Induced pluripotent stem cells for modeling and cell therapy of Parkinson's disease
2016-12-02MáriaCsbnyeiová,uboDaniovi,tefanPolák
PERSPECTIVE
Induced pluripotent stem cells for modeling and cell therapy of Parkinson's disease
Neurodegenerative disorders include a variety of hereditary or sporadic diseases involving the chronic, progressive loss on neural tissue. Parkinson's disease (PD) is the second most common neurodegenerative disease, affecting more than 6 million people worldwide (Wan et al., 2015). Degeneration of nigrostriatal dopamine (DA) neurons is the main pathology in PD, although other dopaminergic and non-dopaminergic systems are also affected. Characteristic symptoms are rigidity, hypokinesia, tremor, and postural instability. The loss of DA neurons is accompanied by lewy bodies and lewy neuritis, which are mainly formed by insoluble aggregates of alpha-synuclein and Tau protein and might restrain the survival and development of newborn neurons. Etiology of PD remains unclear, however interactions between environmental and genetic factors are believed to cause the loss of nigral DA neurons and ensuing locomotor system. Research indicated that increasing level of iron, oxidative stress, mitochondrial and ubiquitin-proteasome system dysfunction, inflammation, and apoptosis may lead to the progression of PD (Pu et al., 2012; Zhu et al., 2016).
The majority of PD cases are sporadic or idiopathic (80—90%), but a minority of cases (10—20%) are familial and can be linked to particular monogenic mutation associated to PD related genes. Monogenic forms of PD account only for small percentage of PD cases. However, understanding how mutation of PD related genes causes the degeneration of DA neurons is essential for the study of disease mechanism. The most frequent mutation represents G2019S mutation of the leucine rich repeat kinase 2 (LRRK2) gene (Nguyen et al., 2011). Many mouse models and postmortem tissue studies have provided insight into pathogenesis of PD, however, the former consistently fail to recapitulate the cardinal features of PD and the latter are end-stage representations (Marchetto et al., 2011)
There is currently no effective medication to treat PD. Drug therapies only provide relief of symptoms and have unpredictable side effects. Although motor symptoms can be treated relatively well with L-3,4-dihydroxyphenylalanine (L-DOPA), DA agonist, dopamine inhibitor carbidopa, and deep brain stimulation to the nucleus subthalamicus, effective therapies for nonmotor symptoms, such as dementia, are lacking, and disease progression cannot be counteracted. Another possibility to cure PD is fetal tissue transplantation. For example, Kordower et al. (2008) published a case report of patient with PD to whom was implanted fetal nigral tissue into the striatum. Study demonstrated that implanted dopamine neurons can survive and reinervate the striatum. However, there are several issues with the use of fetal tissue, such as difficulties in obtaining and ethical concern (Freeman 1997). Stem cell-based therapies for neurodegenerative disorders are particularly attractive, given the limited regenerative capacity of mammalian neurons (Kriks et al., 2011; Han et al., 2015). Clinical trials with transplantation of human fetal ventral midbrain neural stem cells into PD brains have provided proof of principle that neuronal replacement can be effective in some PD patients. However, the ethical issues of human fetal tissue limit its widespread clinical use (Zhu et al., 2016).
The generation of induced pluripotent stem cells (iPSCs) that can be derived from the adult cells of specific patients, has recently revolutionized the field posing hopes that some of the obstacles traditionally associated with stem cells therapy (immune compatibility, ethical issues, purity of cells) could be overcome (Takahashi et al., 2007). Successful reprogramming of somatic cells to a pluripotent state by transient expression of four transcription factors (Oct4, Sox2, Klf4, and c-myc) was achieved for the first time with mouse cells in 2006 (Takahashi and Yamanaka, 2006). However, retroviral insertion can cause genome damage resulting in tumor formation. Since the seminal iPSC work by Dr. Yamanaka and colleagues, the field has rapidly moved forward. In order to retain genome integrity during reprogramming, various techniques have been developed to generate insertion free iPSCs, such as integrative free vectors (piggyBac transposon, plasmid/episomal plasmid vectors, minicircle vectors), and non-integrating methods (direct protein/microRN delivery, small molecules) (Tanabe et al., 2014). However, there still remain safety concerns in the terms of more suitable and harmful endogenous genetic and epigenetic alterations that may occur during reprogramming of iPSCs, since cell growth pathway could be activated and tumor suppressor pathways could be also inhibited after using small molecule cocktail (Oh et al., 2012). The causes of mutagenesis resulting in cellular dysfunction or tumorigenesis are usually retro- or lenti-viral transduction systems using in generation of iPSCs. Moreover, iPSCs can retain gene expression pattern of the cell type of origin. Recently were reported also high variability among iPSC lines including increased levels of aneuploidy, defects in X-chromosome inactivation and presence of point mutations. Thus, in order to use iPSCs for treating human disease, it is necessary to assess the safety of the cells before clinical applications (Marchetto et al., 2011; Cai et al., 2014).
The advent of iPCSs transforms the negative outlook associated with neurodegenerative disease. Reprogramming technology allows researchers to study the development and progression of neurodegeneration in a human system and may enable the discovery of new diagnostics and cell-based therapies. The human neurodevelopmental and neurodegenerative disease can be studied in live neurons in a controlled environment (Figure 1). The major advantage of iPSCs approach is the potential to develop human cell-based disease models of sporadic and genetically complex disease such as PD. Moreover, PD is very interesting model for application of iPSC technology, because protocols for generating DA neurons are relatively robust and reproducible (Soldner et al., 2009; Kriks et al., 2011).
Soldner et al. (2009) derived human iPSCs from skin biopsies obtained from patients with idiopathic PD and developed a robust reprogramming protocol allowing reproducible generation of patient-specific stem cells with efficient removal of transgene sequences. From patient-specific iPSCs were generated of midbrain DA neurons that have the same genetic composition as the patients and share many important properties with the nigral DA neurons (Pu et al. 2012).
One of the first iPSCs model of genetic PD involved a patient triplication in the alpha-synuclein gene was reported by Byers et al. (2011). In the study, DA neurons showed accumulation of alpha-synuclein, overexpression of markers of oxidative stress and enhanced sensitivity to cell toxicity induced by hydrogen peroxide compartment with controls. Triplication of alpha-synuclein gene causes a fully penetrant, aggressive form of PD with dementia because of alpha-synuclein dysfunction.
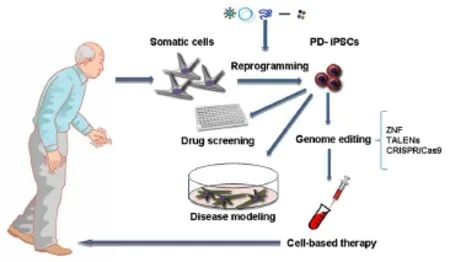
Figure 1 Potential therapeutic applications of PD-iPSCs.
Nguyen et al. (2011) described the first biologically relevant cellular phenotype from iPSCs derived neurons from PD patients. In this study, iPSCs were derived from one 60-year-old female PD patient carryinga point mutation in LRRK2, the most common PD-related mutation. iPSCs were differentiated into DA neurons in parallel with iPSCs from one control patient. Researchers found that DA neurons from PD patient expressed increased levels of alpha-synuclein, and showed increased sensitivity to cellular stressors, such as hydrogen peroxide, MG-132 and 6-hydroxydopamine. Comparably, PARK2 iPSC-derived neurons exhibited mitochondrial dysfunction associated with increased oxidative stress and alpha-synuclein accumulation, resembling pathogenic changes in patient brains (Mochizuki et al., 2014).
iPSC technology may also facilitate identification of therapeutic compounds by elucidating authentic signaling pathways in diseased human neurons rather than artificial models. For example, Cooper et al. (2012) focused on mitochondrial functions in PD associated with mutation in PINK1 and LRRK2 genes. They found that iPSC-derived neurons are more sensitive to chemical toxins valinomycin and concanamycin A. Subsequently, the iPSC-derived neurons were treated with the antioxidant coenzyme Q10, rapamycin or the LRRK2 inhibitor GW5074 caused partial protection against neural degeneration. More recently, nitrosative/oxidative stress was found to cause mitochondrial dysfunction and apoptotic cell death in DA neurons with A53T alpha-synuclein mutation through S-nitrosylation of transcription factor MEF2C, which could be well targeted by a small molecule, isoxazole (Ryan et al., 2013).
Clinical application of iPSC-derived neurons for treatment of PD is still distant option. The challenge for a more comprehensive study of epigenetic and genetic characteristic of iPSC-derived neurons mainly lies in the necessity to generate a cell population that allows purification based on highly specific midbrain DA marker. An outstanding concern is whether it is prudent to use a patient's own cells to derive DA neurons for therapy, in view of their presumed susceptibility to developing PD pathology. Another challenge of modeling late-onset age-related disease, such as PD or AD, is that culture models can typically last only a couple of months, whereas the human disease likely progress very slowly, over decades. Recently, artificial ageing, including long-term culture and expression of progerin, was utilized to induce phenotypes in iPSC- derived neurons to reproduce features of PD. Investigators found that expression of progerin in iPSC-derived fibroblasts and neurons induced multiple aging-related markers and characteristics, including dopamine-specific phenotypes such as neuromelanin accumulation (Miller et al., 2013).
It will be critical to select differentiation protocol that produce the right type of DA neurons both in vivo and in vitro in animal model. During characterization of cells through differentiation stages, there is a need to show robust expression of midbrain DA neuron lineage markers (TH, Nurr1, Foxa2, Lmx1a, Pitx3, Engrailed1, Engrailed2), as well as mature neuronal markers in the final product. Recently has arisen field of gene-editing tools involving zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and RNA-guided clusters regularly interspaced short palindromic repeats (CRISPR)/associated protein 9 nuclease (Cas9) (Cong et al., 2013; Cao et al., 2014). The ZNFs and TALENs consist of endonucleases fused to DNA-binding domain, while the CRISPR/Cas9 system uses guide RNAs to target bacterial Cas9 endonuclease to desired genome location. The common mechanism of these gene-editing techniques are a specific DNA recognition component linked to an enzyme inducing strand breaks in DNA (Fontes and Lakshmipathy, 2013; Pellagatti et al., 2016). The successful correction of the G2019S point mutation in two patient-derived iPSCs by ZFN technology was published by Reinhardt et al., (2013). Their results suggest possible targets for the development of new therapeutic for patient with PD. Despite promising evidence of differentiation, maturation, and integration of grafted cells into the neural circuitry in animal models, more extensive research needs to be done to further characterize patient-specific iPSC-derived DA neurons in term of completeness and stability of their differentiated state.
The work was supported by grant VEGA No. 1/0153/15. Lubos Danisovic is CEO of REGENMED Ltd. Mária Csöbönyeiová and Štefan Polák declares no conflict of interest.
Mária Csöbönyeiová, Ľuboš Danišovič*, Štefan Polák
Institute of Histology and Embryology, Faculty of Medicine, Comenius University in Bratislava, Bratislava, Slovakia (Csöbönyeiová M, Polák Š) Institute of Medical Biology, Genetics and Clinical Genetics, Faculty of Medicine, Comenius University in Bratislava, Bratislava, Slovakia (Danišovič L)
*Correspondence to: Ľuboš Danišovič, Ph.D., lubos.danisovic@fmed.uniba.sk.
Accepted: 2016-04-12
orcid: 0000-0002-5074-9621 (Ľuboš Danišovič)
Byers B, Cord B, Nguyen HN, Schüle B, Fenno L, Lee PC, Deisseroth K, Langston JW, Pera RR, Palmer TD (2011) SNCA triplication Parkinson's patient's iPSC-derived DA neurons accumulate α-synuclein and are susceptible to oxidative stress. PLoS One 6:e26159.
Cai S, Chan YS, Shum DK (2014) Induced pluripotent stem cells and neurological disease models. Sheng Li Xue Bao 66:55-66.
Cao L, Tan L, Jiang T, Zhu XC, Yu JT (2015) Induced pluripotent stem cells for disease modeling and drug discovery in neurodegenerative diseases. Mol Neurobiol 52:244-255.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819-823.
Cooper O, Seo H, Andrabi S, Guardia-Laguarta C, Graziotto J, Sundberg M, McLean JR, Carrillo-Reid L, Xie Z, Osborn T, Hargus G, Deleidi M, Lawson T, Bogetofte H, Perez-Torres E, Clark L, Moskowitz C, Mazzulli J, Chen L, Volpicelli-Daley L, et al. (2012) Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson's disease. Sci Transl Med 4:141ra90.
Fontes A, Lakshmipathy U (2013) Advances in genetic modification of pluripotent stem cells. Biotechnol Adv 31:994-1001.
Freeman TB (1997) From transplants to gene therapy for Parkinson's disease. Exp Neurol 144:47-50.
Han F, Baremberg D, Gao J, Duan J, Lu X, Zhang N, Chen Q (2015) Development of stem cell-based therapy for Parkinson's disease. Transl Neurodegener 4:16.
Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB (2008) Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord 23:2303-2306.
Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L (2011) Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480:547-551.
Marchetto MC, Brennand KJ, Boyer LF, Gage FH (2011) Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Hum Mol Genet 20:R109-R115.
Miller JD, Ganat YM, Kishinevsky S, Bowman RL, Liu B, Tu EY, Mandal PK, Vera E, Shim JW, Kriks S, Taldone T, Fusaki N, Tomishima MJ, Krainc D, Milner TA, Rossi DJ, Studer L (2013) Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell 13:691-705
Oh Y, Wei H, Ma D, Sun X, Liew R (2012) Clinical application of patientspecific induced pluripotent stem cells in cardiovascular medicine. Heart 98:443-449.
Pellagatti A, Dolatshad H, Yip BH, Valletta S, Boultwood J (2016) Application of genome editing technologies to the study and treatment of hematological disease. Adv Biol Regul 60:122-134.
Pu J, Hiang H, Zhang B, Feng J (2012) Redefining Parkinson's disease research using induced pluripotent stem cells. Curr Neurol Neurosci Rep 12:392-398.
Reinhardt P, Schmid B, Burbulla LF, Schöndorf DC, Wagner L, Glatza M, Höing S, Hargus G, Heck SA, Dhingra A, Wu G, Müller S, Brockmann K, Kluba T, Maisel M, Krüger R, Berg D, Tsytsyura Y, Thiel CS, Psathaki OE, et al. (2013) Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell 12:354-367.
Ryan SD, Dolatabadi N, Chan SF, Zhang X, Akhtar MW, Parker J, Soldner F, Sunico CR, Nagar S, Talantova M, Lee B, Lopez K, Nutter A, Shan B, Molokanova E, Zhang Y, Han X, Nakamura T, Masliah E, Yates JR 3rd, et al. (2013) Isogenic human iPSC Parkinson's model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription. Cell 155:1351-1364.
Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O, Jaenisch R (2009) Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136:964-977.
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663-676.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861-872.
Tanabe K, Takahashi K, Yamanaka S (2014) Induction of pluripotency by defined factors. Proc Jpn Acad Ser B Phys Biol Sci 90:83-96.
Wan W, Cao L, Kalionis B, Xia S, Tai X (2015) Applications of induced pluripotent stem cells in studying the neurodegenerative diseases. Stem Cells Int 2015:382530.
Zhu B, Caldwell M, Song B (2016) Development of stem cell-based therapies for Parkinson's disease. Int J Neurosci 29:1-13.
10.4103/1673-5374.182692 http∶//www.nrronline.org/
How to cite this article: Csöbönyeiová M, Danišovič L, Polák Š (2016) Induced pluripotent stem cells for modeling and cell therapy of Parkinson’s disease. Neural Regen Res 11(5):727-728.
杂志排行
中国神经再生研究(英文版)的其它文章
- Possible application of apolipoprotein E-containing lipoproteins and polyunsaturated fatty acids in neural regeneration
- Recovery of injured fornical crura following neurosurgical operation of a brain tumor: a case report
- Antibody-based neuronal and axonal delivery vectors for targeted ligand delivery
- Coordination of the axonal cytoskeleton during the emergence of axon collateral branches
- Alzheimer's disease: the silver tsunami of the 21stcentury
- Clinical trial perspective for adult and juvenile Huntington's disease using genetically-engineered mesenchymal stem cells
