Responses from two firing patterns in inferior colliculus neurons to stimulation of the lateral lemniscus dorsal nucleus
2016-12-02XiaotingLiNingyuWangYanjunWangZhiqingXuJinfengLiuYunfeiBaiJinshengDaiJingyiZhao
Xiao-ting Li, Ning-yu Wang,, Yan-jun Wang, Zhi-qing Xu, Jin-feng Liu, Yun-fei Bai, Jin-sheng Dai, Jing-yi Zhao
1 Department of Otorhinolaryngology Head and Neck Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
2 Department of Neurophysiology, Capital Medical University, Beijing, China
RESEARCH ARTICLE
Responses from two firing patterns in inferior colliculus neurons to stimulation of the lateral lemniscus dorsal nucleus
Xiao-ting Li1, Ning-yu Wang1,*, Yan-jun Wang1, Zhi-qing Xu2, Jin-feng Liu1, Yun-fei Bai2, Jin-sheng Dai1, Jing-yi Zhao1
1 Department of Otorhinolaryngology Head and Neck Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
2 Department of Neurophysiology, Capital Medical University, Beijing, China
Graphical Abstract
orcid: 0000-0002-3080-2093 (Ning-yu Wang)
The γ-aminobutyric acid neurons (GABAergic neurons) in the inferior colliculus are classified into various patterns based on their intrinsic electrical properties to a constant current injection. Although this classification is associated with physiological function, the exact role for neurons with various firing patterns in acoustic processing remains poorly understood. In the present study, we analyzed characteristics of inferior colliculus neurons in vitro, and recorded responses to stimulation of the dorsal nucleus of the lateral lemniscus using the wholecell patch clamp technique. Seven inferior colliculus neurons were tested and were classified into two firing patterns: sustained-regular (n = 4) and sustained-adapting firing patterns (n = 3). The majority of inferior colliculus neurons exhibited slight changes in response to stimulation and bicuculline. The responses of one neuron with a sustained-adapting firing pattern were suppressed after stimulation, but recovered to normal levels following application of the γ-aminobutyric acid receptor antagonist. One neuron with a sustained-regular pattern showed suppressed stimulation responses, which were not affected by bicuculline. Results suggest that GABAergic neurons in the inferior colliculus exhibit sustained-regular or sustained-adapting firing patterns. Additionally, GABAergic projections from the dorsal nucleus of the lateral lemniscus to the inferior colliculus are associated with sound localization. The different neuronal responses of various firing patterns suggest a role in sound localization. A better understanding of these mechanisms and functions will provide better clinical treatment paradigms for hearing deficiencies.
nerve regeneration; inferior colliculus; GABAergic neuron; firing pattern; sustained-regular firing pattern; sustained-adapting firing pattern; precedence effect; long-lasting inhibition; dorsal nucleus of the lateral lemniscus; inhibitory projection; neural regeneration
Introduction
The inferior colliculus (IC) is a vital relay station in the central auditory pathway; it integrates acoustic information from lower brainstem nuclei, such as the dorsal cochlear nucleus complex, the superior olivary complex, and the lateral lemniscal nuclei (Adams, 1979; Malmierca et al., 2005; Oliver, 2005). These projections provide either excitatory or inhibitory innervation, or both of them, and neurons in the IC are responsible for interactions between excitatory and inhibitory inputs (Davis, 2002; Malmierca et al., 2005).
Previous studies have shown that inhibition occupies a dominant role in the processing of acoustic information (Kelly and Caspary, 2005; Nataraj and Wenstrup, 2005; Fuzessery et al., 2006; Pollak et al., 2011; Geis and Borst, 2013). In the IC, γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter (González-Hernández et al., 1996; Chen et al., 1999; Merchán et al., 2005); GABA affects auditory processing in many ways, including duration tuning, selectivity of sweep direction of frequency modulation, and coding of interaural time or intensity differences (Yin et al., 2008; Pollak et al., 2011). Within the IC, GABA is mainly originated from the bilateral dorsal nucleus of the lateral lemniscus (DNLL), especially in the contralateral side (51% in a study) (Adams, 1979; Shneiderman et al., 1993; González-Hernández et al., 1996; Zhang et al., 1998; Burger and Pollak, 2001; Oliver, 2005; Winter and Schreiner, 2005; Pollak et al., 2011). The intrinsic projections in the IC subdivisions also contribute to the GABA source, although levels are much less than in the DNLL (Oliver, 2005).
GABAergic neurons in the IC are classified into various patterns, known as regular-sustained neurons and buildup neurons (Le Beau et al., 1996; Bal et al., 2002; Ono et al., 2005). This classification is based on neuronal responses after constant current injection (depolarization or hyperpolarization) (Peruzzi et al., 2000; Sivaramakrishnan and Oliver, 2001; Tan et al., 2007; Sun and Wu, 2008a). Electrophysiological studies have primarily focused on the distinction of ion channels in the various patterns, although the physiological role in acoustic information processing remains unknown.
In the present study, whole-cell patch clamp was used to classify IC neurons. The stimulation responses in the DNLL were recorded in neurons with various firing patterns, while the current was injected to the IC neuron to modify the discharge to a natural sound. The antagonist of the GABAergic receptor bicuculline was applied to validate the neurotransmitter responses. This study sought to find the relationship between electrophysiological patterns and acoustic processing.
Materials and Methods
Experimental animals
The animal studies were approved by the Committee for Institutional Animal Care and Use Committee of Institute of Beijing Chaoyang Hospital and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment. Seven specific-pathogen-free Sprague-Dawley rats (13—17 days old, either sex) were provided by the Animal Service Center of Capital Medical University in Peking of China (license No. SCXK2011-0011).
Preparation of brain slices
Rat pups were decapitated following deep anesthesia with 4 mL ether. The brain was rapidly removed from the skull, transferred into ice-cooled artificial cerebrospinal fluid, and continuously saturated with 5% CO2and 95% O2. The pH was adjusted to 7.3. The brain block was then cut into coronal slices of 300-µm thickness with a vibratome (VT1000S; Leica, Nussloch, Germany), which was filled with the cold artificial cerebrospinal fluid. The prepared slices, which contained the IC and DNLL, were incubated in oxygenated artificial cerebrospinal fluid at room temperature at least for 1 hour. One to two slices were prepared from each animal. After incubation, the individual slices were transferred to a recording chamber, and bathed with oxygenated artificial cerebrospinal fluid at 3—4 mL/min by gravity-fed perfusion at 31 ± 1°C. The slices were covered with a platinum-nylon net to prevent movement.
Artificial cerebrospinal fluid contained the following composition: 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 25 mM D-glucose, 2 mM CaCl2, and 1.5 mM MgCl2. NaH2PO4was from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Solutions of 25 µM D-(-)-2-animo-5-phosphonopentanoic acid (D-AP5) and 10 µM 6,7-dinitroquinoxaline-2,3-dione (DNQX) were applied to normal artificial cerebrospinal fluid for the entire experiment. In some experiments, 10 µM bicuculline was used to verify GABAAreceptor-medicated currents after recording action potential. All chemicals were supplied by Sigma (St. Louis, MO, USA).
Whole-cell patch clamp recording
Glass microelectrodes were used to record responses of injected current and evoked postsynaptic currents. The microelectrodes were prepared from thin-walled glass pipettes (GB 150F-8P, Sutter instrument, Novato, CA, USA) with a two-stage vertical puller (PC-10; Narishige Scientific Instrument Lab, Tokyo, Japan). The electrode resistance amounted to 4—6 MΩ after filling with intracellular solution. The internal solution contained the following composition: 140 mM K-gluconate, 2 mM MgCl2, 10 mM HEPES, 8 mM KCl, 2 mM Na2ATP, and 0.2 mM MgGTP. The pH was adjusted to 7.3 with KOH. All chemicals were provided by Sigma.
A light microscope (BX51WI; Olympus, Tokyo, Japan) and a 40× long-working distance objective (NA 0.8) were used to view the slices and to locate DNLL neurons and IC neurons. Whole-cell recording was performed with an EPC-10 double amplifier (HEKA Instruments, Lambrecht, Germany) on IC neurons. Fast capacitance, slow capacitance, and series resistance were compensated until the baseline remained straight at a membrane potential of —60mV. All records were digitized using HEKA Patchmaster software (HEKA Elektronik, Lambrecht, Germany). The signals were filtered at 3 kHz.
Action potential recording of GABAergic neurons in IC
Whole-cell configuration was made in the current clamp mode and the holding current was set to 0 mA. The recording took place for at least 5 minutes after whole-cell configuration. In each recording, 100 pA current was injected into the neurons.
Action potentials of IC neurons after evoking ipsilateral DNLL neurons
A concentric stimulating electrode was placed on the DNLL. The synaptic response was evoked by square wave pulses of 100 µs duration and variable intensity. A certain threshold of stimulus intensity, below which no response was detected, was defined to record the evoked inhibitory postsynaptic currents. The recording stimulus intensity was maintained at 1.5 fold of this threshold. A stimulus series containing three single stimuli at a 500-µs interval was applied before 100-pA current inspiring action potentials. The duration of the series of pulses was 1,300 µs. The postsynaptic currents in IC neurons were subsequently recorded to ensure that the recorded inhibitory postsynaptic currents were mediated by the GABAergic receptor. The inhibitory postsynaptic currents mediated by the GABAergic receptor were diminished following application of bicuculline.
Action potential responses of IC neurons after extracellular application of bicuculline
The perfusion of bicuculline (10 µM; Sigma) in artificial cerebrospinal fluid allowed for observation of action potential changes in IC neurons under stimulation of DNLL neurons.
Data acquisition and analysis
All recordings were performed after applying whole-cell clamp patch and allowing the IC neuron membrane to reach a stable resting potential. Spikes were elicited by current injection to IC neurons. Changes in amplitude and firing frequency were measured after simulating in DNLL and application of the GABAA receptor antagonist bicuculline. Postsynaptic currents were measured after paired-stimulation to DNLL. Data were expressed as the mean ± SEM and were recorded and analyzed using the PatchMaster software.
Results
Seven neurons were analyzed. These neurons were located in the IC, according to the Paxinos and Watson atlas (Paxinos and Watson, 2005). All neurons were recorded using the whole-cell patch clamp method and values remained as stable membrane potentials (Table 1).
Identification of neuronal types according to discharge patterns
All tested IC neurons exhibited inhibitory discharges (n = 7). Classification was based on neuronal responses to continuous injections of depolarizing currents. All neurons exhibited sustained discharge to suprathreshold currents, and continuously fired over the entire period of injected current. The inter-spike intervals were measured, and the ratio of the last interval to the first interval was defined as the adaptation ratio (Sun and Wu, 2008b).
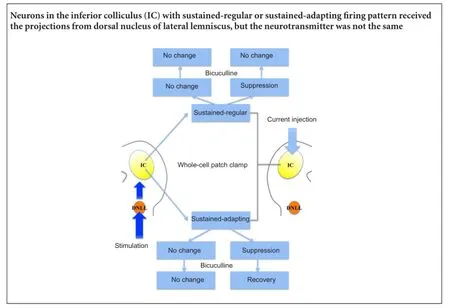
The tested neurons were classified into two types according to adaptation ratio. Four out of seven neurons exhibited continuous pulses with relatively constant inter-spike intervals (sustained-regular firing pattern; Figure 1A). The remaining neurons responded to continuous discharge as well, although the inter-spike intervals were prolonged, suggesting that the later interval was longer than the previous one (sustained-adapting neurons; Figure 2A). The average adaptation ratio for adapting neurons was 1.34 (ranging from 1.09—1.60), while the average adaptation ratio for regular neurons was 1.19 (ranging from 0.98—1.57; Table 1).
Pattern responses of IC neurons to stimulation in the ipsilateral DNLL
The stimulating electrode was placed in the area ventral lateral to the IC, where fibers from the DNLL projected to the IC. Continuous current was injected to imitate the response of IC neurons to acoustic stimuli (Peruzzi et al., 2000; Pecka et al., 2007). A short train of three pulses was then stimulated in the area.
One neuron with sustained-regular firing pattern showed suppressed responses to the stimulation, with a reduced frequency of discharge (Figure 1B). The responses of the remaining three neurons in this pattern were facilitated or slightly suppressed by the stimulation. In the sustained-adapting firing pattern, the responses of one neuron were obviously suppressed (Figure 2B), while the remaining neurons exhibited relatively constant responses.
The response width was presented by the action potential duration at 50%. The average action potential duration from 50% of neurons with sustained-regular pattern was greater than those with the sustained-adapting firing pattern, although both patterns exhibited an increased value after stimulation (Table 2).
Response pharmacology of two patterns in IC neurons
As mentioned above, two IC neurons with distinct firing patterns showed suppressed responses to the stimulation. Because the DNLL is the main origin of inhibitory projections to the IC and the majority of the fibers were GABAergic (Oliver, 2005), the GABAergic receptor antagonist bicuculline was applied. The amplitude of the sustained-adapting neuron increased to normal levels after bicuculline application, while amplitude of the sustained-regular neuron continued to decrease.
The responses to bicuculline in the remaining neurons were also recorded. The sustained-regular neurons exhibited increased responses, and responses of the sustained-adapting neurons became normal (Figure 1C, 2C,Table 2).
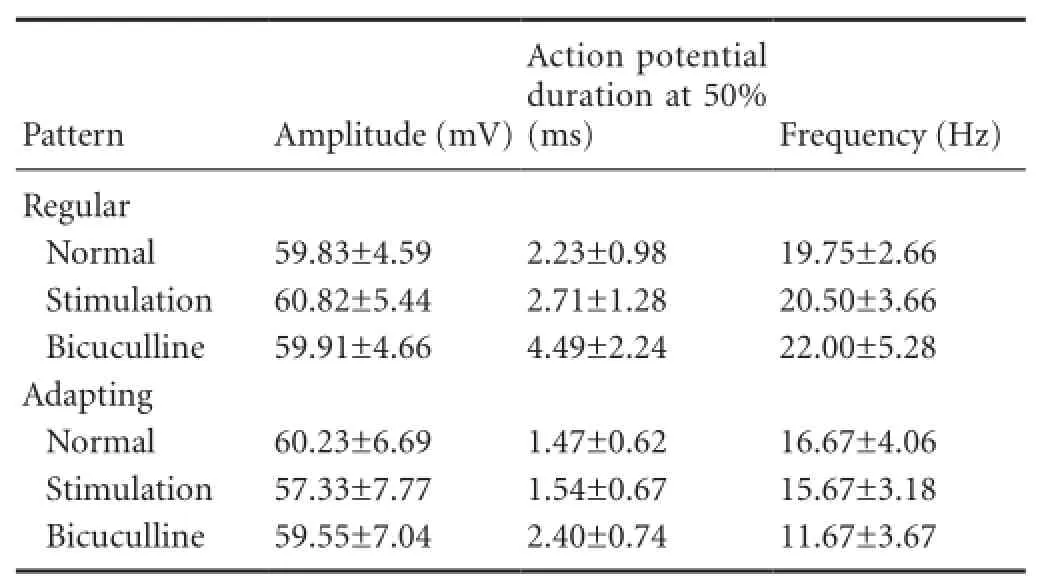
Table 2 Action potential properties of neurons in the IC with two patterns after stimulation and application of bicuculline

Table 3 Adaptation ratio of neurons with two firing patterns

Figure 1 Responses of IC neurons with regular firing pattern.
Adaptation ratio of IC neurons with two firing patterns
The standards to identify the regular and adapting firing patterns were various in the previous studies. The first standard was the adaptation ratio. The second standard was the ratio of each following interval to the first interval, and if the following interval was as twice as the first interval, the neuron was identified as the adapting firing pattern. (Peruzzi et al., 2000; Sivaramakrishnan and Oliver, 2001; Bal et al., 2002; Ono et al., 2005; Tan et al., 2007; Sun and Wu, 2008a).
The present study chose the adaptation ratio to quantify the two firing patterns. The adaptation ratio in the sustained-regular firing pattern was relatively constant and was not affected by stimulation or bicuculline (Table 3). The adaptation ratio in the sustained-adapting firing pattern, however, exhibited an obvious increase following stimulation and bicuculline (Table 3), and the maximal value was twice the normal value.

Figure 2 Responses of IC neurons with adapting firing pattern.
Discussion
Classification of IC neurons
IC neurons with sustained-regular or sustained-adapting firing patterns were identified in the present study, which was consistent with previous studies (Oliver et al., 1994; Peruzzi et al., 2000; Sivaramakrishnan and Oliver, 2001). IC neurons are classified into flat or less-flat categories according to morphology of the shape and orientation of the dendritic tree (Oliver et al., 1994; Malmierca et al., 1995; Peruzzi et al., 2000; Winter and Schreiner, 2005). However, some physiological studies have suggested that identification of IC neurons by the firing pattern is possibly associated with their functions. Peruzzi et al. (2000) identified at least three physiological types of IC neurons. Sivaramakrishnan and Oliver (2001) extended the firing patterns to six types, including sustained-regular, sustained-adapting, onset, pause-buildup, rebound, and rebound regular, and these types were present not only in the central nucleus, but also in the cortex of the IC (Peruzzi et al., 2000; Sun and Wu, 2008a, b). Tan et al. (2007) performed similar experiments using in vivo patch-clamp in the IC of rats and mice; results showed five patterns without the onset-firing pattern that were previously reported in in vitro studies. Tan et al. (2007) argued that this was due to the absence of recording the Kv1.1 potassium channel, which is thought to elicit the onset pattern.
A similar result was observed in a previous study of firing patterns of GABAergic neurons in the IC. Ono et al. (2005) labeled IC neurons with green fluorescent protein fluorescence to discriminate GABAergic from non-GABAergic neurons; the GABAergic neurons were classified into two distinct types according to responses to depolarized currents injection. The first type was a tonic-type neuron that exhibited sustained and regular firing (with slight adapting) to a long period of depolarized current injection, which was consistent with our results. The second type was a transient-type neuron with discharge in the beginning of current injection, which was not observed in our study.
Sivaramakrishnan and Oliver (2001) suggested that the various firing patterns in IC neurons was due to the distinct potassium ion channel on the neuronal membrane. Delayed-rectifying 4-AP-sensitive potassium currents result in sustained discharge in the sustained-regular firing pattern during stimulation, indicating that neurons provide information about input duration. The sustained-adapting firing pattern is determined by apamin and charybdotoxin-sensitive calcium-activated potassium currents, which possibly underlie the sensitivity of neurons to interaural phase modulation.
Although electrophysiological classification of IC neurons is associated with physiological function, the exact role of each pattern is not clear. The rebound-firing pattern is observed by preceding hyperpolarization before depolarized current injection. Some studies have shown that membrane excitability becomes modified after pre-hyperpolarization. Because IC neurons converge projections from lower or higher auditory nuclei, this modification was proposed as a possible mechanism for integrating excitatory and inhibitory projections (Kuwada and Batra, 1999).
GABA effects on responses of IC neurons
Although the average response of sustained-regular neurons was facilitated by stimulation in the DNLL, we noticed that one neuron of this type exhibited an evident reduction in amplitude (from 60.91 mV to 54.14 mV). This suppression, however, did not recover to a normal level after bicuculline application, indicating that GABAA was not acting as the transmitter.
The IC contains two types of inhibitory neurotransmitters— glycine and GABA (Nakamoto et al., 2014; Ono and Oliver, 2014). Glycine is produced mainly by the ipsilateral lateral superior olivary nuclei, and accounts for 26% of synaptic endings in the IC (Oliver, 2005). Because the lateral superior olivary nucleus is separated from the DNLL and there is no possibility to confuse these two nuclei, it meant that glycine cannot be the transmitter in our study. GABA, a vital inhibitory neurotransmitter in the IC, is mainly produced by the bilateral DNLL and intrinsic inputs from subdivisions in the IC (Shneiderman et al., 1993; González-Hernández et al., 1996; Winter and Schreiner, 2005; Pollak et al., 2011; Orton and Rees, 2014; Sturm et al., 2014). Three types of GABA receptors have been described: GABAA, GABAB, and GABAc (Caspary et al., 2008). GABAAelicits hyperpolarization of membrane potential by opening the chloride channel to inhibit evoking of the action potential (Sun and Wu, 2008a). The GABABreceptor can act as presynaptic or postsynaptic receptor. The postsynaptic GABABreceptor elicits postsynaptic inhibitory potential and modulates membrane excitability, which is similar to GABAAfunctions. The presynaptic GABABreceptor modulates synaptic response mediated by GABAA, suggesting that it modulates synaptic transmission (Ma et al., 2002; Sun and Wu, 2009; Jamal et al., 2012). Therefore, GABABremains the most likely neurotransmitter, although we did not administer GABABreceptor agonist to verify this hypothesis.
In this study, a neuron with a sustained-adapting firing pattern exhibited obvious suppression to stimulation in the DNLL, with the amplitude ranging from 56.95 mV to 47.30 mV. However, the amplitude recovered to a normal level (54.32 mV) after application of bicuculline. These results were inconsistent with a previous study by Ono et al. (2005). The neuron in the present study exhibited a remarkable ability to adapt to the firing pattern (adaptation ratio = 1.608), which suggested that GABAergic neurons also exhibit sustained-adapting firing.
The majority of neurons in this study exhibited relatively constant discharge to stimulation and application of bicuculline, which was consistent with a previous study (Ono et al., 2005). The sustained property acts as a distinct function in input modulation to the IC. Tan et al. (2007) investigated the phase-locked responses of IC neurons with three different firing patterns to the signals; their results indicated that the sustained pattern exhibits a phaselocked discharge to the signal, and the membrane properties modulated by the delayed-rectifier potassium current dominate the phase-locked discharge, which is potentially the intrinsic mechanism.
Auditory function of GABAergic neurons in the IC
Previous studies have shown that GABA contributes to sound localization in the reverberant environment, especially in the precedence effect, which refers to the phenomenon of the auditory system to improve localization of initial sound by suppressing directional information of reflected sound (Wallach et al., 1949; Yang and Grantham, 1997; Litovsky et al., 1999; Li and Yue, 2002; Tollin et al., 2004; Pecka et al., 2007; Tolnai et al., 2014; Brown et al., 2015b). Some physiological studies in animals (e.g., cats, rats, and rabbits) showed that most IC neurons exhibit suppression to lag responses in a short interstimulus delay, which is similar to human behavioral studies, and the suppression remains obvious even if the lead does not elicit responses (Yin, 1994; Fitzpatrick et al., 1995, 1999; Litovsky et al., 1997; Litovsky and Yin, 1998a, b; Litovsky and Delgutte, 2002; Dent et al., 2009; Song et al., 2011; Wang et al., 2014). The suppression takes place as along-lasting inhibition (as long as 30 ms), which exceeds the refractory period of IC (Yin, 1994; Fitzpatrick et al., 1995; Litovsky and Yin, 1998b; Bauer et al., 2000). These results indicate that inhibition, rather than the refractory period, causes suppression to lag in the IC (Litovsky and Yin, 1998b; Litovsky et al., 1999). Several in vivo studies have shown that phenobarbital and GABA prolong the half maximal interstimulus delay to paired-stimuli and reduce the peak of responses to lag in the rat IC, indicating that suppression to lag is enhanced by GABA (Covey et al., 1996; Kidd and Kelly, 1996; Tollin et al., 2004; Song et al., 2011; Wang et al., 2014; Brown et al., 2015a).
The long-lasting inhibition refers to a phenomenon where, IC neurons show an inhibitory effect that persists for milliseconds beyond the end of the leading stimuli when a paired lead-lag sound is performed. This has been observed in some in vivo physiological studies that recorded discharge of a single unit to a paired-sound separated by various interaural time differences and interaural intensity differences in different mammals (bat, rabbit, and cat). Results showed that IC neurons exhibit long-lasting inhibition that exceeds the period of the leading sound, and this suppression is affected by the duration and relative intensity of paired stimulus (Li and Kelly, 1992; Yin, 1994; Fitzpatrick et al., 1995; Covey et al., 1996; Li et al., 1998; Litovsky and Yin, 1998b; Wang et al., 2014). Similar findings have been observed in wholecell patch clamp in conscious bats, where inhibition beyond the end of the sound stimuli lasted for ~27 ms (Covey et al., 1996).
Although the origin of long-lasting inhibition is not well understood, the possibilities are discussed below. Similar to previous studies, the inhibition observed in the present study was eliminated by bicuculline, a blocker of GABAergic receptor, indicating that GABA is at least partially responsible for the generation of long-lasting inhibition. In accordance with the multiple sources of GABAergic projections to the IC, we will discuss three possibilities for the generation of long-lasting inhibition.
1) The IC contains three subdivisions that include the central nucleus of the IC, the dorsal cortex of the IC, and the external cortex of the IC; the bilateral IC connects each of these with the commissure of the IC (Covey et al., 1996; Li et al., 1998; Pollak et al., 2011). The inhibitory projections are GABAergic, although the functions of these intrinsic connections remain poorly understood. In the present study, we blocked the GABA receptors in a whole brain slice. Therefore, it cannot be ruled out that intrinsic GABAergic projections are involved in the generation of long-lasting inhibition.
2) The bilateral DNLL is connected by the commissure of Probst. Physiological studies have recorded persistent inhibition from the DNLL that lasts for tens of milliseconds and originates in the contralateral DNLL. Pecka et al. (2007) suggested that persistent inhibition contributes to the mechanisms underlying the precedence effect. This persistent inhibition lasts for milliseconds, which is similar to the duration of long-lasting inhibition observed in the previous studies, indicating that long-lasting inhibition likely comes from persistent inhibition in the DNLL.
3) Kidd and Kelly (1996) investigated the contribution of the DNLL to in vivo binaural responses in the rat IC. By testing different neural responses in the IC with various interaural time differences, they found that long-lasting inhibition was elicited by ipsilateral sound stimulation, which lasted for 10—20 ms in different neurons and was possibly affected by sound intensity. Kynurenic acid was then injected in the contralateral DNLL to block the N-methyl-D-aspartic acid (NMDA) receptor, resulting in reduced long-lasting inhibition. However, this suppression was not observed after injection into the ipsilateral DNLL, indicating that NMDA contributes to long-lasting inhibition only in the IC. They then compared changes before and after administration of excitatory amino acid receptor antagonists, resulting in similar results to a previous study (Chen et al., 1999). Chen et al. suggested that NMDA contributes to extended GABA release in the DNLL, which subsequently increases inhibition to the contralateral IC. Together, these studies suggest that GABAergic projections from DNLL to the ipsilateral IC mediate long-lasting inhibition, and GABA release is modulated by NMDAergic projections from the contralateral DNLL. Further studies are needed to support this hypothesis.
Although the responses of two IC neurons were suppressed by stimulation in the ipsilateral DNLL, an obvious inhibition that lasted for milliseconds was not recorded. There are three possible reasons for this. First, the simple volume was small in this study, and we recorded responses of IC neurons with only two firing patterns. It is possible that the other types of neurons accounted for the cellular basis of long-lasting inhibition. For example, the rebound-firing pattern exhibits discharge after depolarization, which is similar to a neuron that receives inhibitory inputs preceding excitatory inputs. The rebound neuron is critical for controlling spiking patterns, and possibly underlies the encoding of acoustic temporal information (Malmierca et al., 1995). Second, we chose the incorrect site for stimulation, and this was subsequently excluded. Prior to recording, we stimulated the site and recorded the inhibitory currents. These currents were diminished by bicuculline. Third, although the IC receives projections from the bilateral DNLL, some physiological studies suggest that the contralateral DNLL is the major origin of GABAergic input fibers of the IC. We stimulated the contralateral DNLL, but did not record the IC responses. It is possible that the commissure connecting the bilateral DNLL was not included in the brain slice or was damaged during preparation.
In conclusion, two types of IC neurons were observed, including the sustained-regular and sustained-adapting firing pattern. One neuron from each type exhibited a suppressed response to stimulation in the ipsilateral DNLL, and responses of the neuron with the adapting firing pattern recovered to a normal level, suggesting that GABAergic neurons exhibit sustained-adapting firing. Further pre-hyperpolarization studies are needed to observe neurons with other firing patterns. Responses of neurons with different firingpatterns may indicate a physiological relation to acoustic stimuli, especially directional information.
Acknowledgments: We thank Yan Li for the assistance with data analysis. We are very grateful to Xing-lu Yin from No.6 People Hospital Affiliated to Shanghai Jiao Tong University School of Medicine of China for assistant with the performance of the PatchMaster software.
Author contributions: XTL carried out the experiment, analyzed the data, graphed the figures, and wrote the paper. NYW served as a principle investigator, designed the study and obtained the funding. YJW participated in data acquisition and analysis. ZQX and YFB were responsible for the design and performance of electrophysiological experiment. JFL, JSD and JYZ participated in data collection and analysis. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Adams JC (1979) Ascending projections to the inferior colliculus. J Comp Neurol 183:519-538.
Bal R, Green GGR, Rees A, Sanders DJ (2002) Firing patterns of inferior colliculus neurons-histology and mechanism to change firing patterns in rat brain slices. Neurosci Lett 317:42-46.
Bauer EE, Klug A, Pollak GD (2000) Features of contralaterally evoked inhibition in the inferior colliculus. Hear Res 141:80-96.
Brown AD, Stecker GC, Tollin DJ (2015a) The precedence effect in sound localization. J Assoc Res Otolaryngol 16:1-28.
Brown AD, Jones HG, Kan A, Thakkar T, Stecker GC, Goupell MJ, Litovsky RY (2015b) Evidence for a neural source of the precedence effect in sound localization. J Neurophysiol 114:2991-3001.
Burger RM, Pollak GD (2001) Reversible inactivation of the dorsal nucleus of the lateral lemniscus reveals its role in the processing of multiple sound sources in the inferior colliculus of bats. J Neurosci 21:4830-4843.
Caspary DM, Ling L, Turner JG, Hughes LF (2008) Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol 211:1781-1791.
Chen L, Kelly JB, Wu SH (1999) The commissure of probst as a source of GABAergic inhibition. Hear Res 138:106-114.
Covey E, Kauer JA, Casseday JH (1996) Whole-cell patch-clamp recording reveals subthreshold sound-evoked postsynaptic currents in the inferior colliculus of awake bats. J Neurosci 16:3009-3018.
Davis KA (2002) Evidence of a functionally segregated pathway from dorsal cochlear nucleus to inferior colliculus. J Neurophysiol 87:1824-1835.
Dent ML, Tollin DJ, Yin TC (2009) Influence of sound source location on the behavior and physiology of the precedence effect in cats. J Neurophysiol 102:724-734.
Fitzpatrick DC, Kuwada S, Batra R, Trahiotis C (1995) Neural responses to simple simulated echoes in the auditory brain stem of the unanesthetized rabbit. J Neurophysiol 74:2469-2486.
Fitzpatrick DC, Kuwada S, Kim DO, Parham K, Batra R (1999) Responses of neurons to click-pairs as simulated echoes: auditory nerve to auditory cortex. J Acoust Soc Am 106:3460-3472.
Fuzessery ZM, Richardson MD, Coburn MS (2006) Neural mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the inferior colliculus of the pallid bat. J Neurophysiol 96:1320-1336.
Geis HR, Borst JG (2013) Intracellular responses to frequency modulated tones in the dorsal cortex of the mouse inferior colliculus. Front Neural Circuits 7:7.
González-Hernández T, Mantolán-Sarmiento B, González-González B, Pérez-González H (1996) Sources of GABAergic input to the inferior colliculus of the rat. J Comp Neurol 372:309-326.
Jamal L, Khan AN, Butt S, Patel CR, Zhang H (2012) The level and distribution of the GABA(B)R1 and GABA(B)R2 receptor subunits in the rat's inferior colliculus. Front Neural Circuits 6:92.
Kelly JB, Caspary DM (2005) Pharmacology of the inferior colliculus. In: The Inferior Colliculus (Winer JA, Schreiner CE, eds). New York: Springer New York.
Kidd SA, Kelly JB (1996) Contribution of the dorsal nucleus of the lateral lemniscus to binaural responses in the inferior colliculus of the rat: interaural time delays. J Neurosci 16:7390-7397.
Kuwada S, Batra R (1999) Coding of sound envelopes by inhibitory rebound in neurons of the superior olivary complex in the unanesthetized rabbit. J Neurosci 19:2273-2287.
Le Beau FE, Rees A, Malmierca MS (1996) Contribution of GABA-and glycine-mediated inhibition to the monaural temporal response properties of neurons in the inferior colliculus. J Neurophysiol 75:902-919.
Li L, Kelly JB (1992) Inhibitory influence of the dorsal nucleus of the lateral lemniscus on binaural responses in the rat's inferior colliculus. J Neurosci 12:4530-4539.
Li L, Yue Q (2002) Auditory gating processes and binaural inhibition in the inferior colliculus. Hear Res 168:98-109.
Li Y, Evans MS, Faingold CL (1998) In vitro electrophysiology of neurons in subnuclei of rat inferior colliculus. Hear Res 121:1-10.
Litovsky RY, Yin TC (1998a) Physiological studies of the precedence effect in the inferior colliculus of the cat. I. Correlates of psychophysics. J Neurophysiol 80:1285-1301.
Litovsky RY, Yin TC (1998b) Physiological studies of the precedence effect in the inferior colliculus of the cat. II. Neural mechanisms. J Neurophysiol 80:1302-1316.
Litovsky RY, Delgutte B (2002) Neural correlates of the precedence effect in the inferior colliculus: effect of localization cues. J Neurophysiol 87:976-994.
Litovsky RY, Rakerd B, Yin TCT, Hartmann WM (1997) Psychophysical and physiological evidence for a precedence effect in the median sagittal plane. J Neurophysiol 77:2223-2226.
Litovsky RY, Colburn HS, Yost WA, Guzman SJ (1999) The precedence effect. J Acoust Soc Am 106:1633-1654.
Ma CL, Kelly JB, Wu SH (2002) Presynaptic modulation of GABAergic inhibition by GABAB receptors in the rat's inferior colliculus. Neuroscience 114:207-215.
Malmierca MS, Seip KL, Osen KK (1995) Morphological classification and identification of neurons in the inferior colliculus: a multivariate analysis. Anat Embryol (Berl) 191:343-350.
Malmierca MS, Saint Marie RL, Merchan MA, Oliver DL (2005) Laminar inputs from dorsal cochlear nucleus and ventral cochlear nucleus to the central nucleus of the inferior colliculus: two patterns of convergence. Neuroscience 136:883-894.
Merchán M, Aguilar LA, Lopez-Poveda EA, Malmierca MS (2005) The inferior colliculus of the rat: quantitative immunocytochemical study of GABA and glycine. Neuroscience 136:907-925.
Nakamoto KT, Mellott JG, Killius J, Storey-Workley ME, Sowick CS, Schofield BR (2014) Ultrastructural characterization of GABAergic and excitatory synapses in the inferior colliculus. Front Neuroanat 8:108.
Nataraj K, Wenstrup JJ (2005) Roles of inhibition in creating complex auditory responses in the inferior colliculus: facilitated combination-sensitive neurons. J Neurophysiol 93:3294-3312.
Oliver DL (2005) Neuronal Organization in the Inferior Colliculus. In: The Inferior Colliculus (Winer JA, Schreiner CE, eds). New York: Springer New York.
Oliver DL, Winer JA, Beckius GE, Marie RLS (1994) Morphology of GABAergic neurons in the inferior colliculus of the cat. J Comp Neurol 340:27-42.
Ono M, Oliver DL (2014) The balance of excitatory and inhibitory synaptic inputs for coding sound location. J Neurosci 34:3779-3792.
Ono M, Yanagawa Y, Koyano K (2005) GABAergic neurons in inferior colliculus of the GAD67-GFP knock-in mouse: electrophysiological and morphological properties. Neurosci Res 51:475-492.
Orton LD, Rees A (2014) Intercollicular commissural connections refine the representation of sound frequency and level in the auditory midbrain. Elife 3:e03764.
Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates. London: Academic Press.
Pecka M, Zahn TP, Saunier-Rebori B, Siveke I, Felmy F, Wiegrebe L, Klug A, Pollak GD, Grothe B (2007) Inhibiting the inhibition: a neuronal network for sound localization in reverberant environments. J Neurosci 27:1782-1790.
Peruzzi D, Sivaramakrishnan S, Oliver DL (2000) Identification of cell types in brain slices of the inferior colliculus. Neuroscience 101:403-416.
Pollak GD, Xie R, Gittelman JX, Andoni S, Li N (2011) The dominance of inhibition in the inferior colliculus. Hear Res 274:27-39.
Shneiderman A, Chase MB, Rockwood JM, Benson CG, Potashner SJ (1993) Evidence for a GABAergic projection from the dorsal nucleus of the lateral lemniscus to the inferior colliculus. J Neurochem 60:72-82.
Sivaramakrishnan S, Oliver DL (2001) Distinct K currents result in physiologically distinct cell types in the inferior colliculus of the rat. J Neurosci 21:2861-2877.
Song P, Wang N, Wang H, Xie Y, Jia J, Li H (2011) Pentobarbital anesthesia alters neural responses in the precedence effect. Neurosci Lett 498:72-77.
Sturm J, Nguyen T, Kandler K (2014) Development of intrinsic connectivity in the central nucleus of the mouse inferior colliculus. J Neurosci 34:15032-15046.
Sun H, Wu SH (2008a) Physiological characteristics of postinhibitory rebound depolarization in neurons of the rat's dorsal cortex of the inferior colliculus studied in vitro. Brain Res 1226:70-81.
Sun H, Wu SH (2008b) Modification of membrane excitability of neurons in the rat's dorsal cortex of the inferior colliculus by preceding hyperpolarization. Neuroscience 154:257-272.
Sun H, Wu SH (2009) The physiological role of pre- and postsynaptic GABABreceptors in membrane excitability and synaptic transmission of neurons in the rat's dorsal cortex of the inferior colliculus. Neuroscience 160:198-211.
Tan ML, Theeuwes HP, Feenstra L, Borst JG (2007) Membrane properties and firing patterns of inferior colliculus neurons: an in vivo patch-clamp study in rodents. J Neurophysiol 98:443-453.
Tollin DJ, Populin LC, Yin TCT (2004) Neural correlates of the precedence effect in the inferior colliculus of behaving cats. J Neurophysiol 92:3286-3297.
Tolnai S, Litovsky RY, King AJ (2014) The precedence effect and its buildup and breakdown in ferrets and humans. JAS 135:1406-1418.
Wallach H, Newman EB, Rosenzweig MR (1949) The precedence effect in sound localization. Am J Psychol 62:315-336.
Wang YJ, Wang NY, Wang D, Jia J, Liu JF, Xie Y, Wen XH, Li XT (2014) Local inhibition of GABA affects precedence effect in the inferior colliculus. Neural Regen Res 9:420-429.
Winter JA, Schreiner CE (2005) The Inferior Colliculus. New York: Springer New York.
Yang X, Grantham DW (1997) Echo suppression and discrimination suppression aspects of the precedence effect. Percept Psychophys 59:1108-1117.
Yin S, Chen Z, Feng Y, Wang J, Yin S, Chen Z, Feng Y, Wang J (2008) The roles of local inhibition mediated by γ-aminobutyric acid (GABA)-A receptor in duration tuning in the inferior colliculus of guinea pigs. Acta Oto-Laryngol 128:1101-1109.
Yin TC (1994) Physiological correlates of the precedence effect and summing localization in the inferior colliculus of the cat. J Neurosci 14:5170-5186.
Zhang DX, Li L, Kelly JB, Wu SH (1998) GABAergic projections from the lateral lemniscus to the inferior colliculus of the rat. Hear Res 117:1-12.
Copyedited by Cooper C, Yajima W, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.182706 http://www.nrronline.org/
How to cite this article: Li XT, Wang NY, Wang YJ, Xu ZQ, Liu JF, Bai YF, Dai JS, Zhao JY (2016) Responses from two firing patterns in inferior colliculus neurons to stimulation of the lateral lemniscus dorsal nucleus. Neural Regen Res 11(5)∶787-794.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81271090.
Accepted: 2016-04-06
*Correspondence to: Ning-yu Wang, M.D., wny1128@hotmail.com.
杂志排行
中国神经再生研究(英文版)的其它文章
- Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Antibody-based neuronal and axonal delivery vectors for targeted ligand delivery
- Coordination of the axonal cytoskeleton during the emergence of axon collateral branches
- Alzheimer's disease: the silver tsunami of the 21stcentury
- Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells
