term potentiation production at synapses (desaturation). Long-term potentiation and longterm depression compose a neural network to learn (Elgersma and Silva, 1999). Morris et al. (1997) believed that longterm potentiation of experience-dependent hippocampal excitatory synaptic transmission has already formed the basis of some forms of memory. Mcnaughton et al. (1986) found that the saturation in synapses led to severe learning disabilities. The premise of the recovery of learning ability with the recovery of synaptic transmission was that synaptic transmission recovered to a normal level (Morris et al., 1986).
Acupuncture is an effective method to prevent and treat AD (Cui, 2014). Baihui (DU20) and Shenshu (BL23) are acupoints used in the treatment of AD. Zhu et al. (2013) considered that acupuncture at Baihui and its subsidiary acupoints were able to improve the behaviors of rats with vascular dementia by mammalian target of the rapamycin (mTOR) signaling pathway. Meng et al. (2009) considered that acupuncture pretreatment at Baihui and Shenshu was able to ameliorate the learning and memory abilities in rats with vascular dementia. Luo et al. (2013) stated that acupuncture at Baihui and Shenshu in the prevention and treatment of AD was probably associated with the effects of inhibiting overexpression of ethanol dehydrogenase protein in the mitochondria of hippocampal neurons, elevating cytochrome oxidase IV activity, and improving mitochondrial energy metabolism. The effects of electroacupuncture on AD have been strongly associated with electroacupuncture parameters and acupoints (Yu et al., 2014). The currently accepted frequency range of electroacupuncture is 2—5 Hz (low frequency) and 50—100 Hz (high frequency). This study sought to compare the effects of electroacupuncture at different frequencies (2 Hz and 50 Hz) at Baihui and Shenshu on synaptic transmission in AD rats.
Materials and Methods
Animals
Fifty specific-pathogen-free male Wistar rats aged 4—5 months and weighing 300—350 g were provided by the Hubei Research Center of Laboratory Animals of China (license No. SCXK (E) 2008-0005). All rats were housed at 24 ± 2°C in 12-hour light/dark cycles. The protocols were approved by the Animal Ethics Committee of Hubei University of Chinese Medicine of China.
Establishment of AD models
Two unqualified rats were excluded in the water maze test. Exclusion criteria: in normal rats, the upper limit was onefold the standard deviation of their mean escape latency, and the lower limit was two-fold the standard deviation (Du et al., 2011). The remaining 48 rats were equally and randomly divided into normal group, sham surgery group, model group, sham acupuncture group, 2 Hz electroacupuncture group and 50 Hz electroacupuncture group.
AD models were established in accordance with O'Hare et al.'s method (1999). β-Amyloid 1—42 (Aβ1—42) (Sigma, St. Louis, MO, USA) was prepared into 1 µg/µL solution with PBS, and incubated in a 37°C water tank for 7 days. In the model, sham acupuncture, 2 Hz electroacupuncture and 50 Hz electroacupuncture groups, the rats were weighed and intraperitoneally anesthetized with 10% chloral hydrate (0.3 g/kg). 5 µL of oligomeric Aβ1—42was injected into bilateral hippocampal dentate gyrus (anteroposterior: 3.2 mm, lateral/rostral: 2.5 mm, height: 3.5 mm). In the sham surgery group, 5 µL of 0.9% NaCl was injected into bilateral dentate gyrus. Rats in the normal group did not receive any injection.
Electroacupuncture
15 days after model establishment, rats in the 2 Hz electroacupuncture and 50 Hz electroacupuncture groups underwent electroacupuncture using the Hans electronic acupuncture apparatus (HANS-100A, Beijing Huayun Ante Science and Technology Co., Ltd., Beijing, China). Two needles were pricked into the Shenshu and Baihui points as positive and negative electrodes, respectively. Shenshu was alternately stimulated on the left and right sides every day. Parameters were as follows: continuous wave, voltage 2—4 V, current 1—2 mA. The needle was maintained in place for 20 minutes. Electroacupuncture was performed once a day, over a course of 7 days for two courses, with a day off between courses. The frequencies in the 2 Hz electroacupuncture group were 2 Hz and 50 Hz in the 50 Hz electroacupuncture group. In the sham acupuncture group, only the surfaces of Baihui and Shenshu were stimulated, but the current was not connected.
Morris water maze
After electroacupuncture, learning and memory abilities were measured using Morris water maze (Chengdu Taimeng Technology Co., Ltd., Chengdu, Sichuan Province, China). Rats swam freely twice to orient themselves before the test for 120 seconds each time. The Morris water maze consisted of a navigation test and spatial probe test for 5 days. For the navigation test, the tank was arbitrarily divided into southeast, northeast, northwest and southwest quadrants. The time needed for the rats to search and climb the platform in the water was recorded within 2 minutes and this was regarded as the escape latency. Mean escape latency was calculated, and the results were recorded for 4 days. If an animal failed to climb onto the platform within 120 seconds, it was manually guided onto the platform and made to stay for 10 seconds. Spatial probe test: the platform was removed from the pool on day 5, and mice were allowed to search for the platform for 120 seconds. The numbers of platform crossings in 120 seconds, and the first time of crossing the platform were recorded (Du et al., 2011).
Preparation of hippocampal slices
After the Morris water maze, all rats were intraperitoneally anesthetized with chloral hydrate, and decapitated. By continuously washing with an artificial cerebrospinal fluid (NaCl 7.25 g, KCl 0.22 g, MgSO4·7H2O 0.25 g, CaCl20.22 g, KH2PO40.17 g, NaHCO32.18 g and glucose 1.80 g in 1 L of ultrapure water) aerated with 95% O2+ 5% CO2, the brains were rapidly extracted and placed on artificial cerebrospinal fluid-coated filter paper. After removal of the cerebellum, the cerebrum was cut open along the median line. Along the sagittal line at 45°, the hemisphere was cut with a blade, fixed on agarose gel, and sliced into 500 µm-thick sections with a microtome (Leica, Freiburgim Breisgau, Germany) in mixed gas and 4°C artificial cerebrospinal fluid. The sections were placed in 30—32°C artificial cerebrospinal fluid aerated with 95% O2+ 5% CO2for 1 hour.
Electrophysiological experiments
After 1 hour of incubation, all sections were placed in a recording chamber (0.3—0.4 mL) and continuously aerated with 95% O2+ 5% CO2and 4°C artificial cerebrospinal fluid at a speed of 1.5—2.5 mL/min at 31—32°C. A stimulating electrode was placed on perforating fibers anterior to the entorhinal area, and a recording electrode was placed on the hippocampal dentate gyrus. MED64 Mobius software (Alpha Med Science, Osaka, Japan) was used to record postsynaptic population spike.
Long-term potentiation and long-term depression recording
Conditioned stimulus was first given at pulse width 150 µs, 0.5 Hz frequency, constant current (0.15—1.0 mA), square wave. Input/output (I/O) curves were measured. The conditioned stimulus intensity was the intensity that evoked 50% of the maximum population spike amplitude. 30 minutes after the population spike was stable (amplitude changes <10%), the conditioned stimulus was given and recorded for 30 minutes as a baseline. High-frequency stimulus was then given: twenty 200-Hz pulses served as a group, with an interval of 2 seconds, 10 groups in total, with the intensity that evoked 75% of the maximum population spike amplitude. Afterwards, conditioned stimulus was given and amplitude changes were recorded within 60 minutes. In the long-term depression test, low-frequency stimulus was given: the intensity of conditioned stimulus, nine hundred 1-Hz train stimuli, 5 pulses at 250 Hz in each train. Population spike amplitude was measured with the distance of the minimum of the first descent phase to the midpoint of two rising peaks. The percentage of population spike amplitude before and after stimulation was used to indicate the altered degree of the plasticity of synaptic transmission (long-term potentiation and long-term depression).
Statistical analysis
All data were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA) and expressed as the mean ± SD. Intergroup data were compared using one-way analysis of variance. A value of P < 0.05 was considered statistically significant.
Results
Effects of different frequencies of electroacupuncture on the behavior of AD rats
Navigation test
The escape latency was significantly longer in the model group than in the normal group (P < 0.01). The escape latency was significantly shorter in the 2 Hz and 50 Hz electroacupuncture groups than in the model and sham acupuncture groups (P < 0.01). The escape latency was significantly shorter in the 50 Hz electroacupuncture group than in the 2 Hz electroacupuncture group (P < 0.01; Figure 1).
Spatial probe test
The first time of crossing the platform was significantly later in the model group than in the normal group (P < 0.01). The first time of crossing the platform was significantly shorter (P < 0.01), but the number of times test animals crossed the platform was significantly more (P < 0.01) in the 2 Hz and 50 Hz electroacupuncture groups compared with the model and sham acupuncture groups. The escape latency was shorter (P < 0.01), but the number of times test animals crossed the platform was more (P < 0.01) in the 50 Hz electroacupuncture group compared with the 2 Hz electroacupuncture group (Figure 2).
Effects of different frequencies of electroacupuncture on hippocampal synaptic transmission in AD rats
After high-frequency stimulus, the population spike amplitude increased stably in each group. At 60 minutes, the increased population spike amplitude was 253.21 ± 26.10% of the conditioned stimulus in the normal group, 163.58 ± 17.32% in the model group, 250.27 ± 27.12% in the sham surgery group, 167.74 ± 18.42% in the sham acupuncture group, 190.45 ± 23.64% in the 2 Hz electroacupuncture group, and 210.34 ± 21.25% in the 50 Hz electroacupuncture group. Compared with the normal group, the increased population spike amplitude was significantly lower in the model group (P < 0.05). The population spike amplitude in the 2 Hz and 50 Hz electroacupuncture groups was higher than in the model group, but lower than in the normal group (P < 0.05). The increased population spike amplitude was higher in the 50 Hz electroacupuncture group than in the 2 Hz electroacupuncture group (P < 0.05). The increased population spike amplitude was similar between the normal and sham surgery groups and sham acupuncture and model groups (P > 0.05; Figure 3).
Following low-frequency stimulus, the population spike amplitude was similar to baseline in the normal group. The population spike amplitude decreased and stabilized in the model group. 60 minutes later, compared with the model group, the population spike amplitude was lower in the 2 Hz and 50 Hz electroacupuncture groups (P < 0.05). The decreased population spike amplitude was less in the 50 Hz electroacupuncture group than in the 2 Hz electroacupuncture group (P < 0.05). No significant difference in thedecreased population spike amplitude was detected between the normal and sham surgery groups and sham acupuncture and model groups (P > 0.05; Figure 4).

Figure 1 Effects of electroacupuncture (EA) on mean escape latency in the Morris water navigation test in Alzheimer's disease rats.

Figure 2 Effects of electroacupuncture (EA) on spatial probe test in Alzheimer's disease rats.
Discussion
The possible mechanisms underlying acupuncture in the treatment of AD to improve learning and memory abilities are: neurotransmitter release regulation, neuron protection, increase in neurotrophic factor content, regulation of protein kinase activity in the hippocampus, inhibition of inflammatory reaction of brain tissue, adjusting abnormal protein levels, and up-regulation of autophagic activity levels (Zhu et al., 2012). These mechanisms are strongly associated with synaptic plasticity, and can improve learning and memory abilities by altering synaptic plasticity (Feng et al., 2014). Glutamate has the highest content of any excitatory neurotransmitter in the central nervous system. An overdose of glutamate has toxic effects on the nervous system. Acupuncture was able to regulate glutamate transmitter and glutamate receptor expression in the cerebral cortex and hippocampus, affect synaptic transmission and improve learning and memory abilities (Shi et al., 1998; Meng et al., 2008). Neurotrophic factors participate in axon growth and brain protection, and play an important role in synaptic plasticity. Acupuncture improves synaptic ultrastructure, enhances synaptic transmission, and restores memory function by promoting neurotrophic factor and its mRNA expression (Huang et al., 2004; Tang et al., 2005; Yi et al., 2006). Results from this study demonstrated that 2 Hz or 50 Hz electroacupuncture was able to shorten escape latency and the first time of crossing the platform, and increase the number of times crossed the platform within 120 seconds in AD rats. Moreover, the effects of 50 Hz electroacupuncture were better than that of 2 Hz electroacupuncture. These findings suggest that electroacupuncture with frequencies of 2 Hz and 50 Hz was able to improve learning and memory abilities of AD rats, and the degree of improvement was associated with the frequency.
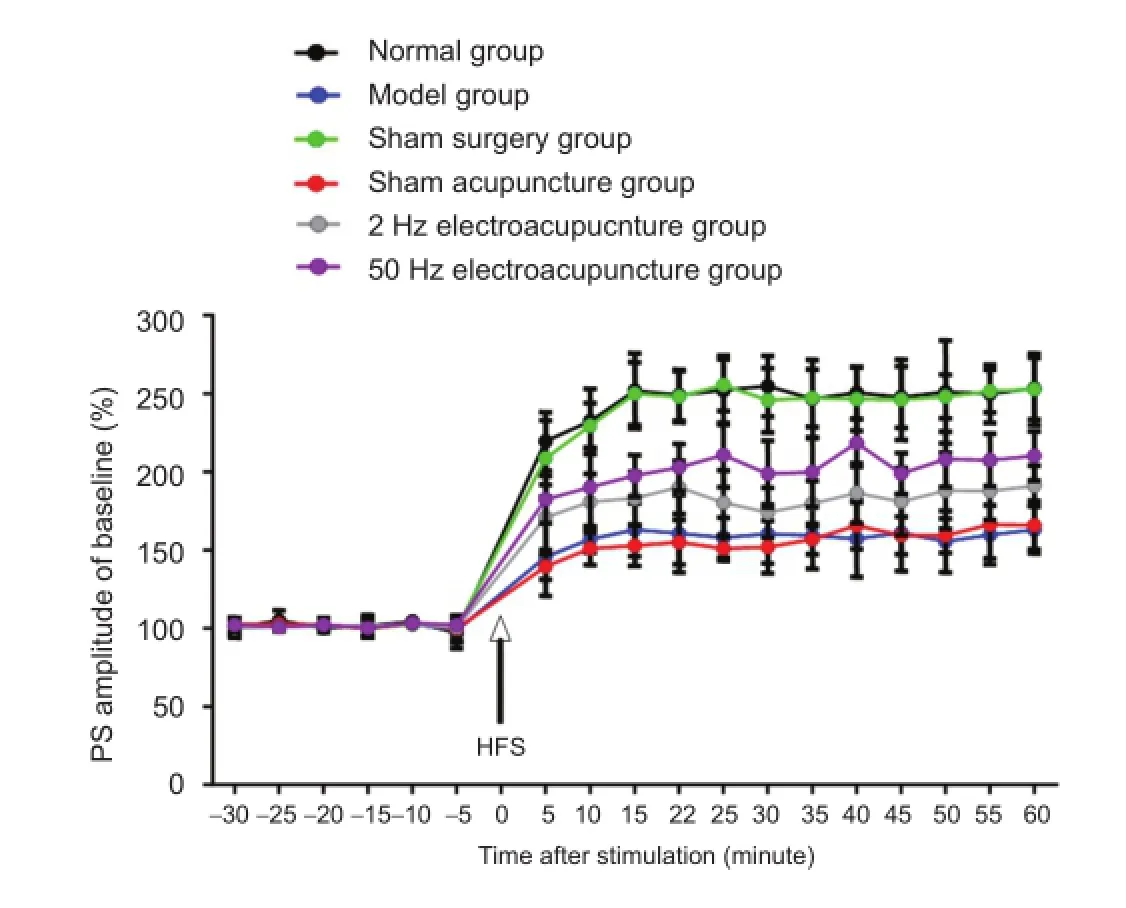
Figure 3 Effects of electroacupuncture on long-term potentiation in Alzheimer's disease rats.
Long-term synaptic plasticity is often presented as longterm potentiation/long-term depression. Xu et al. (2014) stated that 2 Hz electroacupuncture was able to improve the learning and memory abilities in senescence-accelerated mice, with the possible mechanism being the down-regulation of the β-amyloid precursor protein mRNA expression. Shen et al. (2010) asserted that electroacupuncture was able to improve the learning and memory abilities in AD rats, and its mechanism is probably associated with the recovery of long-term potentiation by electroacupuncture. Shen et al. (2013) confirmed that 2 Hz electroacupuncture improved long-term potentiation and elevated the learning and memory abilities by enhancing cyclic AMP response element-binding protein in AD rats. Wei et al. (2013) verified that 50 Hz electroacupuncture improved the learning and memory abilities in AD rats; and its mechanism may be associated with increasing protein kinase C expression and enhancing long-term potentiation. We found that electroacupuncture was able to enhance synaptic transmission in AD rats, and the effects of 50 Hz electroacupuncture were better than those of 2 Hz electroacupuncture, which was consistent with the results of a previous study (Wang, 2013). Simultaneously, electroacupuncture was able to reverse the suppression of synaptic transmission; and the effects of 50 Hz electroacupuncture were better than those of 2 Hz electroacupuncture. In conclusion, electroacupuncture strengthens synaptic transmission and improves memory disorders in AD rats. The degree of improvement is associated with the frequency of electroacupuncture.
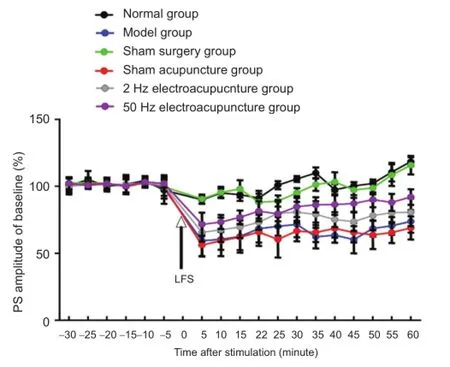
Figure 4 Effects of electroacupuncture on long-term depression in Alzheimer's disease rats.
Author contributions: WL wrote the paper, provided the data and ensured the integrity of the data. LHK obtained the funding, conceived and designed the study, and was in charge of paper authorization. HW participated in the experiments. FS oversaw the water maze test. YWW oversaw the electrophysiological experiments. HZ oversaw the establishment of AD models. GJS was in charge of the paper authorization. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Amici M, Doherty A, Jo J, Jane D, Cho K, Collingridge G, Dargan S (2009) Neuronal calcium sensors and synaptic plasticity. Biochem Soc Trans 37:1359-1363.
Bliss TV (1999) Young receptors make smart mice. Nature 401:25-27.
Collingridge GL, Peineau S, Howland JG, Wang YT (2010) Long-term depression in the CNS. Nat Rev Neurosci 11:459-473.
Cui Y (2014) Meta-analysis on acupuncture for treatment of dementia. Neimenggu Zhongyiyao 33:55.
Du YJ, Zhou H, Sun GJ, Chen BG (2011) Effect of acupuncture and moxbustion pre-disposal on Alzheimer's disease rats' hippocamp tissue ultramicro-morphological. Zhonghua Zhongyiyao Xuekan 29:1234-1236.
Elgersma Y, Silva AJ (1999) Molecular mechanisms of synaptic plasticity and memory. Curr Opin Neurobiol 9:209-213.
Feng M, Hou TS, Yu ML, Wu QF, Lu SF, Yu SG (2014) Effect and mechanism of acupuncture on synaptic plasticity. Shizhen Guoyi Guoyao 25:172-174.
Han TZ (2005) Progress in research of synaptic plasticity and longterm potentiation. Xi'an Jiaotong Daxue Xuebao: Yixue Ban 26:305-308.
Huang HF, Wang FC, Shi Y (2004) Deprivation effect of acupuncture against amblyopia and its mechanism. Zhongguo Linchuang Kangfu 8:8036-8037.
Lister JP, Barnes CA (2009) Neurobiological changes in the hippocampus during normative aging. Arch Neurol 66:829-833.
Luo L, Sun GJ, Du YJ (2013) Effects of acupuncture and moxibustion on energy metabolism-related protein of hippocampai neuron mitochondria in Alzheimer's disease rats. Zhongguo Zhenjiu 33:913-918. McNaughton BL, Barnes CA, Rao G, Baldwin J, Rasmussen M (1986) Long-term enhancement of hippocampal synaptic transmission and the acquisition of spatial information. J Neurosci 6:563-571.
Meng PY, Sun GJ, Liu SH, Yan HM (2008) Effect of electroacupuncture pretreatment on glutamate-NMDAR signal pathway in hippocampal neurons of vascular dementia rats. Zhenci Yanjiu 33:103.
Meng PY, Sun GJ, Mao JJ, Su FR, Zhou CL (2009) A Study of the effect of acupuncture pretreatment on learning and memory abilities in vascular dementia rats. Shanghai Zhenjiu Zazhi 28:293-296.
Morris RG, Frey U (1997) Hippocampal synaptic plasticity: role in spatial learning or the automatic recording of attended experience? Philos Trans R Soc Lond B Biol Sci 352:1489-1503.
Morris RG, Anderson E, Lynch GS, Baudry M (1986) Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319:774-776.
O'Hare E, Weldon DT, Mantyh PW, Ghilardi JR, Finke MP, Kuskowski MA, Maggio JE, Shephard RA, Cleary J (1999) Delayed behavioral effects following intrahippocampal injection of aggregated A beta (1-42). Brain Res 815:1-10.
Peng JA, Guo P (2008) Research progress of learning, memory and LTP. Henan Zhigong Yixueyuan Xuebao 20:315-318.
Shen F, Du YJ, Wang F, Kong LH, Sun GJ (2013) Effect of electro-acupuncture on CREB with Alzheimer's disease in rats. Liaoning Zhongyi Zazhi 40:531-533.
Shen MH, Tang QQ, Li ZR, Ma C (2010) Effect of electroacupuncture on hippocampal LTP in Alzheimer' s disease rats induced by Aβ_(25-35). Zhenci Yanjiu 35:3-7.
Shi XM, Han JX, Li P, Zhao JH, Wang S, Liu QZ, Yang ZR, Lai LP, Zhao LR (1998) Effect of acupuncture on the level of excitatory amino acid in the brain of aging dementia rats. Zhongguo Zhenjiu 18:689-692.
Tang L, Tang RW, Tang J, Ren YY (2012) Research progress of the relationship between synaptic plasticity and learning & memory. Chuanbei Yixueyuan Xuebao 27:89-92.
Tang Y, Yu SG, Chen J (2005) Enhanced synapstic plasticity in Parkinson's disease mice sunbstantla nigra by electroacupuncture therapy. Chengdu Zhongyiyao Daxue Xuebao 28:29.
Wang ZD (2013) Molecular mechanism of electroacupuncture with two different frequency on the spatial learning and memory ability in AD model rats. Harbin: Heilongjiang University of Chinese Medicine.
Wei DM, Jia XM, Yin XX, Jiang WW (2013) Effect of electroacupuncture on learning-memory ability and protein kinase C expression in hippocampal nerve cells of vascular dementia in rats. Zhongguo Laonian Xue Zazhi 33:328-330.
Xu AP, Tang YS, Chen WS, Mo YP, Yao HJ, Saiyinchaoketu, Li ZG (2014) Effect of electroacupuncture intervention on learning-memory ability, APP and ApoE mRNA expression in cerebral cortex of SAMP8. Zhenjiu Linchuang Zazhi 30:62-65.
Yi W, Xu NG, Wang GB, Xu ZH, She SF, Huang ZY, Lai XS (2006) Experimental study on effects of electro-acupuncture in improving synaptic plasticity in focal cerebral ischemia rats. Zhongguo Zhongxiyi Jiehe Zazhi 26:710.
Yu HJ, Zhang X, Tan QW (2014) The different frequency of electro-acupunture's knowledge. Jiangxi Zhongyiyao 45:48-50.
Zhu J, Guo HD, Shao SJ (2012) Progress of researches on mechanisms of acupuncture intervention of Alzheimer's disease. Zhenci Yanjiu 37:422-427.
Zhu Y, Zeng Y, Wang X, Ye X (2013) Effect of electroacupuncture on the expression of mTOR and eIF4E in hippocampus of rats with vascular dementia. Neurol Sci 34:1093-1097.
Copyedited by Paul P, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.182708 http://www.nrronline.org/
How to cite this article: Li W, Kong LH, Wang H, Shen F, Wang YW, Zhou H, Sun GJ (2016) High-frequency electroacupuncture evidently reinforces hippocampal synaptic transmission in Alzheimer's disease rats. Neural Regen Res 11(5)∶801-806.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81373741.
Accepted: 2016-03-07
*Correspondence to: Li-hong Kong, xiyu1618@sina.com.
杂志排行
中国神经再生研究(英文版)的其它文章
- Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Antibody-based neuronal and axonal delivery vectors for targeted ligand delivery
- Coordination of the axonal cytoskeleton during the emergence of axon collateral branches
- Alzheimer's disease: the silver tsunami of the 21stcentury
- Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells
