Cell transplantation for the treatment of spinal cord injury — bone marrow stromal cells and choroid plexus epithelial cells
2016-12-01ChizukaIdeNorihikoNakanoKenjiKanekiyoCentralResearchLaboratoryAinoUniversitySchoolofHealthScienceIbarakiOsakaJapan
Chizuka Ide, Norihiko Nakano, Kenji KanekiyoCentral Research Laboratory, Aino University School of Health Science, Ibaraki, Osaka, Japan
Cell transplantation for the treatment of spinal cord injury — bone marrow stromal cells and choroid plexus epithelial cells
Chizuka Ide*, Norihiko Nakano, Kenji Kanekiyo
Central Research Laboratory, Aino University School of Health Science, Ibaraki, Osaka, Japan
How to cite this article: Ide C, Nakano N, Kanekiyo K (2016) Cell transplantation for the treatment of spinal cord injury — bone marrow stromal cells and choroid plexus epithelial cells. Neural Regen Res 11(9):1385-1388.
Funding: This work was supported in part by grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (No. 2300125 to CI, No. 15K10957 to NN, and No. 26870744 to KK).
Chizuka Ide, M.D., Ph.D.,
c-ide@ot-u.aino.ac.jp.
Accepted: 2016-06-15
Transplantation of bone marrow stromal cells (BMSCs) enhanced the outgrowth of regenerating axons and promoted locomotor improvements of rats with spinal cord injury (SCI). BMSCs did not survive long-term, disappearing from the spinal cord within 2—3 weeks after transplantation. Astrocyte-devoid areas, in which no astrocytes or oligodendrocytes were found, formed at the epicenter of the lesion. It was remarkable that numerous regenerating axons extended through such astrocyte-devoid areas. Regenerating axons were associated with Schwann cells embedded in extracellular matrices. Transplantation of choroid plexus epithelial cells (CPECs) also enhanced axonal regeneration and locomotor improvements in rats with SCI. Although CPECs disappeared from the spinal cord shortly after transplantation, an extensive outgrowth of regenerating axons occurred through astrocyte-devoid areas, as in the case of BMSC transplantation. These findings suggest that BMSCs and CPECs secret neurotrophic factors that promote tissue repair of the spinal cord, including axonal regeneration and reduced cavity formation. This means that transplantation of BMSCs and CPECs promotes “intrinsic” ability of the spinal cord to regenerate. The treatment to stimulate the intrinsic regeneration ability of the spinal cord is the safest method of clinical application for SCI. It should be emphasized that the generally anticipated long-term survival, proliferation and differentiation of transplanted cells are not necessarily desirable from the clinical point of view of safety.
bone marrow stromal cell; choroid plexus epithelial cell; spinal cord injury; axonal regeneration; locomotor improvement; intrinsic regeneration ability
Introduction
Many kinds of somatic cells have been studied as transplants for the treatment of spinal cord injury, which include bone marrow stromal cells (BMSCs) (Ohta et al., 2004), Schwann cells (Williams and Bunge, 2012), olfactory ensheathing cells (Li et al., 2003; Iwatsuki et al., 2008), dental pulp-derived cells (Sakai et al., 2012), adipose-derived stromal cells (Arboleda et al., 2011), epidermal neural crest stem cells (Sieber-Blum et al., 2006), skin-derived precursor cells (Biernaskie et al., 2007), and choroid plexus epithelial cells (Ide et al., 2001). Among them, we have been studying the transplantation of BMSCs and choroid plexus epithelial cells (CPECs) for the treatment of the contusion-injured spinal cord of rats. BMSCs and CPECs were transplanted either directly into the spinal cord lesion, or indirectly by infusing them through the cerebrospinal fluid (CSF) via the 4thventricle.
With either method of transplantation, engrafted cells do not survive long-term, but disappear from the spinal cord 2—3 weeks after transplantation. Nevertheless, locomotor improvements and tissue repair, including axonal regeneration, were enhanced, suggesting that transplanted cells do not serve as scaffolds for regenerating axons, but secrete some neurotrophic factors effective for the promotion of the“intrinsic” regeneration capacity of the spinal cord.
Some somatic cells, such as Schwann cells, reportedly survive, but, in many cases, gradually decrease in number after transplantation. Usually, they show moderate proliferation, migration, and some differentiation after transplantation. They are well-integrated in the host spinal cord tissue (Deng et al., 2015). These properties of somatic cells guarantee their safety after transplantation in the host spinal cord. In comparison, immature cells such as neural stem/progenitor cells (NSPCs) exhibit an extensive capacity for proliferation, differentiation, and migration after transplantation in the spinal cord. The methods, by which the behaviors of NSPCs can be properly manipulated and controlled to be appropriately integrated into the host spinal cord should be developed before NSPCs can be used for clinical purposes.
Bone Marrow Stromal Cells
BMSCs are cells that adhere to the dish on culture of a bone marrow perfusate. Ohta et al. (2004) studied the indirect transplantation of BMSCs through the CSF in rats with acute SCI, showing that transplanted BMSCs were conveyed through the CSF to the spinal cord, where most BMSCs attached to the spinal cord surface, while a few invaded the lesion. Locomotor behaviors of rats were improved, and cavity formation in the spinal cord lesion was suppressed. Transplanted BMSCs did notsurvive long-term to be integrated into the host spinal cord, but disappeared from the spinal cord within 3 weeks after injection. There were no findings suggesting the proliferation, differentiation, or migration of transplanted BMSCs. The CSF harvested from the rats in which BMSCs had been injected 2 days previously promoted the neurosphere cells to adhere to the culture dish and spread to the periphery. These findings suggest that BMSCs exert effects by releasing some trophic factors into the CSF. Nakano et al. (2010) showed that conditioned medium (CM) of bone marrow stromal cells contained insulin-like growth factor-1 (IGF-1), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and transforming growth factor beta-1 (TGFβ-1).
Subsequently, a study on the direct transplantation of BMSCs into the spinal cord lesion of sub-acute SCI (2 weeks post-injury) showed that locomotor behaviors of rats clearly improved, and cavity formation in the spinal cord was markedly reduced (Ide et al., 2010). The spinal cord lesion, in which engrafted BMSCs were located, was not stained by immunohistochemistry for glial fibrillary acidic protein (GFAP). Accordingly, the lesion appeared as a large, empty cavity by immunohistochemistry. However, such astrocyte-devoid areas were, in fact, filled with extracellular matrices, through which numerous axons associated with Schwann cells extended longitudinally (Figure 1). The finding that axons extended through the extracellular matrices in the astrocyte-devoid areas was an unexpected one: regenerating axons with characteristics of peripheral nerve fibers extended through the astrocyte-devoid area, and further grew beyond the borders of the astrocyte-devoid area into the surrounding host spinal cord tissue.
To examine whether the indirect transplantation of BMSCs was effective for sub-acute (1—2 weeks post-injury) and chronic (4 weeks post-injury) SCI, BMSCs were injected three times (once weekly) into the CSF via the 4thventricle (Nakano et al., 2013). Although transplanted BMSCs disappeared from the spinal cord within 7 days after transplantation, numerous axons were found extending longitudinally in the astrocyte-devoid areas of the spinal cord lesion filled with extracellular matrices, including collagen fibrils. Numerous axons surrounded by Schwann cells extended through the extracellular matrices. Locomotor functions were improved, and the cavity formation was reduced. These findings were the same as those obtained in the previous studies. These studies show that, although they do not survive long-term disappearing shortly after transplantation, BMSCs exert an effect on axonal outgrowth and locomotor improvement. It is hypothesized that BMSCs might secrete some neurotrophic factors effective for tissue repair, including axonal regeneration, in the spinal cord lesion.
The effect of transplantation of bone marrow mononuclear cells (BMNCs) for spinal cord injury was also studied. BMNCs were separated by density-gradient centrifugation from a bone marrow perfusate, and used for transplantation without cell culture. The indirect transplantation of BMNCs through the CSF enhanced locomotor improvement and tissue repair, while they disappeared shortly after transplantation, as in the case of BMSC transplantation (Yoshihara et al., 2008). These basic studies of transplantation of BMSCs and BMNCs led to the clinical application of these cells. Cells were indirectly transplanted into the CSF by lumbar puncture for patients. So far, the transplantation of BMSCs has been performed for 5 patients (Saito et al., 2012), and the transplantation of BMNCs has been performed for 10 patients (Suzuki et al., 2014). These series of cell transplantation studies confirmed the safety of the clinical transplantation of BMSCs and BMNCs. Unlike BMSCs, BMNCs can be used for transplantation without cell culture. This is a great benefit for BMNCs. BMNCs can be separated from a bone marrow perfusate of a patient, and transplanted autologously without cell culture to the patient by lumbar puncture in an operation room. BMNCs do not need an expensive cell processing facility.
Choroid Plexus Epithelial Cells
The choroid plexus (CP) takes part in the formation of the ventricular wall of the brain. The CP consists of epithelial cells and the underlying vascular-rich pia mater. The CP, by producing the CSF, may contribute to the maintenance of the normal environment of the central nervous system (CNS). It has been reported that the CP is the primary gate for trafficking immune cells from the vascular system to the CSF in CNS impairment (Kunis et al., 2015). The transplantation study using minced CP as a transplant showed that the CP enhanced axonal regeneration in the spinal cord (Ide et al., 2001). This result suggests that the CP can be used as a transplant to efficiently promote axonal regeneration in the spinal cord. The co-culture of CPECs with neurons showed that neurons associated with CPECs extended extensive neurites on the surface of CPECs (Chakrabortty et al., 2000; Kimura et al., 2004). Another study showed that conditioned medium of CPEC culture enhanced neuronal survival and neurite extension of hippocampal neurons (Watanabe et al., 2005). These studies show that CPECs have dual effects, i.e., cell contact and humoral trophic effects, to enhance neurite extension. Matsumoto et al. (2010) demonstrated that the infusion of cultured CPECs through the CSF markedly reduced the size of ischemic lesions caused by middle cerebral artery ligation. In this experiment, no CPECs entered the ischemic areas, suggesting that cultured CPECs exerted their effects by releasing some neurotrophic factors in the CSF.
Recently, we carried out a study on CPEC transplantation, in which cultured CPECs were directly injected into the spinal cord lesion (Kanekiyo et al., 2016). The spinal cord was contusion-injured at T8—9, and, 1 week after injury, the cultured CPECs were transplanted into the spinal cord lesion. CPECs, although they are neuronal cells, showed only shortterm survival (Figure 2), and disappeared from the spinal cord within 2—3 weeks. This finding was unexpected for us. Before experiments, it was anticipated that CPECs, ependymal—lineage neural cells, could survive long-term in the spinal cord. The disappearance of CPECs may be due to a harsh environment of the lesion, and/or an immunological reaction, since the rats used for the experiment were the closed colony SD strain. However, the locomotor functions were significantly improved, and cavity formation was reduced. Numerous axons extended through the astrocyte-devoid areas, as described in the case of BMSC transplantation (Figure 3). There was no finding suggesting an astrocyte scar at the border of the lesion. Regenerating axons appeared to extend smoothly through the border areas. These findings are the same as those found in the case of BMSC transplantation. CPECs exhibited no differentiation after transplantation. The important findings are that, although both BMSCs andCPECs do not survive long-term to be integrated into the host spinal cord tissue, locomotor improvements and axonal regeneration are promoted by their transplantation. The transplantation of these cells might elicit and promote the“intrinsic” ability of the spinal cord to regenerate. It is suggested that neurotrophic factors secreted from BMNCs and CPECs are responsible for these regenerating effects.
The fact that BMSCs and CPECs did not survive longterm, disappearing shortly after transplantation, appeared to be a disadvantage. However, on the contrary, these properties are desirable from the clinical point of view of safety: transplants do not exert undesirable extra-effects to threaten the safety of recipients.

Figure 1 One week after bone marrow stromal cells (BMSC) transplantation.
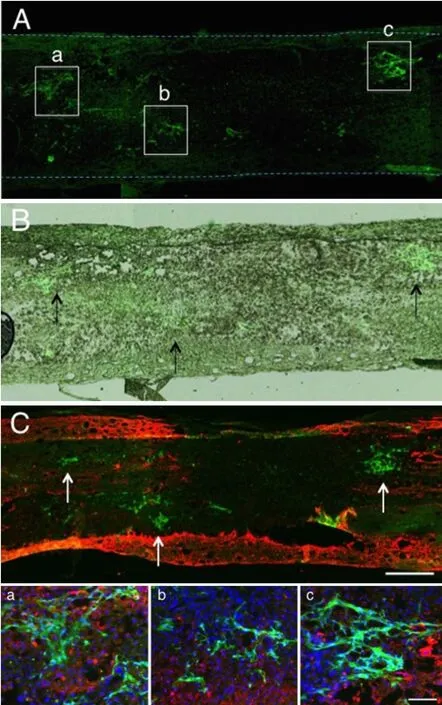
Figure 2 Two days after green fluorescent protein (GFP)-labeled choroid plexus epithelial cell (CPEC) transplantation.

Figure 3 Two weeks after choroid plexus epithelial cell (CPEC) transplantation.
Neural Stem/Progenitor Cells
Our previous studies of the transplantation of NSPCs showed the problems of immature cell transplantation. NSPCs survived long-term, proliferated, and differentiatedinto neuronal cells (Bai et al., 2003; Wu et al., 2003). Lu et al. (2014) demonstrated that human induced pluripotent stem cells-derived NSPCs survived long term, proliferated, and differentiated into neurons that extended axons over long distances in spinal cord injury. These properties appeared to be an advantage of transplants. In fact, cell transplantation studies have a premise that the transplanted cells should survive to be integrated into the host spinal cord, and contribute to axonal regeneration, leading to the establishment of neural circuits in the host spinal cord. However, at present, the proliferation, differentiation, and migration of the transplants cannot be manipulated and controlled to allow NSPCs to be appropriately integrated into the host spinal cord tissue. In addition, regarding functional recovery, NSPC transplantation is not necessarily effective for locomotor improvement. Locomotor improvement is an essential parameter for the clinical application of cell transplantation.
Conclusion
The transplantation of somatic cells such as bone marrow stromal cells and choroid plexus epithelial cells promotes axonal regeneration and enhances locomotor improvements. However, they do not survive long term after transplantation into the spinal cord. This suggests that some neurotrophic factors are released from those transplants to accelerate axonal regeneration through the astrocyte-devoid area formed in the epicenter of the lesion. Neurotrophic factors stimulate the “intrinsic“ ability of the spinal cord to regenerate. This concept is important for the treatment of SCI. The intrinsic regeneration ability of the spinal cord includes the outgrowth of regenerating axons that are associated with Schwann cells through extracellular matrices in astrocyte-devoid areas formed at the epicenter of the lesion.
Author contributions: CI, NN, and KK discussed an outline of this review. CI wrote the manuscript, NN and KK prepared data and micrographs, and analyzed related references.
Conflicts of interest: None declared.
References
Arboleda D, Forostyak S, Jendelova P, Marekova D, Amemori T, Pivonkova H, Masinova K, Sykova E (2011) Transplantation of predifferentiated adipose-derived stromal cells for the treatment of spinal cord injury. Cell Mol Neurobiol 31:1113-1122.
Bai H, Suzuki Y, Noda T, Wu S, Kataoka K, Kitada M, Ohta M, Chou H, Ide C (2003) Dissemination and proliferation of neural stem cells on the spinal cord by injection into the fourth ventricle of the rats. J Neurosci Methods 124:181-187.
Biernaskie J, Sparling JS, Liu J, Shannon CP, Plemel JR, XieY, Miller FD, Tetzlaff W (2007) Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci 27:9545-9559.
Chakrabortty SL, Kitada M, Matsumoto N, Taketomi M, Kimura K, Ide C (2000) Choroid plexus ependymal cells enhance neurite outgrowth from dorsal root ganglion neurons in vitro. J Neurocytol 29:707-717.
Deng LX, Walker C, Xu XM (2015) Schwann cell transplantation and descending propriospinal regeneration after spinal cord injury. Brain Res 1619:104-114.
Ide C, Kitada M, Chakrabortty S, Taketomi M, Matsumoto N, Kikukawa S, Mizoguchi A, Kawaguchi S, Endo K, Suzuki Y (2001)Grafting of choroid plexus ependymal cells promotes the growth of regenerating axons in the dorsal funiculus of rat spinal cord- a preliminary report. Exp Neurol 167:242-251.
Ide C, Nakai Y, Nakano N, Seo TB, Yamada Y, Endo K, Noda T, Saito F, Suzuki Y, Fukushima M, Nakatani T (2010) Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res 1332:32-47.
Iwatsuki K, Yoshimine T, Kishima H, Aoki M, Yoshimura K, Ishihara M, Ohnishi Y, Lima C (2008) Transplantation of olfactory mucosa following spinal cord injury promotes recovery in rats. Neuroreport 19:1249-1259.
Kanekiyo K, Nakano N, Noda N, Yamada Y, Suzuki Y, Ohta M, Yokota A, Fukushima M, Ide C (2016) Transplantation of choroid plexus epithelial cells into contusion-injured spinal cord of rats. Restor Neurol Neurosci 34: 347-366.
Kimura K, Matsumoto N, Kitada M, Mizoguchi A, Ide C (2004) Neurite outgrowth from hippocampal neurons is promoted by choroid plexus ependymal cells in vitro. J Neurocytol 33:465-476.
Kunis G, Baruch K, Miller O, Schwartz M (2015) Immunization with a myelin-derived antigen activates the brain’s choroid plexus for recruitment of immunoregulatory cells to the CNS and attenuates disease progression in a mouse model of ALS. J Neurosci 35:6381-6393.
Li Y, Decherchi G, Raisman G (2003) Transplantation of olfactory ensheathing cells into spinal cord lesion restores breathing and climbing. J Neurosci 23:727-731.
Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, Goldstein LSB, Tuszynski MH (2014) Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 83:789-796.
Matsumoto N, Taguchi A, Kitayama A, Watanabe Y, Ohta M, Yoshihara T, Itokazu Y, Dezawa M, Suzuki Y, Sugimoto H, Noda M, Ide C (2010) Transplantation of cultured choroid plexus epithelia cells via cerebrospinal fluid shows prominent neuroprotective effects against acute ischemic brain injury in the rat. Neurosci Lett 496:283-288.
Nakano N, Nakai Y, Seo TB, Yamada Y, Ohno T, Yamanaka A, Nagai Y, Fukushima M, Suzuki Y, Nakatani T, Ide C (2010) Characterization of conditioned medium of cultured bone marrow stromal cells. Neurosci Lett 483:57-61.
Nakano N, Nakai Y, Seo TB, Homma T, Yamada Y, Ohta M, Suzuki Y, Nakatani T, Fukushima M, Hayashibe M, Ide C (2013) Effects of bone marrow stromal cells transplantation through CSF on the subacute and chronic spinal cord injury in rats. PLoS One 8:e73494.
Ohta M, Suzuki Y, Noda T, Ejiri Y, Dezawa M, Kitaoka K, Chou H, Ishikawa N, Matsumoto N, Iwashita Y, Mizuta E, Kubo S, Ide C (2004) Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol 187:266-278.
Saito F, Nakatani T, Iwase I, Maeda M, Murao M, Suzuki Y, Fukushima M, Ide C (2012) Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: A pilot study. Restor Neurol Neurosci 30:127-136.
Sakai K, Yamamoto A, Matsubara K, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, HIbi H, Kadomatsu K, Ishiguro N, Ueda M (2012) Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clinic Invest 122:80-90.
Sieber-Blum M, Schnell L, Grim M, Hu TF, Schneider R, Schwab ME (2006) Characterization of epidermal neural crest stem cell (EPI-NCNC) grafts in the lesioned spinal cord. Mol Cell Neurosci 32:67-81.
Suzuki Y, Ishikawa N, Omae K, Ohnishi K, Nakano N, Nishida H, Nakatani T, Fukushimas M, Ide C (2014) Bone marrow-derived mononuclear cell transplantation in spinal cord injury patients by lumbar puncture. Restor Neurol Neurosci 32:473-482.
Watanabe Y, Matsumoto N, Dezawa M, Itokazu Y, Yoshihara T, Ide C (2005) Conditioned medium of the primary culture of rat choroid plexus epithelial (modified ependymal) cells enhances neurite outgrowth and survival of hippocampal neurons. Neurosci Lett 379:158-163.
Williams RR, Bunge MB (2012) Schwann cell transplantation: A repair strategy for spinal cord injury? Prog Brain Res 201:295-312.
Wu S, Suzuki Y, Kitada M, Kitaura M, Kataoka K, Takahashi J, Ide C, Nishimura Y (2003) Migration, integration, and differentiation of hippocampus-derived neurosphere cells after transplantation into injured spinal cord. Neurosci Lett 312:173-176.
Yoshihara T, Ohta M, Itokazu Y, Matsumoto M, Dezawa M, Suzuki Y, Tauchi A, Watanabe Y, Adachi Y, Ikehara S, Sugimoto H, Ide C (2007) Neuroprotective effect of bone marrow-derived mononuclear cells promoting functional recovery from spinal cord injury. J Neurotrauma 24:1026-1036.
10.4103/1673-5374.191198
*Correspondence to:
杂志排行
中国神经再生研究(英文版)的其它文章
- Utilizing pharmacotherapy and mesenchymal stem cell therapy to reduce inflammation following traumatic brain injury
- Recovery of corticospinal tract injured by traumatic axonal injury at the subcortical white matter: a case report
- Blood microRNAs as potential diagnostic and prognostic markers in cerebral ischemic injury
- Intra-axonal protein synthesis - a new target for neural repair?
- Nanobiomaterials for neural regeneration
- Harnessing neural activity to promote repair of the damaged corticospinal system after spinal cord injury
