Bumetanide promotes neural precursor cell regeneration and dendritic development in the hippocampal dentate gyrus in the chronic stage of cerebral ischemia
2016-12-02WangshuXuXuanSunChengguangSongXiaopengMuWenpingMaXinghuZhangChuanshengZhao
Wang-shu Xu, Xuan Sun, Cheng-guang Song, Xiao-peng Mu Wen-ping Ma, Xing-hu Zhang Chuan-sheng Zhao
1 Department of Neurology, First Affiliated Hospital of China Medical University, Shenyang, Liaoning Province, China
2 Neuroinfection and Neuroimmunology Center, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
3 Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
4 Department of Neurology, Benxi Central Hospital of China Medical University, Benxi, Liaoning Province, China
5 Department of Medical Genetics, School of Basic Medicine, Peking University, Beijing, China
RESEARCH ARTICLE
Bumetanide promotes neural precursor cell regeneration and dendritic development in the hippocampal dentate gyrus in the chronic stage of cerebral ischemia
Wang-shu Xu1,2,#, Xuan Sun3,#, Cheng-guang Song1,4, Xiao-peng Mu1, Wen-ping Ma5, Xing-hu Zhang2, Chuan-sheng Zhao1,*
1 Department of Neurology, First Affiliated Hospital of China Medical University, Shenyang, Liaoning Province, China
2 Neuroinfection and Neuroimmunology Center, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
3 Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
4 Department of Neurology, Benxi Central Hospital of China Medical University, Benxi, Liaoning Province, China
5 Department of Medical Genetics, School of Basic Medicine, Peking University, Beijing, China
Graphical Abstract
#These authors contributed equally to this study.
orcid: 0000-0002-4746-2064 (Wang-shu Xu)
Bumetanide has been shown to lessen cerebral edema and reduce the infarct area in the acute stage of cerebral ischemia. Few studies focus on the effects of bumetanide on neuroprotection and neurogenesis in the chronic stage of cerebral ischemia. We established a rat model of cerebral ischemia by injecting endothelin-1 in the left cortical motor area and left corpus striatum. Seven days later, bumetanide 200 µg/kg/day was injected into the lateral ventricle for 21 consecutive days with a mini-osmotic pump. Results demonstrated that the number of neuroblasts cells and the total length of dendrites increased, escape latency reduced, and the number of platform crossings increased in the rat hippocampal dentate gyrus in the chronic stage of cerebral ischemia. These findings suggest that bumetanide promoted neural precursor cell regeneration, dendritic development and the recovery of cognitive function, and protected brain tissue in the chronic stage of ischemia.
nerve regeneration; cerebral ischemia; bumetanide; Na+-K+-2Cl—cotransporter 1; hippocampal dentate gyrus; neurogenesis; neural precursor cells; dendritic development; cognitive function; neural regeneration
Introduction
The subventricular and subgranular zones of the hippocampal dentate gyrus are brain regions where neurogenesis continues throughout adulthood. They can continue to produce new neurons, and form synaptic contacts with the existing neural circuitry (Danzer, 2008; Eisch et al., 2008). Robust neurogenesis alters noticeably after brain injury (Zhang et al., 2008; Zhao et al., 2008). The nervous system shows great plasticity after cerebral ischemia (Walther et al., 2009), increasing nerve regeneration, but the long-term viability of newborn neurons is relatively limited (Thored et al., 2006). These results indicate that the local microenvironment is not conducive to self repair after a stroke. It is of important clinical significance to explore new drugs with neuroprotective action against the effects of cerebral ischemia.
The Slc12a2 gene, which encodes the Na+-K+-2Cl—cotransporter 1 (NKCC1), is extensively expressed in different animals and tissues, including the cell membrane of cortical neurons of the central nervous system (Lee et al., 2010). Bumetanide is a specific antagonist of NKCC1 that can reduce the influx of chloride ions into cells of the central nervous system (Kahle and Staley, 2008). Bumetanide reduced cerebral edema and the infarct area by suppressing NKCC1 in the acute stage of cerebral ischemia (O'Donnell et al., 2004; Lu et al., 2007; Wang et al., 2014). Shulga et al. (2012) confirmed that bumetanide reduced the damage to nerve cells in rats. The above results verified that bumetanide exerted a certain neuroprotective effect in the brain. Nevertheless, the effects of bumetanide on neurogenesis and behavioral recovery in the chronic stage of cerebral ischemia in rats remain poorly understood. Therefore, we investigated the effects of bumetanide on neurogenesis and the recovery of learning and memory functions in rats with focal cerebral ischemia.
Materials and Methods
Ethics statement
All experimental protocols were approved by the Animal Ethics Committee of Medical University of China. The animal studies were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, USA. Precautions were taken to minimize suffering and the number of animals used in each experiment.
Animals
Adult male Wistar rats weighing 200—250 g were provided by the Liaoning Changsheng Biotechnology Co., Ltd., Benxi, Liaoning Province, China (animal license No. SCXK (Liao) 2010-0001). The rats were housed at 18—23°C and 30—45% humidity and allowed free access to food and water in a periodically ventilated room with natural light.
Establishment of a rat model of focal cerebral ischemia by intracranial injection of endothelin 1 (ET-1)
Twenty-two rats were intraperitoneally injected with 10% chloral hydrate 350 mg/kg. On a stereotaxic apparatus, after shaving and sterilizing the skin, a median incision was made and subcutaneous tissue was removed to expose the skull.
The skull and dura were drilled with a 2-mm-diameter electric drill. ET-1 (Sigma-Aldrich, St. Louis, MO, USA; purity≥ 97%, specification 0.1 mg, solid powder) was diluted with sterile saline, 0.5 µg/µL. In accordance with anatomical atlas of the rat brain (Paxinos and Watson, 2005), ET-1 was injected at 0.5 µL/min with a microinjector into the following three points (Soleman et al., 2010): taking anterior fontanelle as a center, (1) anteroposterior = +0.7 mm, mediolateral = +2.2 mm, dorsoventral = -2.0 mm; (2) anteroposterior = +2.3 mm, mediolateral = +2.5 mm, dorsoventral = -2.3 mm; (3) anteroposterior = +0.7 mm, mediolateral = +3.8 mm, dorsoventral = -5.8 mm (Figure 1). Points (1) and (2) were in the left cortical motor region; point (3) was in the left corpus striatum. At each point, 2 µL of drug was injected, totalling 6 µL. There was a 1-minute rest between each 1-µL injection. The needle was maintained in place for 3 minutes. After injection, the hole was covered with sterile gelfoam, and the incision was sutured. At 3 hours after injection (when the rats regained consciousness), the rats presenting the following manifestations were considered successful ischemic models: right upper limb flexion to the chest when the tail was lifted, or tilting to the right when walking, or circling to the right, for 24 consecutive hours. Physiological saline (2 µL) was injected into the same region of 18 sham-operated rats. Of the 22 rats injected with ET-1, 4 rats were excluded, because 2 rats died and 2 rats did not suffer from cerebral ischemia. The successful ischemic models were equally and randomly divided into ischemia group (ISC group) and ischemia + bumetanide group (ISC + BUM group) (n = 9). 18 sham-operated rats were equally and randomly divided into sham operation group (SHAM group) and sham operation + bumetanide group (SHAM + BUM group).
Intracranial sustained administration of bumetanide with a mini-osmotic pump
Seven days after injury, NKCC1 antagonist bumetanide was injected in rats of the ISC + BUM and SHAM + BUM groups with the mini-osmotic pump (Model 2004; Alzet, Palo Alto, CA, USA). The mini-osmotic pump was immersed in 37°C sterile water overnight before implantation. Bumetanide (25 µg/µL), diluted by sterile water, was injected into the pump, which was connected to a 8-cm catheter. After anesthesia with 10% chloral hydrate, a rat was fixed in the stereotaxic apparatus (RWD Life Science, Shenzhen, China). After shaving, a 2.5-cm-long longitudinal incision was made behind the eye to expose the skull. The periosteum on the skull was exposed by bluntly shaving with tweezers before a hole was made on the lateral ventricle (anteroposterior -0.9, mediolateral +1.5) with a 1.2-mm cranial drill. A channel between the incision on the head and scapula on the back was bluntly opened with a hemostat for pump implantation. The catheter, connected to the pump, was inserted into the hole in the lateral ventricle. Dental cement was used to cover the connecting zone of the catheter base and the skull. Four minutes later, the scalp was sutured after the cement was dry. On average, 50 µg of bumetanide was pumped every day, for 21 consecutive days (Figure 2).
Morris water maze test
Nine rats from each group were given a Morris water maze test 28 days after injury. The changes in spatial learning and memory were detected with the water maze image analysis system (Beijing Shuolinyuan Technology Co., Ltd., Beijing, China). The escape latencies within 120 seconds were recorded for 3 consecutive days. The platform was removed from the pool on the last day, and the spatial probe test was conducted. The number of platform crossings within 60 seconds was recorded (Furuta et al., 1997).
Preparation of frozen sections
Thirty-one days after ischemia, rats were intraperitoneally anesthetized with 10% chloral hydrate 350 mg/kg, fixed with 150—200 mL of 4% paraformaldehyde through the heart, and then decapitated. After the skull was opened, brain tissue was fixed in 4% paraformaldehyde at 4°C overnight and stored in 30% sucrose solution (0.1 M PBS, pH 7.2—7.4) at 4°C. The fixed tisssue was embedded with an optimal cutting temperature compound, and serially sliced into 40-µm-thick coronal frozen sections with a cryostat (Model CM1900; Leica, Munich, Germany). The above sections were stored in an anti-freezing medium at -20°C for further use.
Measurement of infarct volume
The infarct volume was measured after Nissl's staining in the ISC and ISC + BUM groups (n = 6). Serial coronal sections (40 µm thick) were collected at 1-mm intervals from +4.5 mm anterior to anterior fontanelle and -7.5 mm posterior to anterior fontanelle. The sections were soaked in distilled water, stained with aqueous solutions of cresyl violet for 10 minutes, washed with distilled water until colorless. They were differentiated with 95% alcohol for 30 seconds, rapidly dehydrated with absolute alcohol, permeabilized with xylene for 2 minutes, and mounted with neutral resin. Sections on ipsilateral and contralateral hemispheres were measured with ImageJ (NIH, Bethesda, MD, USA). The total infarct volume was calculated by the whole hemisphere area - infarct hemisphere area × the interval.
Immunofluorescence staining
Six rats were measured in each group. Using the free-floating method (Wang et al., 2015), brain sections were washed three times with 0.01 M PBS for 10 minutes each, blocked with 5% goat serum (Bioss Biotechnology, Beijing, China) for 90 minutes, incubated with Guinea pig anti-DCX polyclonal antibody (1:800; Millipore, Billerica, MA, USA) at 4°C overnight, and then washed three times with 0.01 M PBS for 10 minutes each. All samples were incubated with Alexa Fluor 488 goat anti-guinea pig IgG (1:800; Invitrogen, Carlsbad, CA, USA) at room temperature for 2 hours in the dark, and washed three times with 0.01 M PBS for 10 minutes each. Afterwards, all sections were mounted with anti-fluorescence quenching mounting medium. Five sections through the dentate gyrus of each rat were quantified with NIH ImageJ software (Kralic et al., 2005). The dendritic development of doublecortin (DCX)-positive cells in the hippocampal dentate gyrus was observed with a laser scanning confocal microscope (FV1000; Olympus, Tokyo, Japan). The three-dimensional structure of dendrites was reconstructed after a series of layers of DCX-positive cells in the Z-axis direction were magnified 200-fold under the confocal microscope. Dendrites and their branches were measured and described with NIH ImageJ software (Stumm and Höllt, 2007).
Statistical analysis
Data were expressed as the mean ± SD and analyzed with SPSS 17.0 for windows (SPSS, Chicago, IL, USA). One-way analysis of variance or repeated measures one-way analysis of variance was used to judge the statistical significance of the difference. A value of P < 0.05 was considered statistically significant.
Results
Bumetanide could not reduce the infarct size in rats with focal cerebral ischemia
ET-1 injection could evidently induce infarction. The infarct volume was similar between the ISC and BUM + ISC groups (125.8 ± 8.2 mm3vs. 129.8 ± 15.2 mm3; P > 0.05; Figure 3).
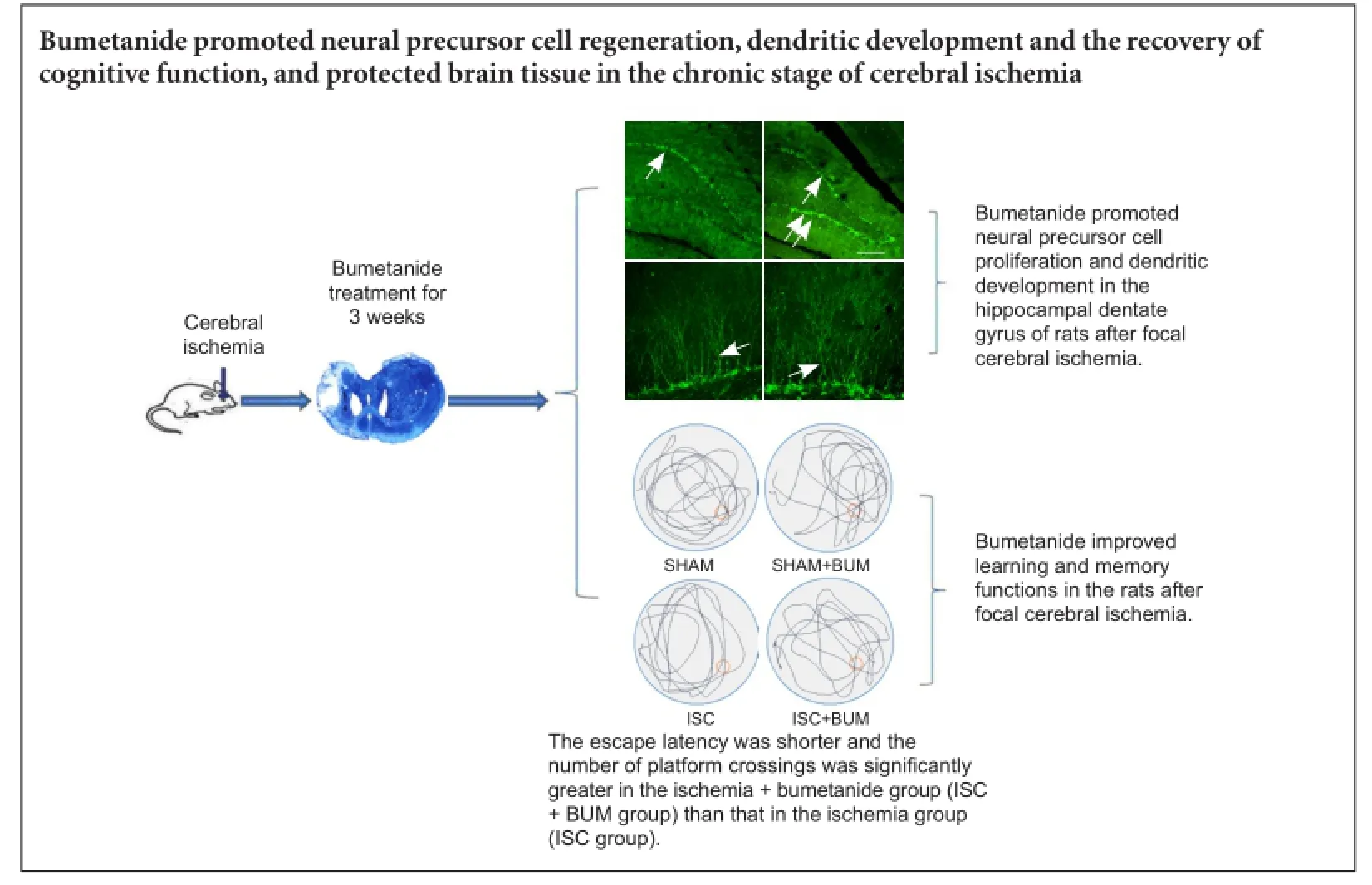
Bumetanide promoted neural precursor cell proliferation and dendritic development in the hippocampal dentate gyrus of rats after focal cerebral ischemia
DCX is a marker for neural precursor cells (Zhao et al., 2013). The number of DCX-positive cells and their dendritic morphology could be used to observe neural stem cell proliferation and dendritic development. Compared with the SHAM group, the number of DCX-positive cells and the total length of dendrites were significantly higher in the hippocampal dentate gyrus in the ISC group (P < 0.01). Compared with the ISC group, the number of DCX-positive cells and the total length of dendrites were significantly greater in the ISC + BUM group (P < 0.01). No significant difference in the number of DCX-positive cells and the total length of dendrites was detected between the SHAM and SHAM + BUM groups (P > 0.05; Figure 4).
Bumetanide improved learning and memory functions in rats after focal cerebral ischemia
Compared with the SHAM and SHAM + BUM groups, escape latency was longer and the number of platform crossings was significantly less in the ISC group (P < 0.05). The escape latency was shorter and the number of platform crossings was significantly more in the ISC + BUM group than in the ISC group (P < 0.05; Figure 5).
Discussion
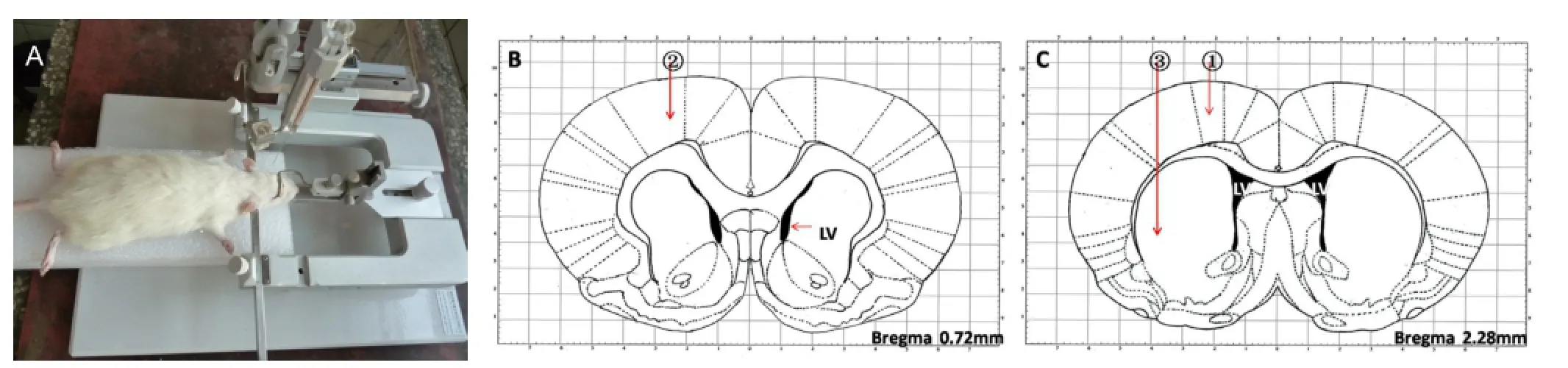
Figure 1 Rat model of cerebral ischemia by intracranial injection with ET-1.
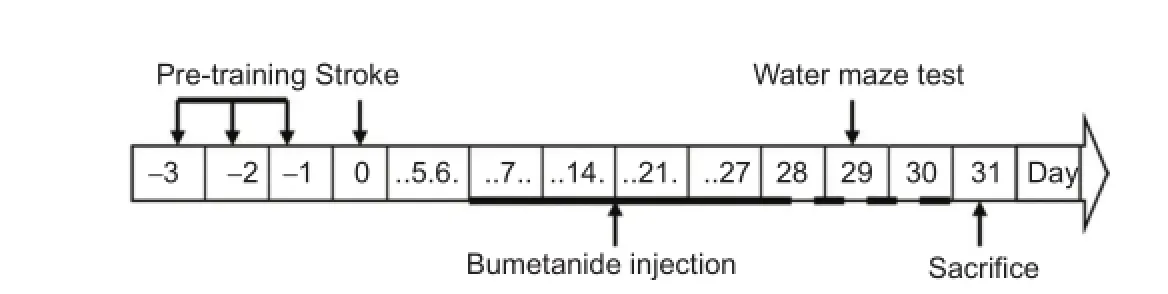
Figure 2 Time chart of the whole study.
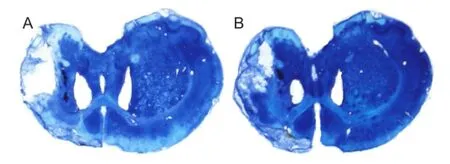
Figure 3 Effects of bumetanide on infarct volume in rats with focal cerebral ischemia (Nissl staining).

Figure 5 Bumetanide effects on learning and memory functions in rats with cerebral ischemia.
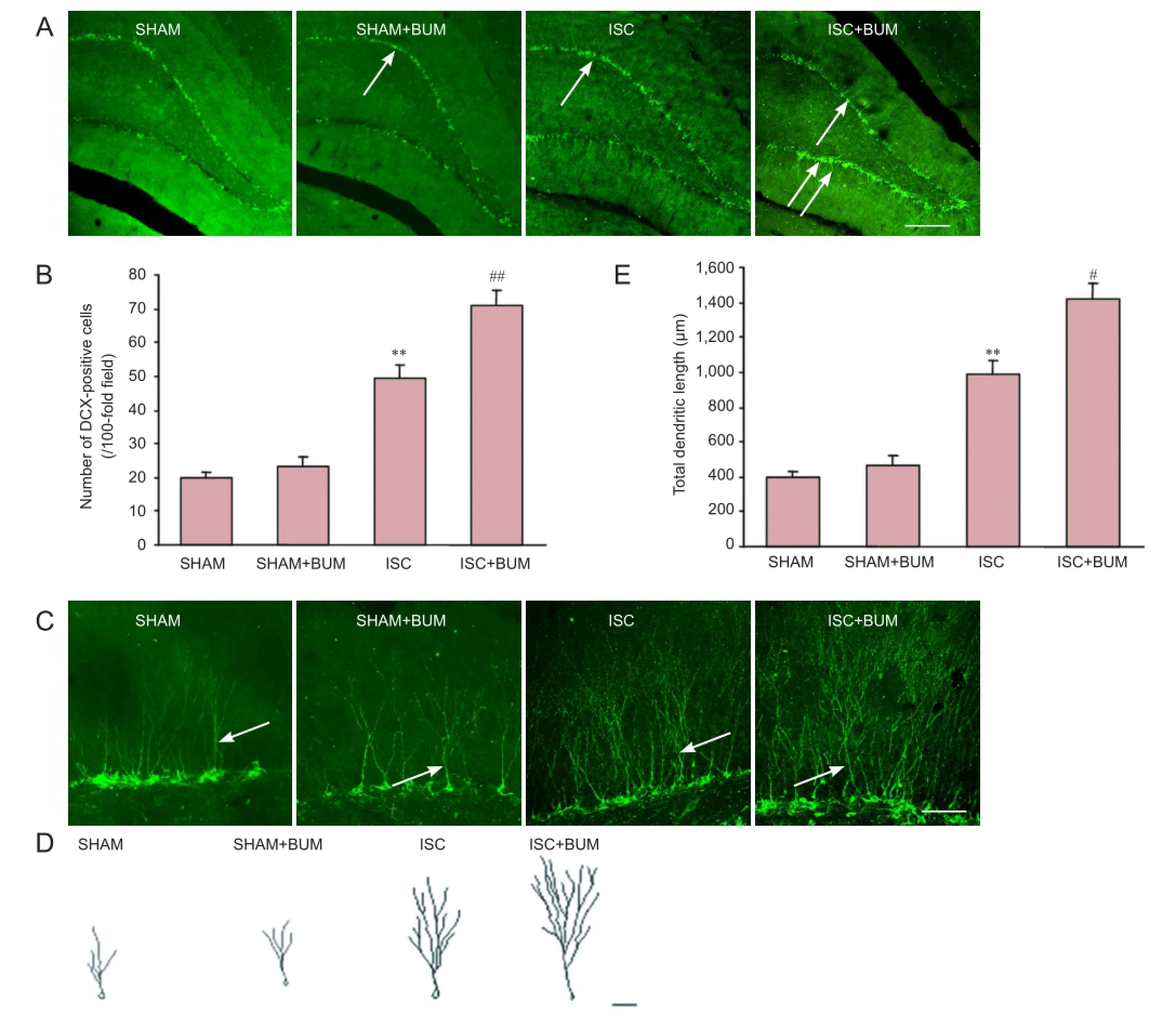
Figure 4 Bumetanide effects on neural precursor cell proliferation and dendritic development in the hippocampal dentate gyrus of rats with cerebral ischemia.
Cerebral ischemia has been shown to promote nerve regeneration in the hippocampal dentate gyrus (Jin et al., 2001; Takasawa et al., 2002; Türeyen et al., 2004; Wang et al., 2011). Wurm et al. (2007) considered that cerebral ischemia contributed to the dendritic development and altered dendritic morphology in newborn nerve cells of the hippocampal dentate gyrus. We also confirmed that nerve regeneration and dendritic development appeared in the hippocampal dentate gyrus after focal cerebral ischemia. The application of bumetanide could further promote the above changes, but bumetanide did not exert its effect in sham operation, which suggested that bumetanide does not promote nerve regeneration under normal conditions. These results indicate that after cerebral ischemia, the application of bumetanide produced a microenvironment, which was beneficial to the regeneration and survival of nerve cells, exerting a neuroprotective effect.
We explored the underlying mechanism, that is to say, the presence of NKCC1 that mediates Cl-influx and KCC2 that mediates Cl-efflux on the cell membrane of the central nervous system (Kahle and Staley, 2008). KCC2 is expressed in mature neurons, whereas NKCC1 is mainly expressed in immature neurons (Vannucci and Perlman, 1997). GABA is an important neurotransmitter in the central nervous system, and plays a biological role by binding to a GABAAreceptor. The ionotropic receptor GABAAis in a class of ligand-gated ion channels, and expressed on cell membranes. Because KCC2 is mainly expressed in normal mature neurons, after GABAAreceptor is activated, extracellular Cl-concentration increases and membrane hyperpolarization occurs, playingthe role of an inhibitory synapse (Ge et al., 2007).
Jaenisch et al. (2010) confirmed that NKCC1 expression increased, but KCC2 expression reduced after focal cerebral ischemia. After the GABAAreceptor was activated, the Clchannel on the cell membrane was opened, allowing a large influx of Cl-from the high extracellular concentration to low intracellular concentration and caused membrane depolarization; here GABA plays the role of an excitatory synapse (Nabekura et al., 2002; Blaesse et al., 2009).
A large number of neural precursor cells proliferate after cerebral ischemia, but only a few neural precursor cells differentiate further and survive for a long period (Arvidsson et al., 2002; Ohab et al., 2006; Thored et al., 2006). The expression of NKCC1 was found mainly in cell membranes of immature neurons (Jaenisch et al., 2010). Bumetanide, an antagonist of NKCC1, blocks NKCC1 on the cell membrane in the central nervous system, and reduces the influx of Cl-(Blaesse et al., 2009). Therefore, bumetanide has greater effects on regenerating neural precursor cells. NKCC1 activation-induced intracellular Cl-concentration increase is the main reason for GABA-mediated immature neuronal excitability. Depolarization of GABAA-mediated immature neurons may be the reason for the low survival rate of regenerated nerve cells after cerebral infarction.
GABA-mediated depolarization can cause nerve cell regeneration and migration (Ben-Ari, 2002; Owens and Kriegstein, 2002). However, cell membrane depolarization can lead to Ca2+influx (Nabekura et al., 2002; Toyoda et al., 2003). This leads to a large amount of Ca2+influx. Intracellular calcium overload is detrimental to cell survival. Kristián and Siesjö (1998) verified that after cerebral ischemia, increased Ca2+could trigger the death of nerve cells. Nerve cells begin to regenerate after cerebral infarction, but the survival rate of nerve cells is low over a longer period, which is consistent with the above mechanism. Bumetanide produces factors that are not conducive to nerve regeneration and survival, possibly by inhibiting NKCC1 and blocking GABA depolarization, but the precise mechanism remains poorly understood.
Our water maze test results demonstrated that bumetanide contributed to the recovery of learning and memory functions of rats with cerebral ischemia. It is unclear whether the regeneration of hippocampal dentate gyrus neurons is associated with learning and memory functions. Previous studies have confirmed that neural stem cells in the subventricular zone and hippocampal dentate gyrus could proliferate, migrate and differentiate into neurons and glial cells to replace damaged neurons after cerebral ischemia (Zhang et al., 2008; Zhao et al., 2008). The hippocampus is a part of the brain that is involved in learning and memory formation. The maintenance of normal hippocampus-dependent learning and memory function requires the involvement of new neurons, and nerve regeneration in the hippocampus can enhance learning and memory functions (Gould et al., 1999). Nerve regeneration probably promotes functional recovery, and plays an important role in the generation of spatial memory and object recognition memory (Broadbent et al., 2004). The above findings are consistent with our results. It remains controversial whether neurogenesis is directly associated with functional improvement (Ohab et al., 2006; Jaenisch et al., 2010; Minnerup et al., 2011).
In conclusion, bumetanide promoted nerve cell regeneration in the hippocampal dentate gyrus and dendritic development of neural precursor cells. It also improved learning and memory functions in rats with cerebral ischemia. These findings provide a theoretical basis for the application of bumetanide in the treatment of cerebral ischemia.
Author contributions: WSX designed the study, collected data and wrote the paper. XS participated in immunofluorescence staining, data analysis and literature consultation. CGS and XPM participated in animal model establishment, sample collection and water maze test. WPM participated in statistical analysis of water maze test data. XHZ analyzed experimental data and oversaw the experiment. CSZ designed and conceived the study, and served as a principle investigator. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963-970.
Ben-Ari Y (2002) Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci 3:728-739.
Blaesse P, Airaksinen MS, Rivera C, Kaila K (2009) Cation-chloride cotransporters and neuronal function. Neuron 61:820-838.
Broadbent NJ, Squire LR, Clark RE (2004) Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A 101:14515-14520.
Danzer SC (2008) Postnatal and adult neurogenesis in the development of human disease. Neuroscientist 14:446-458.
Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS (2008) Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci 28:11785-11791.
Furuta A, Rothstein JD, Martin L (1997) Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci 17:8363-8375.
Ge S, Pradhan DA, Ming GL, Song H (2007) GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci 30:1-8.
Gould E, Tanapat P, Hastings NB, Shors TJ (1999) Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci 3:186-192.
Jaenisch N, Witte OW, Frahm C (2010) Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke 41:e151-159.
Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA (2001) Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A 98:4710-4715.
Kahle KT, Staley KJ (2008) The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures. Neurosurg Focus 25:E22.
Kralic JE, Ledergerber DA, Fritschy JM (2005) Disruption of the neurogenic potential of the dentate gyrus in a mouse model of temporal lobe epilepsy with focal seizures. Eur J Neurosci 22:1916-1927.
Kristián T, Siesjö BK (1998) Calcium in ischemic cell death. Stroke 29:705-718.
Lee HA, Hong SH, Kim JW, Jang IS (2010) Possible involvement of DNA methylation in NKCC1 gene expression during postnatal development and in response to ischemia. J Neurochem 114:520-529.
Lu KT, Wu CY, Yen HH, Peng JH, Wang CL, Yang YL (2007) Bumetanide administration attenuated traumatic brain injury through IL-1 overexpression. Neurol Res 29:404-409.
Minnerup J, Kim JB, Schmidt A, Diederich K, Bauer H, Schilling M, Strecker JK, Ringelstein EB, Sommer C, Schöler HR, Schäbitz WR (2011) Effects of neural progenitor cells on sensorimotor recovery and endogenous repair mechanisms after photothrombotic stroke. Stroke 42:1757-1763.
Nabekura J, Ueno T, Okabe A, Furuta A, Iwaki T, Shimizu-Okabe C, Fukuda A, Akaike N (2002) Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J Neurosci 22:4412-4417.
O'Donnell ME, Lam TI, Tran L, Anderson SE (2004) The role of the blood-brain barrier Na-K-2Cl cotransporter in stroke. Adv Exp Med Biol 559:67-75.
Ohab JJ, Fleming S, Blesch A, Carmichael ST (2006) A neurovascular niche for neurogenesis after stroke. J Neurosci 26:13007-13016.
Owens DF, Kriegstein AR (2002) Is there more to gaba than synaptic inhibition? Nat Rev Neurosci 3:715-727.
Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates. London: Academic Press.
Shulga A, Magalhães AC, Autio H, Plantman S, di Lieto A, Nykjær A, Carlstedt T, Risling M, Arumäe U, Castrén E, Rivera C (2012) The loop diuretic bumetanide blocks posttraumatic p75NTR upregulation and rescues injured neurons. J Neurosci 32:1757-1770.
Soleman S, Yip P, Leasure JL, Moon L (2010) Sustained sensorimotor impairments after endothelin-1 induced focal cerebral ischemia (stroke) in aged rats. Exp Neurol 222:13-24.
Stumm R, Höllt V (2007) CXC chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J Mol Endocrinol 38:377-382.
Türeyen K, Vemuganti R, Sailor KA, Bowen KK, Dempsey RJ (2004) Transient focal cerebral ischemia-induced neurogenesis in the dentate gyrus of the adult mouse. J Neurosurg 101:799-805.
Takasawa K, Kitagawa K, Yagita Y, Sasaki T, Tanaka S, Matsushita K, Ohstuki T, Miyata T, Okano H, Hori M, Matsumoto M (2002) Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J Cereb Blood Flow Metab 22:299-307.
Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O (2006) Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 24:739-747.
Toyoda H, Ohno K, Yamada J, Ikeda M, Okabe A, Sato K, Hashimoto K, Fukuda A (2003) Induction of NMDA and GABAA receptor-mediated Ca2+ oscillations with KCC2 mRNA downregulation in injured facial motoneurons. J Neurophysiol 89:1353-1362.
Vannucci RC, Perlman JM (1997) Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics 100:1004-1114.
Walther M, Juenger H, Kuhnke N, Wilke M, Brodbeck V, Berweck S, Staudt M, Mall V (2009) Motor cortex plasticity in ischemic perinatal stroke: a transcranial magnetic stimulation and functional MRI study. Pediatr Neurol 41:171-178.
Wang C, Zhang M, Sun C, Cai Y, You Y, Huang L, Liu F (2011) Sustained increase in adult neurogenesis in the rat hippocampal dentate gyrus after transient brain ischemia. Neurosci Lett 488:70-75.
Wang G, Huang H, He Y, Ruan L, Huang J (2014) Bumetanide protects focal cerebral ischemia-reperfusion injury in rat. Int J Clin Exp Pathol 7:1487-1494.
Wang S, Zhang XQ, Song CG, Xiao T, Zhao M, Zhu G, Zhao CS (2015) In vivo effects of bumetanide at brain concentrations incompatible with NKCC1 inhibition on newborn DGC structure and spontaneous EEG seizures following hypoxia-induced neonatal seizures. Neuroscience 286:203-215.
Wurm F, Keiner S, Kunze A, Witte OW, Redecker C (2007) Effects of skilled forelimb training on hippocampal neurogenesis and spatial learning after focal cortical infarcts in the adult rat brain. Stroke 38:2833-2840.
Zhang RL, Zhang ZG, Chopp M (2008) Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology 55:345-352.
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645-660.
Zhao SS, Zhao Y, Xiao T, Zhao M, Jolkkonen J, Zhao CS (2013) Increased neurogenesis contributes to the promoted behavioral recovery by constraint-induced movement therapy after stroke in adult rats. CNS Neurosci Ther 19:194-196.
Copyedited by Dawes EA, Haase R, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.182700 http://www.nrronline.org/
How to cite this article: Xu WS, Sun X, Song CG, Mu XP, Ma WP, Zhang XH, Zhao CS (2016) Bumetanide promotes neural precursor cell regeneration and dendritic development in the hippocampal dentate gyrus in the chronic stage of cerebral ischemia. Neural Regen Res 11(5)∶745-751.
Accepted: 2016-02-25
*Correspondence to: Chuan-sheng Zhao, M.D., xws921@sina.com; cszhao@mail.cmu.edu.cn.
杂志排行
中国神经再生研究(英文版)的其它文章
- Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Antibody-based neuronal and axonal delivery vectors for targeted ligand delivery
- Coordination of the axonal cytoskeleton during the emergence of axon collateral branches
- Alzheimer's disease: the silver tsunami of the 21stcentury
- Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells
