Rhesus monkey neural stem cell transplantation promotes neural regeneration in rats with hippocampal lesions
2016-12-01LijuanYeHuiBianYaodongFanZhengboWangHualinYuYuanyeMaFengChenDepartmentofPathologyThirdAffiliatedHospitalofKunmingMedicalUniversityKunmingYunnanProvinceChinaKunmingInstituteofZoologyChineseAcademyofSciencesKunmingY
Li-juan Ye, Hui Bian, Yao-dong Fan Zheng-bo Wang Hua-lin Yu,, Yuan-ye Ma, Feng Chen Department of Pathology, Third Affiliated Hospital of Kunming Medical University, Kunming, Yunnan Province, China Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, Yunnan Province, China Department of Physiology, Kunming Medical University, Kunming, Yunnan Province, China Second Department of Neurosurgery, First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan Province, China5 Department of Radiology, Hainan General Hospital, Haikou, Hainan Province, China
Rhesus monkey neural stem cell transplantation promotes neural regeneration in rats with hippocampal lesions
Li-juan Ye1,2,4, Hui Bian3, Yao-dong Fan1, Zheng-bo Wang2, Hua-lin Yu4,*, Yuan-ye Ma2,*, Feng Chen5,*
1 Department of Pathology, Third Affiliated Hospital of Kunming Medical University, Kunming, Yunnan Province, China
2 Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, Yunnan Province, China
3 Department of Physiology, Kunming Medical University, Kunming, Yunnan Province, China
4 Second Department of Neurosurgery, First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan Province, China
5 Department of Radiology, Hainan General Hospital, Haikou, Hainan Province, China
How to cite this article: Ye LJ, Bian H, Fan YD, Wang ZB, Yu HL, Ma YY, Chen F (2016) Rhesus monkey neural stem cell transplantation promotes neural regeneration in rats with hippocampal lesions. Neural Regen Res 11(9):1464-1470.
Funding: This work was supported by the National Natural Science Foundation of China, No. 31571109, 81460261; the Chinese-Finnish Joint Project Fund, No. 813111172; a grant from the Yunnan Key Program of Science and Technology of China, No. 2014FC005; the Key Science and Technology Research Project Fund of Hainan Province of China, No. ZDYF2016156; the National Clinical Key Subject Construction Project Fund of China.
Feng Chen, Ph.D.,
Yuan-ye Ma, Ph.D., or
Hua-lin Yu, Ph.D.,
fenger0802@163.com,
yuanma0716@sina.com, or yhl308@126.net.
orcid:
0000-0002-9129-7895
(Feng Chen)
0000-0001-5291-9367
(Yuan-ye Ma)
0000-0002-1105-3867
(Hua-lin Yu)
Accepted: 2016-08-02
Graphical Abstract
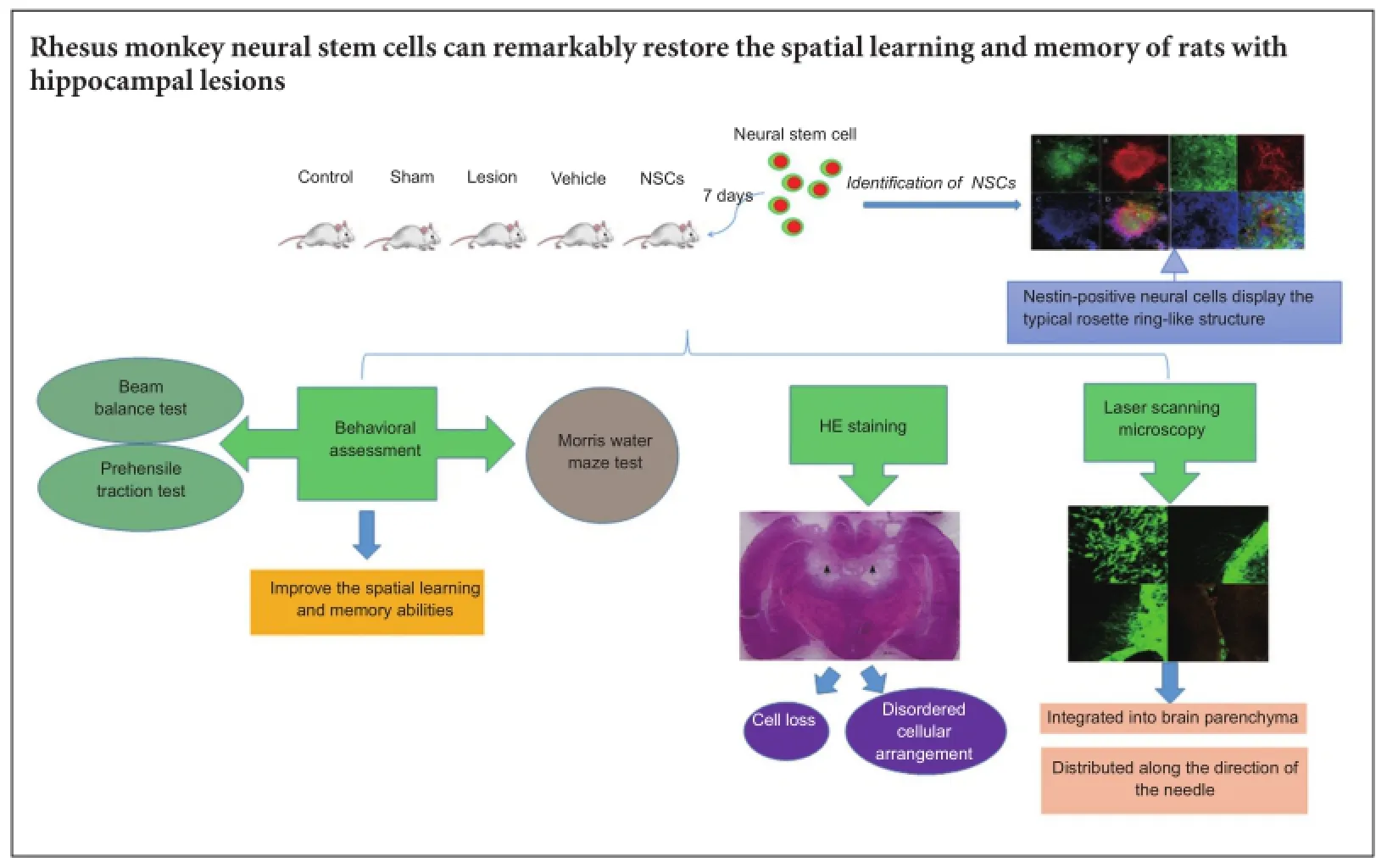
Rhesus monkey neural stem cells are capable of differentiating into neurons and glial cells. Therefore, neural stem cell transplantation can be used to promote functional recovery of the nervous system. Rhesus monkey neural stem cells (1 × 105cells/μL) were injected into bilateral hippocampi of rats with hippocampal lesions. Confocal laser scanning microscopy demonstrated that green fluorescent protein-labeled transplanted cells survived and grew well. Transplanted cells were detected at the lesion site, but also in the nerve fiber-rich region of the cerebral cortex and corpus callosum. Some transplanted cells differentiated into neurons and glial cells clustering along the ventricular wall, and integrated into the recipient brain. Behavioral tests revealed that spatial learning and memory ability improved, indicating that rhesus monkey neural stem cells noticeably improve spatial learning and memory abilities in rats with hippocampal lesions.
nerve regeneration; rhesus monkey; neural stem cells; hippocampal lesion; cell transplantation; spatial learning and memory abilities; neural regeneration
Introduction
Death of many neurons and astrocytes is a common characteristic of nervous system injury and degenerative disease (Decimo et al., 2012). Neural stem cell (NSC) replacement therapy may be a potential approach for treating nervous system injury and degenerative disease (Pluchino et al., 2005; Lindvall and Kokaia, 2006; Imitola, 2007; Simonato et al., 2013), as NSCs are self-renewing, multipotent cells that can give rise to neurons, astrocytes, and oligodendrocytes (Chaubey and Wolfe, 2013; Santamaria et al., 2015; Yuan et al., 2015). In 2012, a Japanese team was the first to transplant NSCs into a Parkinson’s disease model of the primate brain. They found that the implanted cells had an effect on dopaminergic neurons, and improved intention tremor symptoms (Doi et al., 2012). Additionally, American scientists established a spinal cord injury model, and transplanted oligodendrocyte progenitor cells, which originated from human embryonic stem cells, 7 days after injury. Accordingly, motor function recovered in rats transplanted with oligodendrocyte progenitor cells (Sharp et al., 2010). Previously, we obtained a high proportion of rhesus monkey NSCs by establishing a differentiation system using the embryoid body and single layer methods. Further, we found that long-tailed Macaca fascicularis NSCs implanted into the brain of rats or Macaca fascicularis can be kept alive and maintain certain functions (Takagi et al., 2005). However, there are differences among different primates (Conti and Cattaneo, 2010). Therefore, whether rhesus monkey and Macaca fascicularis NSCs show the same function needs to be confirmed.
Hippocampal integrity is necessary for spatial learning and memory (Squire and Zola-Morgan, 1991; Eichenbaum, 2000; D’Hooge and De Deyn, 2001). In this study, we transplanted rhesus monkey NSCs into rats subjected to hippocampal damage. We had two objectives: first, to observe development and differentiation of these cells in the rat hippocampus, and second, to observe recovery of spatial learning and memory in the rats.
Materials and Methods
Ethics statement and animals
Animal studies were approved by the Laboratory Animal Care and Use Committee of Kunming Institute of Zoology, Chinese Academy of Sciences (approval No. SYDW2009013), and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment.
Fifty-four adult male, specific-pathogen-free, Sprague-Dawley rats, aged 8—10 weeks and weighing 180—200 g, were purchased from the Experimental Animal Center of Kunming Medical University of China [license No. SCXK (Dian) 2005-0008]. All rats were allowed to acclimate to their housing environment for 1 week before beginning the study. Rats were kept in animal cages with free access to water and food. The temperature of the housing room was controlled at 23 ± 2°C with a 12:12 light/dark cycle (lights on 07:00—19:00). Rats were divided into the following five groups: control group (no treatment; n = 11), sham group (sham operation only; n = 11), lesion group (hippocampal injury only; n = 14), vehicle group (hippocampal injury + vehicle; n = 8), and NSCs group (hippocampal injury + NSCs; n = 10).
Animal model establishment
Rats were anesthetized with an intraperitoneal injection of sodium chloride solution (45 mg/kg; Sigma, St. Louis, MO, USA), and then placed in a brain stereotaxic instrument (Shenzhen Reward Life Technology Co., Ltd., Shenzhen, Guangdong Province, China). The head of each rat was conventionally shaved and disinfected with iodophor, and the middle scalp incised. Muscle and other tissues were retracted to expose the skull. Four holes (1-mm pore size) were then drilled in the left and right sides of the skull above the hippocampus. Coordinates for the rat dorsal hippocampus were determined according to the rat brain map (Morris, 1981): posterior 4 mm from bregma, mediolateral 2 mm and 3.4 mm from the midline, and 4 mm down from the skull. A single 0.3-mm-diameter stainless steel electrode was used, which was insulated except for its 0.5-mm pointed tip (Beijing Yidian Technology Co., Ltd., Beijing, China). The electrode was slowly inserted into the dorsal hippocampus perpendicular to the zero level of bregma below the skull. Direct current electrolysis (2 mA for 60 seconds) was performed using a direct current electrical damage gauge (Primate Cognitive Science Laboratory of Kunming Institute of Zoology, Chinese Academy of Sciences). The sham group was subjected to an identical procedure except that current was not passed through the electrode when it was inserted into the dorsal hippocampus. On completion of the surgical procedure, the incision was sutured using locally applied erythromycin ointment. Simultaneously, an intramuscular injection of penicillin sodium (4 × 106U) was administered to prevent infection.
Preparation of rhesus monkey NSCs
Rhesus monkey NSCs were provided by the Primate Cognitive Science Laboratory of Kunming Institute of Zoology, Chinese Academy of Sciences. Previously, we obtained a high proportion of NSCs using the embryoid body method (Thomson et al., 1998; Kuo et al., 2003) and single layer method (Salli et al., 2004). Specifically, a clone was selected by hand and cut into cell clusters containing approximately 1,000 cells. The suspension was cultured to form embryoid bodies, which were then transferred into neural differentiation medium defined by the extracellular matrix (Sigma). NSCs with rosette ring structures were selected, and the cell suspension concentration modulated to approximately 1 × 105cells/μL prior to use.
Transplantation of NSCs
Transplantation trials of green fluorescent protein (GFP)-labelled rhesus monkey NSCs (Calhoun et al., 2003) were performed 7 days after delivery of direct electrical current damage. Next, selected 5 μL NSC suspensions (including approximately 1 × 105cells/μL), with a rosettering structure, were slowly injected into the bilateral hippocampus of rats using a microinjector (Hamilton, Bonaduz, Grischun, Switzerland). The coordinates for NSC implantation were: 4 mm posterior from bregma, 2 mm mediolateral from the midline, and 4 mm below the skull. The injection speed was controlled at 1 μL/min. The needle was kept in situ for 3 minutes after transplantation, before slowly removing the microinjector. For the vehicle group, the surgical procedure was identical except for injection of 5 μL sterile PBS (0.1 M) via the microinjector, as the transplantation control. An intramuscular injection of cyclosporine A (Novartis Pharma Stein A G, Bonaduz, Grischun, Switzerland) (5 μg/g/d) was administered for 21 consecutive days from the day of transplantation.
Preparation of brain sections
Upon completion of the experiments, rats were deeply anesthetized with sodium chloride solution (45 mg/kg; Sigma), and perfused with 0.9% sodium chloride solution followed by 4% paraformaldehyde through the left ventricle. Each brain was carefully removed from the skull and postfixed in 4% paraformaldehyde. Brains were stored at 4°C until use, and dehydrated in a gradient sucrose solution (20% and 30%) at 4°C, and cut into 20-μm sections using a freezing-sliding microtome (CMI850, Leica, Frankfurt, Germany).
Observation by confocal laser scanning microscopy
Survival and distribution of the transplanted cells and differentiated rhesus monkey NSCs were examined by confocal laser scanning microscopy. The procedure was as follows: freezing-slides and differentiated rhesus monkey NSCs were dried for 10 minutes at room temperature, immersed in phosphate buffered saline (PBS) for 10 minutes, blocked in 5% normal goat serum (Gibco-Invitrogen, Carlsbad, CA, USA) in 0.1 M PBS with 0.2% Triton™ X-100 (Sigma) for 30 minutes, and then incubated in primary antibodies diluted in blocking solution overnight at 4°C. The primary antibodies used were: mouse anti-rat nestin, class III β-tubulin, and GFP monoclonal antibody (1:200; Invitrogen). After washing, the preparations were incubated in appropriate fluorescent-labeled goat anti-mouse IgG (1:200; Invitrogen) for 1 hour at room temperature. Primary antibodies were omitted in control experiments. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Sigma). Finally, slides were scanned using a confocal laser scanning microscope (LSM 510 META, ZEISS, Dresden, Germany).
现如今随着社会的进步与综合国力的增强,峨眉武术的特色身体文化作为一种通用的身体符号进行沟通、交流,大众可以无碍地参与练习、娱乐、修身,甚至借助于峨眉武术这种身体特色文化来了解巴蜀的文化魅力,探索峨眉武术独特的肢体语言,传承巴蜀文化中习武者所秉承的不服输的文化象征,全面凝结中国特色的传统民族文化。
Hematoxylin-eosin staining
Cryosections were recovered and dried at room temperature for 1 hour. Slides were immersed in distilled water for 2 minutes, stained with hematoxylin for 7 minutes, and then rinsed with water. After differentiation in hydrochloric alcohol and rinsing in water until blue, sections were stained with eosin for 1 minute, dehydrated briefly in 70% and 80% ethanol, then 95% ethanol I and 100% ethanol II for 1 minute each, followed by 100% ethanol I and 100% ethanol II for 2 minutes each. Sections were cleared in xylene I and II for 8 minutes each. To keep the sections wet, excessive xylene was removed from around the slides, and moderate neutral balsam quickly added. Finally, slides were covered with coverslips. Staining was examined by light microscopy (Leica) to identify hippocampal lesions.
Behavioral assessment
Motor ability tests were performed in each group 21 days after hippocampal injury.
Beam balance test
Both ends of a cylindrical beam (length 70 cm and diameter 2.5 cm) were fixed to a stainless steel bracket. The bracket height was adjusted to ensure it was parallel to and elevated 40 cm from the ground, on which cushions were placed to avoid injury to the rats. Rats were placed on the central portion of the beam and observed for 30 seconds. The balancing ability of each rat was evaluated according to a scoring code. Rats with normal motor ability were scored at 3. Lower beam balance test scores reflect poorer balancing ability (Combs and D’Alecy, 1987; Mattiasson et al., 2000).
Prehensile traction test
Both ends of a nylon rope (diameter, 0.5 cm) were fixed to a stainless steel bracket. The bracket height was adjusted to ensure it was parallel to and 40 cm elevated from the ground, on which cushions were placed to avoid injury to the rats. The rat was hung in the air with its forepaws on the nylon rope. Gripping ability of each rat was evaluated according to a scoring code. Rats with normal motor ability were scored at 3. Lower prehensile traction test scores reflect poorer gripping ability (Combs and D’Alecy, 1987; Mattiasson et al., 2000).
Morris water maze test
The Morris water maze test is dependent on spatial learning and memory (Morris, 1981, 1984), which is based on integrity of hippocampal structure and function (Miller et al., 2013). The water maze used in this trial was a circular tank (180 cm in diameter, 50 cm in height, and 31 cm in depth), which was divided equally into four quadrants. The rats were placed at four entry points (northwest, north, east, and southeast), following the procedure of Vorhees and Williams (2006). The escape platform was placed in the center of quadrant II (target quadrant) (Figure 1). Data collection and recording analysis of movement were performed using the automatic tracking system and software developed by the Primate Cognitive Science Laboratory of Kunming Institute of Zoology, Chinese Academy of Sciences.
Rats were placed into the water maze and orientated by facing the wall. They were allowed to swim freely for 120 seconds to adapt to the environment. If the rat did not find the platform within 120 seconds, it was guided to the platform and allowed to remain for 60 seconds. The hidden platform was used for 5 or 7 consecutive days, four times every day. Next, rats were placed into the water from the center ofquadrant III (opposite to the target quadrant) and given a 120-second probe time. Average latency of the rat to find the hidden platform, number of times crossing the platform location in the probe test, percentage of swimming time in the four quadrants (for a duration of 12 seconds), and number of times the rat swam past the escape platform location were recorded (Vorhees and Williams, 2014).
Statistical analysis
Data expressed as the mean ± SEM were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Escape latency in the water maze test was analyzed using repeated measures analysis of variance. Percentage of swimming time in each quadrant in the spatial probe trial was analyzed by one-way analysis of variance. Comparison of both groups and number of times crossing the escape platform in the spatial probe trial were analyzed by non-pairwise t-tests. Statistical significance was set at P ≤ 0.05.
Results
Hippocampal morphology in lesioned rats
Hematoxylin-eosin staining revealed alterations in histological structure of the hippocampus in the lesion group, with destruction of the pyramidal cell layer, cell loss, and a disordered cellular arrangement. The main lesion was located in and surrounding the dorsal hippocampus (Figure 2).
After 12 days, rhesus monkey NSCs had differentiated into nestin-positive neural cells displaying a typical rosette ring-like structure (Figure 3A-D). During dedifferentiation, cells began to differentiate and exhibited a filamentous morphology, characteristic of long neuronal axons. Immunocytochemical staining showed positive expression of class III β-tubulin protein, a cellular neuronal protein marker (Figure 3E-H).
Effect of rhesus monkey NSC transplantation on spatial learning and memory abilities in rats with hippocampal lesions
Behavioral ability
There were no statistical differences between groups in the beam balance test (P > 0.05; data not shown) or prehensile traction test (P > 0.05; data not shown). In the hidden platform trial of the Morris water maze test, average escape latency in each group gradually decreased along with increased training time. Compared with the vehicle and lesion groups, mean escape latency was significantly reduced in the NSC group on days 4 to 8 (P < 0.01 or P < 0.05). Compared with the sham group, mean escape latency in the NSC group increased on days 6 and 7 (P < 0.01 or P < 0.05; Figure 4A).
In the spatial probe trial, there was a significant increase in the number of times a rat crossed the platform in the NSC group compared with the vehicle group (P < 0.01; Figure 4B). Further, percentage of swimming time in the target quadrant was not higher in the NSC group. Accordingly, there was no significant difference compared with the vehicle group (P > 0.05), although there was a significant difference between the NSC and sham groups (P < 0.05; Figure 4C). Hippocampal lesions Confocal laser scanning microscopy showed that a number of cells had long neuronal axons (Figure 5A). GFP-labeled cells with long axons grew on the surface of the brain (Figure 5B). A large amount of GFP-labeled cells grew and integrated into the brain parenchyma (Figure 5C). Some GFP-labelled cells had distributed along the direction of the injection needle (Figure 5D).
Discussion
Effect of rhesus monkey NSC transplantation on spatial learning and memory abilities in hippocampal-lesioned rats
Here, we show that transplantation of rhesus monkey NSCs improves spatial learning and memory in hippocampal-lesioned rats. This is consistent with findings from Japanese and American researchers, who have succeeded in recovering motor ability in animal models by transplanting human embryonic stem cells (Sharp et al., 2010). Multiple studies (Sinson et al., 1996; Riess et al., 2002) have demonstrated that cell replacement therapy contributes to movement recovery in injured animals, yet as movement recovery has been the predominant focus, the influence on the brain is unknown. In our previous study (Dong et al., 2012), we obtained rhesus monkey NSCs that had differentiated from embryonic stem cells in vitro. In particular, at the early stage, these cells could be applied to transplantation research. In our current experiment, reduction of mean escape latency in the NSC group shows that NSC transplantation improves spatial learning ability in lesioned rats. Also, increased number of platform crosses in the NSC group shows enhanced ability to accurately locate the platform in the NSC group, indicating that NSC transplantation may improve spatial memory in injured rats. However, the percentage of swimming time in the target quadrant did not increase. Thus, damaged spatial memory did not completely recover after transplantation, perhaps because it was not so strengthened enough for slow extraction of memory information (Miller et al., 2013), although this needs further confirmation. Our findings are not only conducive to investigating the potential application of NSCs, but also provide an experimental basis and data for treatment of neural regeneration and injury clinically.
Effect of transplantation time on transplantation results
We transplanted rhesus monkey NSCs 1 week after injury. Increasing evidence suggests that successful cell replacement therapy should consider the time window of transplantation (Lindvall and Kokaia, 2010), as there is a serious inflammatory reaction because of acute injury. During this particular pathological state, the signaling balance of the internal environment is destroyed, and the resulting high concentration of inflammatory mediators and many cytokines are not conducive to transplanted cell survival. Consequently, the acute phase is not appropriate for transplantation (Ryu et al., 2009). In the chronic stage of injury, glial scar formation can lead to regeneration difficulties, and is not suitable for transplantation (Calhoun et al., 2003; Mochizuki et al., 2008).About 1 week after injury, the acute phase of injury is over, but the glial scar has not yet formed. Moreover, the local microenvironment is favorable for survival of transplanted cells, and is a good opportunity for transplantation (Harting et al., 2009). Therefore, 1 week after injury is a suitable NSC transplantation time, and likely has an important effect on the success of transplantation. For further in vitro studies, it will be beneficial to examine transplantation time.

Figure 2 Bilateral dorsal hippocampi injured by electrolysis (hematoxylineosin staining).

Figure 3 Identification of rhesus monkey NSCs.
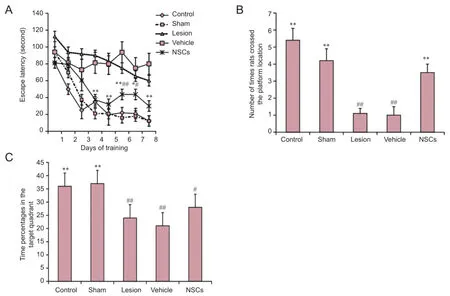
Figure 4 Effect of rhesus monkey NSC transplantation on behavioral ability of hippocampal-lesioned rats in the Morris water maze test.

Figure 5 Effect of rhesus monkey NSC transplantation in hippocampal-lesioned rats.
For heteroplastic transplantation, a high quantity of NSCs is not necessarily beneficial to transplantation. When human-derived NSCs were transplanted into the brain of adult rats in concentrations of 2 × 105, 1 × 106, and 2 × 106cells, Ostenfeld et al. (2000) found that growth and survival in the 2 × 105cells group was best. This may be due to the low concentration of cells in the transplantation group being more conducive to axonal growth, and with a smaller number of cells, there will be a smaller immune rejection (Hernández-Benítez et al., 2012). NSC implantation at the original lesion site can reduce injury time of the second brain injury (Davie and Petersen, 2012; Ramasamy et al., 2013). Relevant measures to ensure full survival of transplanted cells include all of the above in our study.
Future studies must face the challenge of finding the mechanisms underlying the observed improvements: (1) reasons for behavioral improvement; (2) differentiation of cells in the brain post-transplantation and establishment of in vivo function; (3) whether neural growth factor is produced by NSCs; (4) effect of transplanted NSCs and host cells; and (5) how these cells alter behavioral function by interacting other downstream factors.
Author contributions: LJY and HB were responsible for animal surgery, cell preparation, data processing and manuscript preparation. ZBW participated in data integrity, accuracy and statistics. YYM and FC were responsible for study design and study supervision. HLY and YDF were responsible for data collection and manuscript preparation. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Calhoun JD, Lambert NA, Mitalipova MM, Noggle SA, Lyons I, Condie BG, Stice SL (2003) Differentiation of rhesus embryonic stem cells to neural progenitors and neurons. Biochem Biophys Res Commun 306:191-197.
Chaubey S, Wolfe JH (2013) Transplantation of CD15-enriched murine neural stem cells increases total engraftment and shifts differentiation toward the oligodendrocyte lineage. Stem Cells Transl Med 2:444-454.
Combs DJ, D’Alecy LG (1987) Motor performance in rats exposed to severe forebrain ischemia: effect of fasting and 1,3-butanediol. Stroke 18:503-511.
Conti L, Cattaneo E (2010) Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci 11:176-187.
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Rev 36:60-90.
Davie E, Petersen J (2012) Environmental control of cell size at division. Curr Opin Cell Biol 24:838-844.
Decimo I, Bifari F, Krampera M, Fumagalli G (2012) Neural stem cell niches in health and diseases. Curr Pharm Des 18:1755-1783.
Doi D, Morizane A, Kikuchi T, Onoe H, Hayashi T, Kawasaki T, Motono M, Sasai Y, Saiki H, Gomi M, Yoshikawa T, Hayashi H, Shinoyama M, Refaat MM, Suemori H, Miyamoto S, Takahashi J (2012) Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson’s disease. Stem Cells 30:935-945.
Dong JR, Guo LY, Qu JG, Qi RL, Wang WC, Xiao CJ, Wang ZB (2012) Rhesus monkey embryonic stem cells differentiation, proliferation and allotransplantation. Dongwuxue Yanjiu 33:43-48.
Eichenbaum H (2000) A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 1:41-50.
Harting MT, Sloan LE, Jimenez F, Baumgartner J, Cox CS Jr (2009) Subacute neural stem cell therapy for traumatic brain injury. J Surg Res 153:188-194.
Hernández-Benítez R, Ramos-Mandujano G, Pasantes-Morales H (2012) Taurine stimulates proliferation and promotes neurogenesis of mouse adult cultured neural stem/progenitor cells. Stem Cell Res 9:24-34.
Imitola J (2007) Prospects for neural stem cell-based therapies for neurological diseases. Neurotherapeutics 4:701-714.
Kuo HC, Pau KY, Yeoman RR, Mitalipov SM, Okano H, Wolf DP (2003) Differentiation of monkey embryonic stem cells into neural lineages. Biol Reprod 68:1727-1735.
Lindvall O, Kokaia Z (2006) Stem cells for the treatment of neurological disorders. Nature 441:1094-1096.
Lindvall O, Kokaia Z (2010) Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest 120:29-40.
Mattiasson GJ, Philips MF, Tomasevic G, Johansson BB, Wieloch T, McIntosh TK (2000) The rotating pole test: evaluation of its effectiveness in assessing functional motor deficits following experimental head injury in the rat. J Neurosci Methods 95:75-82.
Miller JF, Neufang M, Solway A, Brandt A, Trippel M, Mader I, Hefft S, Merkow M, Polyn SM, Jacobs J, Kahana MJ, Schulze-Bonhage A (2013) Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science 342:1111-1114.
Mochizuki N, Takagi N, Onozato C, Moriyama Y, Takeo S, Tanonaka K (2008) Delayed injection of neural progenitor cells improved spatial learning dysfunction after cerebral ischemia. Biochem Biophys Res Commun 368:151-156.
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47-60.
Morris RG (1981) Spatial localization does not require the presence of local cues. Learn Motiv 12:239-260.
Ostenfeld T, Caldwell MA, Prowse KR, Linskens MH, Jauniaux E, Svendsen CN (2000) Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Exp Neurol 164:215-226.
Pluchino S, Zanotti L, Deleidi M, Martino G (2005) Neural stem cells and their use as therapeutic tool in neurological disorders. Brain Res Rev 48:211-219.
Ramasamy S, Narayanan G, Sankaran S, Yu YH, Ahmed S (2013) Neural stem cell survival factors. Arch Biochem Biophys 534:71-87.
Riess P, Zhang C, Saatman KE, Laurer HL, Longhi LG, Raghupathi R, Lenzlinger PM, Lifshitz J, Boockvar J, Neugebauer E, Snyder EY, McIntosh TK (2002) Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery 51:1043-1054.
Ryu JK, Cho T, Wang YT, McLarnon JG (2009) Neural progenitor cells attenuate inflammatory reactivity and neuronal loss in an animal model of inflamed AD brain. J Neuroinflammation 6:39.
Salli U, Reddy AP, Salli N, Lu NZ, Kuo HC, Pau FK, Wolf DP, Bethea CL (2004) Serotonin neurons derived from rhesus monkey embryonic stem cells: similarities to CNS serotonin neurons. Exp Neurol 188:351-364.
Santamaria S, Garcia-Sanz JA (2015) Insights of the brain damage response using antibodies identifying surface antigens on neural stem cells and neuroblasts. Neural Regen Res 10:1574-1575.
Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS (2010) Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 28:152-163.
Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF, Gray SJ, Lowenstein PR, Vandenberghe LH, Wilson TJ, Wolfe JH, Glorioso JC (2013) Progress in gene therapy for neurological disorders. Nat Rev Neurol 9:277-291.
Sinson G, Voddi M, McIntosh TK (1996) Combined fetal neural transplantation and nerve growth factor infusion: effects on neurological outcome following fluid-percussion brain injury in the rat. J Neurosurg 84:655-662.
Squire L, Zola-Morgan S (1991) The medial temporal lobe memory system. Science 253:1380-1386.
Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N (2005) Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest 115:102-109.
Thomson JA, Marshall VS, Trojanowski JQ (1998) Neural differentiation of rhesus embryonic stem cells. APMIS 106:149-156; discussion 156-157.
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848-858.
Vorhees CV, Williams MT (2014) Assessing spatial learning and memory in rodents. ILAR J 55:310-332.
Yuan LL, Guan YJ, Ma DD, Du HM (2015) Optimal concentration and time window for proliferation and differentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours. Neural Regen Res 10:1516-1522.
Copyedited by James R, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.191221
*Correspondence to:
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Blood microRNAs as potential diagnostic and prognostic markers in cerebral ischemic injury
- Recovery of corticospinal tract injured by traumatic axonal injury at the subcortical white matter: a case report
- Sigma-1 receptor and neuroprotection: current outlook and potential therapeutic effects
- Intra-axonal protein synthesis - a new target for neural repair?
- Nanobiomaterials for neural regeneration
- Cell transplantation for the treatment of spinal cord injury — bone marrow stromal cells and choroid plexus epithelial cells
