Neuroprotective effects of cold-inducible RNA-binding protein during mild hypothermia on traumatic brain injury
2016-12-02GuanWangJianningZhangJiakuiGuoYingCaiHongshengSunKunDongChenggangWu
Guan Wang, Jian-ning Zhang, Jia-kui Guo Ying Cai, Hong-sheng Sun, Kun Dong Cheng-gang Wu
1 Postgraduate Institution, Tianjin Medical University, Tianjin, China
2 Department of Neurosurgery, Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
3 Department of Neurosurgery, General Hospital of Tianjin Medical University, Tianjin, China
4 Department of Neurosurgery, Tianjin Huanhu Hospital, Tianjin, China
RESEARCH ARTICLE
Neuroprotective effects of cold-inducible RNA-binding protein during mild hypothermia on traumatic brain injury
Guan Wang1,2,*, Jian-ning Zhang3, Jia-kui Guo2, Ying Cai4, Hong-sheng Sun4, Kun Dong2, Cheng-gang Wu2
1 Postgraduate Institution, Tianjin Medical University, Tianjin, China
2 Department of Neurosurgery, Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
3 Department of Neurosurgery, General Hospital of Tianjin Medical University, Tianjin, China
4 Department of Neurosurgery, Tianjin Huanhu Hospital, Tianjin, China
Graphical Abstract
orcid: 0000-0001-8310-7142 (Guan Wang)
Cold-inducible RNA-binding protein (CIRP), a key regulatory protein, could be facilitated by mild hypothermia in the brain, heart and liver. This study observed the effects of mild hypothermia at 31 ± 0.5°C on traumatic brain injury in rats. Results demonstrated that mild hypothermia suppressed apoptosis in the cortex, hippocampus and hypothalamus, facilitated CIRP mRNA and protein expression in these regions, especially in the hypothalamus. The anti-apoptotic effect of mild hypothermia disappeared after CIRP silencing. There was no correlation between mitogen-activated extracellular signal-regulated kinase activation and CIRP silencing. CIRP silencing inhibited extracellular signal-regulated kinase-1/2 activation. These indicate that CIRP inhibits apoptosis by affecting extracellular signal-regulated kinase-1/2 activation, and exerts a neuroprotective effect during mild hypothermia for traumatic brain injury.
nerve regeneration; traumatic brain injury; mild hypothermia; cold-inducible RNA-binding protein; mitogen-activated extracellular signal-regulated kinase; anti-apoptosis; neural regeneration
Introduction
Traumatic brain injury (TBI) results in high mortality and the comprehensive therapeutic measures taken at the acute stage are important areas of neurosurgery research. In recent years, mild hypothermia therapy has been widely used as an important treatment at the acute phase in severe TBI (Andresen et al., 2015). Although mild hypothermia therapy has some side effects and shortcomings, long-term clinical and experimental studies have found that mild hypothermia therapy may have neuroprotective effects on reducing intracranial pressure, controlling cerebral edema, and protecting the blood-brain barrier (Idris et al., 2014). This can help the patients go through the acute stage of TBI (Darwazeh and Yan, 2013). However, the regulatory mechanisms of neuroprotective effects of mild hypothermia on TBI patients are not yet clear.
Cold-inducible RNA-binding protein (CIRP), a key regulatory protein, could be facilitated by mild hypothermia in the brain, heart and liver (Kaneko and Kibayashi, 2012). Under mild hypothermia, the synthesis of most proteins was inhibited, but CIRP was significantly enhanced. CIRP can reduce the demand for nutrients of the cells, inhibitapoptosis, participate in the transcription and translation of various genes, and protect the cytoskeleton at low temperatures (Zhang et al., 2015a).
CIRP may participate in the neuroprotective effect of mild hypothermia by regulating the signaling pathway of phosphorylated extracellular signal-regulated kinase (ERK). The neurological dysfunction is associated with the death of nerve cells after brain injury, including necrosis and apoptosis, especially apoptosis (Xiong et al., 2011). Because apoptosis occurs late and lasts longer, it has a more significant impact on neurological function. The ERK pathway plays an irreplaceable role in apoptotic signal transduction and promotes survival of cells (Li et al., 2014). However, various experiments have yielded conflicting conclusions, so there are still many questions over its exact role. ERK expression is excessively activated and significantly prolonged after brain injury (Atkins et al., 2009). Unduly sustained activation of ERK is harmful to cells (Sun et al., 2015).
This study investigated the correlation between CIRP expression and ERK pathway activation, and the mechanism of action of CIRP expression during mild hypothermia in TBI rats.
Materials and Methods
Experimental animals
This study was approved by the Committee for Institutional Animal Care and Use Committee Institution (No. 11300223) and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment. Healthy clean Sprague-Dawley rats aged 11—14 weeks and weighing 300—400 g, half male and half female, were provided by the Experimental Animal Center of Military Medical Science Academy, Beijing, China (license No. SCXK (Jing) 2012-0004). All rats were housed at 25°C and 40% humidity, with 16-hour illumination daily, before each experiment. The rats were randomly divided into a control group (Group 1), a blank AD5-green fluorescent protein (GFP)-transfection group (Group 2) and a AD5-GFP-CIRP-siRNA transfection group (Group 3), with 87 rats in each group. Each of Groups 1, 2 and 3 were subdivided into a sham group with 21 rats, a TBI group with 21 rats, a mild hypothermia group with 21 rats, and a TBI + mild hypothermia group with 21 rats. The remaining three rats, one from each of Groups 1, 2 and 3 were used for histological examination to detect viral transfection.
Viral transfection
For Group 2 and Group 3, viral transfection was conducted first. Group 1 rats were administered 0.1 mL of saline intrathecally. The rats were anesthetized and cut open under sterile conditions. The atlanto-occipital membrane was separated from the rats. Cerebrospinal fluid that flowed out after puncturing was pumped back. In Group 2, rats received a one-time intrathecal injection of 0.1-mL blank AD5-GFP (Genechem Co., Ltd., Shanghai, China). A onetime intrathecal injection of 0.1 mL (1 × 1010pfu/mL) AD5-GFP-CIRP-siRNA (Genechem Co., Ltd.) was administered to Group 3 rats. After the incision was sutured, the rats were fed in their normal environment for 3 days, and then underwent model induction.
TBI model establishment
After anesthesia, the rats were fixed on the stereotaxic apparatus holder (GENE, Beijing, China). The scalp was cut open sagittally under aseptic conditions. A hole was drilled in the right parietal bone at 3 mm from the cranial coronal suture, 3 mm from the right sagittal suture. A bone window of approximately 3 mm2was made. The fluid percussion device (GENE) was used to produce the brain injury, with harmful impact strength of 200 kPa. The incision was then sutured. In the sham group and mild hypothermia group, only a hole was drilled.
Mild hypothermia treatment
After TBI, 21 rats from each group underwent immediately mild hypothermia. The rats were placed on a low-temperature ice blanket, anesthetized by intraperitoneal injection of chloral hydrate, to keep the rats in sustained dormant state. The rectal temperature was maintained at 31 ± 0.5°C for 48 hours, and a rectal thermometer (Eastwest, Beijing, China) was used to detect the changes in body temperature. During mild hypothermia, conventional feeds and water were given via a stomach tube.
Viral transfection detection
After viral vector had been transfected for 3 days, one rat was randomly selected from each of Groups 1, 2 and 3. Immunofluorescence staining was used to confirm whether AD5-GFP in the brain tissue has been successfully transfected. The brain was removed from the skull and the tissue was fixed in 4% paraformaldehyde for approximately 6 hours. It was then dehydrated, fixed in formaldehyde, immersed in paraffin, cut into 2-mm thick tissue, and then sliced into 5-µm thick frozen sections with a freezing microtome. Fluorescent dye, DAPI, was used to stain the nuclei. Results were observed under a fluorescence microscope (200×, Leica, Wetzlar, Germany) using an excitation light. Nuclei emitted a blue light. The cells transfected by GFP fluoresced as green. Cells where the staining overlapped indicated successful transfection of the viral vector. Results were analyzed with Image-pro plus 6.1 software (Media Cybernetics, Inc., Rockville, MD, USA).
Specimen collection
After model induction, three rats were selected from each group respectively at 30 minutes, 6, 12, 24, 48 and 72 hours to detect protein expression. They were sacrificed by removing their vertebral columns. Their brain tissue was taken out and stored in liquid nitrogen. The remaining rats were sacrificed by the same method at 96 hours after the experiment began. The cortex, hippocampus and hypothalamus (Goodrich,2014) were stored for reverse transcription-polymerase chain reaction (RT-PCR) and apoptosis detection.
Apoptosis detection
Cell apoptosis was detected by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay (Wang et al., 2016). Under anesthesia, the rats were perfused with 4% paraformaldehyde via the left ventricle until the bodies were stiff. The brain tissue was taken out and fixed for 24 hours, and incubated for 24 hours with primary antibodies at 4°C. The reaction mixture was labeled with TUNEL, incubated for 1 hour at 37°C, incubated with POD conversion solution for 30 minutes at 37°C, and visualized with 3,3′-diaminobenzidine in the dark. Five axial slices from each tissue were selected and 10 fields were randomly selected under the microscope to count the apoptotic cells. The apoptotic index was calculated by the number of apoptotic cells/total number of cells × 100.
RT-PCR
Total RNA was extracted from the cortex, hippocampus and hypothalamus using TRIzol. The RNA concentration was measured at 260 nm and its purity was calculated according to the OD260/OD280ratio. Centrifugation and electrophoresis were used to purify the RNA samples. cDNA was synthesized by reverse transcription. Primers were designed using GeneRunner software. The GAPDH gene amplification product (synthesized by Beijing Invitrogen Biotechnology Company, Beijing, China) is 110 bp, with the primer sequences of upstream primer 5′-AAC TCC CAT TCT TCC ACC-3′ and downstream primer of 5′-ACC ACC CTG TTG CTG TAG-3′. The CIRP gene amplification product is 167 bp, with the primer sequences of upstream primer 5′-TTA AGG CCA AGC AAG CAT CT-3′ and downstream primer of 5′-CTC CCT GTC CTT TAC CAC CA-3′. After PCR and amplification, data analysis was conducted. The change in cycle threshold (ΔCt) value was obtained from the difference of CIRP mRNA Ct values and internal referenced Ct values. The average ΔCt value was calculated and 2—ΔΔCtvalue represented the difference between relative CIRP mRNA expression and that of the control group.
Western blot assay
Western blot assay was used to detect the expression of CIRP, mitogen-activated ERK kinase (MEK), phospho-MEK (p-MEK), extracellular signal-regulated kinase (ERK-1/2) and p-ERK-1/2 proteins of the part of hypothalamus. The primary antibodies used were as follows: anti-CIRP (1:2,000; Abcam, Cambridge, UK), anti-MEK, anti-p-MEK, anti-ERK-1/2 and anti-p-ERK-1/2 (1:2,000; Cell Signaling Technology, Beverly, MA, USA), anti-β-actin (1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Apart from the anti-CIRP antibody, a purified goat IgG, the other primary antibodies are purified rabbit IgGs. The corresponding secondary antibody of peroxidase-conjugated AffiniPure rabbit anti-goat IgG or goat anti-rabbit IgG was purchased from Zhongshan Golden Bridge Biotechnology Co., Ltd. (1:10,000; Beijing, China). After anesthesia, the rats were decapitated and the brains removed. The hypothalamus was dissected from the brain tissue and placed on ice, finely cut, lysed with lysate, and homogenized in an ice-bath. Subsequently, homogenate was centrifuged at 11,000 × g, at 4°C. The supernatant was taken and the protein level was determined with Coomassie brilliant blue staining. Samples were transferred onto the membrane (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membranes were incubated with primary antibodies overnight at 4°C and with secondary antibodies for 1 hour at room temperature, blocked and visualized by enhanced chemiluminescence reaction. Optical density values were measured with a gel image system (Bio-Rad, Hercules, CA, USA). The ratio of the average optical density of the target band to the internal reference β-actin represented the protein level. Semi-quantitative analysis was then carried out.
Statistical analysis
Data were expressed as the mean ± SEM. Statistical analysis was determined by one-way analysis of the variance followed by Tukey's test. Statistical analysis was performed using SAS 9.1.3 software (SAS Institute, Ins., Cary, NC, USA). A value of P < 0.05 was considered statistically significant.
Results
Viral transfection outcomes
Three days after blank AD5-GFP and AD5-GFP-CIRP-siRNA transfection, green fluorescence under the fluorescence microscope indicated that transfection was successful (Figure 1).
CIRP silencing affected CIRP mRNA expression after TBI under mild hypothermia
TUNEL assay results demonstrated that the number of apoptotic cells in the cerebral cortex, hippocampus and hypothalamus was higher in the TBI group and TBI + mild hypothermia group than in the sham group and mild hypothermia group (P < 0.05). Injury promoted apoptosis, but mild hypothermia reduced apoptosis in the cerebral cortex, hippocampus and hypothalamus (P < 0.05). Mild hypothermia alone did not affect apoptosis in the cortex, hippocampus and hypothalamus (P > 0.05). There were fewer apoptotic cells in the hypothalamus than in the cortex and hippocampus from the TBI + mild hypothermia group (P < 0.05). After CIRP-siRNA interference, the anti-apoptotic effect of mild hypothermia was not significant in TBI rats transfected with AD5-GFP-CIRP-siRNA (Figure 2).
CIRP silencing affected apoptosis after TBI under mild hypothermia
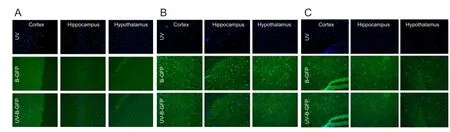
Figure 1 Results of viral transfection (× 200).

Figure 2 Apoptosis in various groups (TUNEL assay, × 400).

Figure 3 CIRP mRNA expression in the cortex, hippocampus and hypothalamus of various groups.
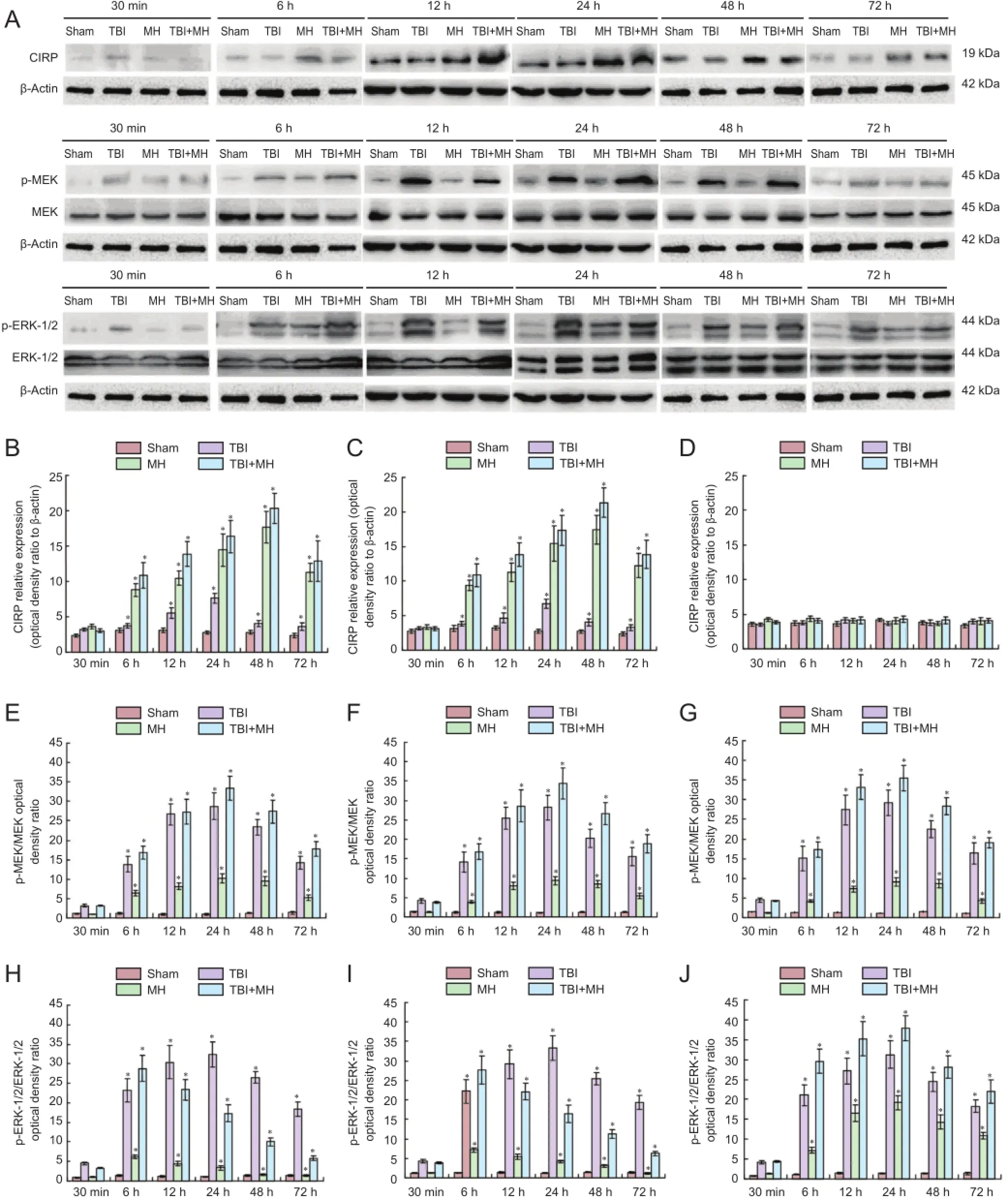
Figure 4 Protein expression of CIRP, MEK and ERK in different groups.
RT-PCR results of the expression of CIRP mRNA in either the cortex or the hippocampus or the hypothalamus revealed that there was no significant difference between the TBI group and the sham group (P > 0.05). Mild hypothermia increasedCIRP mRNA expression in the cortex, hippocampus and hypothalamus (P < 0.05). CIRP mRNA expression was significantly higher in the hypothalamus than in the cortex and hippocampus (P < 0.05). The above changes were also found in the blank AD5-GFP group. After AD5-GFPCIRP-siRNA transfection, CIRP expression was not affected by mild hypothermia and TBI (P > 0.05) (Figure 3).
CIRP silencing affected CIRP expression after TBI under mild hypothermia
Western blot assay results showed that CIRP expression in the hypothalamus increased at 12 and 24 hours after injury (P < 0.05; vs. 30 minutes), and then decreased. In the mild hypothermia group, CIRP protein expression began to significantly increase 6 hours after mild hypothermia (P < 0.05), peaked at 48 hours, and showed a downward trend at 72 hours. Mild hypothermia led to high CIRP expression. In the TBI + mild hypothermia group, CIRP protein expression also began to significantly increase after 6 hours (P < 0.05), peaked at 48 hours, and showed a downward trend at 72 hours. CIRP expression in the groups with blank AD5-GFP had the same tendency, but CIRP protein expression was not affected by mild hypothermia in rats transfected with AD5-GFP-CIRP-siRNA (P> 0.05; Figure 4A-D).
CIRP silencing affected MEK activation after TBI under mild hypothermia
The p-MEK/MEK ratio indicated MEK activation in the hypothalamus. As shown in Figure 4A, E-G, no significant difference in p-MEK/MEK ratio was found in the sham groups of Groups 1, 2 and 3 (P > 0.05). In the TBI group, mild hypothermia group and TBI + mild hypothermia group of Group 1, MEK activation showed progressive increase, and peaked at 24 hours, followed by a progressive decline. MEK activation of all groups in Groups 2 and 3 had very similar trends to that of Group 1. MEK activation was not correlated with CIRP silencing, which indicated that CIRP protein had no effect on MEK activation.
CIRP silencing affected ERK activation after TBI under mild hypothermia
The p-ERK-1/2 to ERK-1/2 ratio indicated ERK-1/2 activation in the hypothalamus. The p-ERK-1/2 / ERK-1/2 did not change significantly in the sham groups (P > 0.05). ERK-1/2 activation significantly increased at 6 hours after injury in all the TBI groups (P < 0.05), and peaked at 24 hours, followed by a decline, suggesting that the injury led to ERK-1/2 activation. Results from Groups 1 and 2 in both the mild hypothermia and TBI + mild hypothermia groups showed that ERK-1/2 activation first significantly increased at 6 hours (P < 0.05), and declined after 12 hours. In the TBI + mild hypothermia of Group 3, the peak of the progressive ERK-1/2 activation appeared at 24 hours, and then began to decline. Mild hypothermia promoted ERK-1/2 activation reaching a peak, but ERK-1/2 activation quickly reduced during CIRP silencing (P < 0.05; Figure 4A, H-J).
Correlation of CIRP expression, MEK activation and ERK1/2 activation
In the TBI + mild hypothermia group, CIRP expression in the hypothalamus peaked at 48 hours; MEK activation peaked at 24 hours; ERK-1/2 activation peaked at 6 hours. When CIRP expression was inhibited, there was no significant change in CIRP expression, MEK and ERK-1/2 activation peaks both appeared at 24 hours. This indicated that, when CIRP expression was silent, ERK-1/2 activation was regulated by MEK, and p-ERK-1/2 expression would increase. However, when CIRP was overexpressed, MEK activation showed no significant change and ERK-1/2 activation decreased rapidly over time, proving that CIRP did not regulate ERK-1/2 activation through MEK, but might affect ERK-1/2 directly, causing its activation to decrease rapidly with time.
Discussion
Mild hypothermia has been widely used in the treatment of brain injury, cerebral ischemic diseases, cardiac surgery, and cardiopulmonary resuscitation. Although mild hypothermia has many shortcomings, long-term clinical practices have confirmed its cytoprotective effect. The protective mechanism of mild hypothermia is not yet clear. Recent studies on the molecular biological mechanisms of mild hypothermia have mainly focused on the temperature-associated regulatory mechanism (Saito et al., 2010). When the body temperature declines, a group of proteins are produced to accommodate the temperature change. CIRP is the first cold-shock protein expressed in mammalian cells (Kaneko and Kibayashi, 2012). It was first isolated from mouse testis cells by Nishiyama et al. (1997), and they found that CIRP has a regulatory effect on cell growth and apoptosis under cold induction. CIRP has many regulatory pathways, and can exert a regulatory effect during transcription, replication, and mRNA translation processes (Artero-Castro et al., 2009). Oishi et al. (2013) have shown that, at normal temperature, CIRP in rat liver cells could be detected, but CIRP mRNA could hardly be detected. However, at low temperature, CIRP mRNA could be found in great quantities, indicating that the stability of CIRP mRNA is regulated by the environmental temperature. When the microbes are in an environment where the temperature drops rapidly, the cold-shock response will lead to the inhibition of translation initiation, the reduction of polyribosomes and increase of 70S monomer. The signal to initiate cold-shock response is at the non-ribosomal translation level. The function of a cold shock protein is to transform the ribosome to promote CIRP expression and makes the cells adapt to low temperatures, which can be regulated at the translational level (Sumitomo et al., 2012). Zhou et al. (2014) confirmed that CIRP expression decreased in the hippocampus of ischemic models and that exogenous drugs inhibited CIRP expression in nerve cells. Liu et al. (2010) considered that at low temperatures, CIRP is overexpressedin cells with cold stress. Li et al. (2012) verified that the reactive oxide radicals were produced after brain ischemia and aggravated brain damage. However, H2O2, as a metabolite of reactive oxide species, could prevent CIRP expression, indicating that CIRP reduction may be one of the mechanisms for the effects of oxide radicals on brain injury. This study measured the apoptosis of nerve cells in different brain tissues under mild hypothermia. It was found that apoptosis decreased significantly in the cortex, hippocampus and hypothalamus after mild hypothermia, compared with the TBI group. Mild hypothermia could evidently reduce apoptosis. The reduction was greater in the hypothalamus than that in the cortex and hippocampus. RT-PCR results revealed that, CIRP mRNA expression was significantly higher in the hypothalamus than in the cortex and hippocampus, suggesting that mild hypothermia noticeably regulates the apoptosis of nerve cells in the hypothalamus, because CIRP mRNA expression was high. After CIRP-siRNA silencing, there was no significant difference in the apoptosis of various regions between the mild hypothermia and TBI groups. There was no significant difference in apoptosis between the hypothalamus and the cortex and hippocampus, indicating that after CIRP silencing, mild hypothermia had no regulatory effect on apoptosis.
The ERK pathway, the core in many signal transduction processes, includes two highly homologous subclasses, ERK-1 and ERK-2. Extracellular stimulation can be activated by G protein receptors, growth factor receptors, tyrosine kinase receptors and Ras-Raf-MEK-ERK cascade signals. There are many ways for ERK activation, but these activation pathways will have many effects on cells (Kumar et al., 2014). Adapter protein (Shc), G protein (Ras), and protein kinase C (PKC) are the three ways to activate ERK. By activating Raf and MEK, they not only activate ERK, but also produce a series of subsequent reactions by the simultaneous activation of Raf, MEK and ERK. On one hand, they generate cell transcriptional activities, which are beneficial to cell survival, but they also activate the programmed cell death pathways (Zhang et al., 2015). After injury, ERK-1/2 shows an obvious sustained activation in the central area of injury, indicating a direct relationship between ERK and cell death (Sticozzi et al., 2013). This study measured ERK pathway activation and CIRP expression at different time points during mild hypothermia. The results showed that CIRP transient expression in the TBI group increased at an early stage and over the following 24 hours. In the mild hypothermia group and the TBI + mild hypothermia group, CIRP expression significantly increased over time. After mild hypothermia ended, namely 48 hours later, CIRP expression declined, indicating that mild hypothermia played a leading role in CIRP expression. Previous studies have demonstrated that the main activation pathway for the ERK pathway is by Ras-Raf-MEK-ERK cascade signals. This study showed that, in the TBI group, MEK activation showed a progressive increase after injury, and peaked at 24 hours, followed by a progressive decline. The degree of ERK-1/2 activation was similar to that of MEK, which indicates the injury activated ERK by the MEK-ERK pathway, resulting in acute brain damage. The degree of MEK activation at different time points is similar between the TBI + mild hypothermia group and the TBI group. CIRP has no significant effect on MEK, but CIRP directly reduces ERK-1/2 activation, and reduces cell damage mediated by the ERK pathway in the acute phase. When CIRP-siRNA was silenced, the curves for activation peak of MEK and ERK-1/2 were similar, which further indicates that ERK-1/2 activation may be inversely regulated by CIRP expression.
This study showed that the protective effect of mild hypothermia on the hypothalamus was greater than that on the cortex and hippocampus. Moreover, CIRP expression in the hypothalamus was higher than that in the cortex and hippocampus. When CIRP expression was silent, the difference in apoptosis frequencies between the cortex, hippocampus and hypothalamus disappeared, suggesting that the neuroprotective effect of mild hypothermia on brain injury may be achieved via CIRP overexpression. The detection of key factors for the ERK signaling pathway and CIRP expression at different time points have found that the anti-apoptosis signaling pathway of CIRP may be realized by directly reducing ERK-1/2 activation. More in-depth studies are needed in the future to find out whether mild hypothermia can exert its neuroprotective effect by other means and whether there are other regulatory pathways for the anti-apoptotic effects of CIRP.
Author contributions: GW designed this study, obtained funding, and wrote the paper. JKG assisited to design this study. JNZ was in charge of paper authorization. YC provided data. HSS analyzed statistical data. KD ensured the integrity of the data. CGW analyzed data. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Al-Fageeh MB, Smales CM (2009) Cold-inducible RNA binding protein (CIRP) expression is modulated by alternative mRNAs. RNA 15:1164-1176.
Andresen M, Gazmuri JT, Marín A, Regueira T, Rovegno M (2015) Therapeutic hypothermia for acute brain injuries. Scand J Trauma Resusc Emerg Med 23:42.
Artero-Castro A, Callejas FB, Castellvi J, Kondoh H, Carnero A, Fernández-Marcos PJ, Serrano M, Ramón y Cajal S, Lleonart ME (2009) Cold-inducible RNA-binding protein bypasses replicative senescence in primary cells through extracellular signal-regulated kinase 1 and 2 activation. Mol Cell Biol 29:1855-1868.
Atkins CM, Falo MC, Alonso OF, Bramlett HM, Dietrich WD (2009) Deficits in ERK and CREB activation in the hippocampus after traumatic brain injury. Neurosci Lett 459:52-56.
Darwazeh R, Yan Y (2013) Mild hypothermia as a treatment for central nervous system injuries: Positive or negative effects. Neural Regen Res 8:2677-2686.
Goodrich JT (2014) Models of functional cerebral localization at the dawning of modern neurosurgery--a perspective on these remarkable events. World Neurosurg 81:300-301.
Idris Z, Zenian MS, Muzaimi M, Hamid WZ (2014) Better Glasgow outcome score, cerebral perfusion pressure and focal brain oxygenation in severely traumatized brain following direct regional brain hypothermia therapy: A prospective randomized study. Asian J Neurosurg 9:115-123.
Kaneko T, Kibayashi K (2012) Mild hypothermia facilitates the expression of cold-inducible RNA-binding protein and heat shock protein 70.1 in mouse brain. Brain Res 1466:128-136.
Koh PO (2015) Ferulic acid attenuates the down-regulation of MEK/ ERK/p90RSK signaling pathway in focal cerebral ischemic injury. Neurosci Lett 588:18-23.
Kumar P, Rao GN, Pal BB, Pal A (2014) Hyperglycemia-induced oxidative stress induces apoptosis by inhibiting PI3-kinase/Akt and ERK1/2 MAPK mediated signaling pathway causing downregulation of 8-oxoG-DNA glycosylase levels in glial cells. Int J Biochem Cell Biol 53:302-319.
Li Q, Chen M, Liu H, Yang L, Yang T, He G (2014) The dual role of ERK signaling in the apoptosis of neurons. Front Biosci (Landmark Ed) 19:1411-1417.
Li S, Zhang Z, Xue J, Liu A, Zhang H (2012) Cold-inducible RNA binding protein inhibits H2O2-induced apoptosis in rat cortical neurons. Brain Res 1441:47-52.
Liu A, Zhang Z, Li A, Xue J (2010) Effects of hypothermia and cerebral ischemia on cold-inducible RNA-binding protein mRNA expression in rat brain. Brain Res 1347:104-110.
Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J (1997) A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol 137:899-908.
Oishi K, Yamamoto S, Uchida D, Doi R (2013) Ketogenic diet and fasting induce the expression of cold-inducible RNA-binding protein with time-dependent hypothermia in the mouse liver. FEBS Open Bio 3:192-195.
Saito K, Fukuda N, Matsumoto T, Iribe Y, Tsunemi A, Kazama T, Yoshida-Noro C, Hayashi N (2010) Moderate low temperature preserves the stemness of neural stem cells and suppresses apoptosis of the cells via activation of the cold-inducible RNA binding protein. Brain Res 1358:20-29.
Sticozzi C, Belmonte G, Meini A, Carbotti P, Grasso G, Palmi M (2013) IL-1β induces GFAP expression in vitro and in vivo and protects neurons from traumatic injury-associated apoptosis in rat brain striatum via NFκB/Ca2+-calmodulin/ERK mitogen-activated protein kinase signaling pathway. Neuroscience 252:367-383.
Sumitomo Y, Higashitsuji H, Higashitsuji H, Liu Y, Fujita T, Sakurai T, Candeias MM, Itoh K, Chiba T, Fujita J (2012) Identification of a novel enhancer that binds Sp1 and contributes to induction of cold-inducible RNA-binding protein (cirp) expression in mammalian cells. BMC Biotechnol 12:72.
Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF (2015) Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct 35:600-604.
Tan HK, Lee MM, Yap MGS, Wang DIC (2008) Overexpression of cold-inducible RNA-binding protein increases interferon-γ production in Chinese-hamster ovary cells. Biotechnol Appl Biochem 49:247-257.
Tong G, Endersfelder S, Rosenthal LM, Wollersheim S, Sauer IM, Bührer C, Berger F, Schmitt KRL (2013) Effects of moderate and deep hypothermia on RNA-binding proteins RBM3 and CIRP expressions in murine hippocampal brain slices. Brain Res 1504:74-84.
Wang H, Yang LL, Ji YL, Chen YH, Hu J, Zhang C, Zhang J, Xu DX (2016) Different fixative methods influence histological morphology and TUNEL staining in mouse testes. Reprod Toxicol 60:53-61.
Xiong M, Cheng GQ, Ma SM, Yang Y, Shao XM, Zhou WH (2011) Post-ischemic hypothermia promotes generation of neural cells and reduces apoptosis by Bcl-2 in the striatum of neonatal rat brain. Neurochem Int 58:625-633.
Zhang HT, Xue JH, Zhang ZW, Kong HB, Liu AJ, Li SC, Xu DG (2015a) Cold-inducible RNA-binding protein inhibits neuron apoptosis through the suppression of mitochondrial apoptosis. Brain Res 1622:474-483.
Zhang YH, Belegu V, Zou Y, Wang F, Qian BJ, Liu R, Dai P, Zhao W, Gao FB, Wang L, Cao LM, McDonald JW, Liu S, Lin N, Wang TH (2015b) Endoplasmic reticulum protein 29 protects axotomized neurons from apoptosis and promotes neuronal regeneration associated with Erk signal. Mol Neurobiol 52:522-532.
Zhou M, Yang WL, Ji Y, Qiang X, Wang P (2014) Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia. Biochim Biophys Acta 1840:2253-2261.
Copyedited by Dawes EA, Hindle A, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.182704 http://www.nrronline.org/
How to cite this article: Wang G, Zhang JN, Guo JK, Cai Y, Sun HS, Dong K, Wu CG (2016) Neuroprotective effects of cold-inducible RNA-binding protein during mild hypothermia on traumatic brain injury. Neural Regen Res 11(5)∶771-778.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81303091.
Accepted: 2016-04-23
*Correspondence to: Guan Wang, Doctoral candidate, neurocrown@163.com.
杂志排行
中国神经再生研究(英文版)的其它文章
- Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Antibody-based neuronal and axonal delivery vectors for targeted ligand delivery
- Coordination of the axonal cytoskeleton during the emergence of axon collateral branches
- Alzheimer's disease: the silver tsunami of the 21stcentury
- Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells
