超临界正丙醇回收炭纤维增强环氧树脂复合材料
2016-10-31吕春祥经德齐常春报刘纳新侯相林
严 华, 吕春祥, 经德齐, 常春报, 刘纳新, 侯相林
(1.山西钢科碳材料有限公司,山西 太原030100;2.中国科学院山西煤炭化学研究所 碳纤维制备技术国家工程实验室,山西 太原030001;3.中国科学院山西煤炭化学研究所 煤转化国家重点实验室,山西 太原030001)
超临界正丙醇回收炭纤维增强环氧树脂复合材料
严华1,2,吕春祥2,经德齐2,常春报1,刘纳新1,侯相林3
(1.山西钢科碳材料有限公司,山西 太原030100;2.中国科学院山西煤炭化学研究所 碳纤维制备技术国家工程实验室,山西 太原030001;3.中国科学院山西煤炭化学研究所 煤转化国家重点实验室,山西 太原030001)
研究了降解温度、反应时间和添加剂对超临界正丙醇中炭纤维增强环氧树脂基复合材料回收的影响。利用扫描电镜、热重、X射线光电子能谱、接触角和单丝拉伸对回收炭纤维进行表征。结果表明,随温度的升高,复合材料降解速率加快,但回收炭纤维力学性能略微降低。随反应时间的延长,复合材料降解速率降低,回收炭纤维力学性能降低。1%质量含量的KOH能明显提高复合材料的回收效率。伴随KOH含量增加,复合材料降解速率没有明显提高,而使回收炭纤维力学性能变差。合适的反应条件对回收具有清洁表面、良好热稳定性和力学性能完好保留的炭纤维至关重要。回收炭纤维表面化学的微弱变化使回收炭纤维同环氧树脂的接触角略增加。超临界正丙醇是一种回收炭纤维复合材料的有效方法。
炭纤维; 回收; 临界正丙醇; 环氧树脂; 复合材料
1 Introduction
Carbon fiber reinforced polymer composites (CFRP) have been extensively used as load-bearing structural materials in aerospace and automotive sectors as well as many industrial, consumer, and sports goods owing to an interesting combination of low weight, high strength and excellent corrosion resistance[1]. However, disposing of the composites will be a problem when the CFRP reach their service lives[2]. Both landfill and incineration of epoxy based CFRP wastes are not optimal since these will cause environmental pollution from the chemicals released after polymers are degraded[3].
Therefore, researchers and engineers around the world made much effort to develop various recycling technologies[4-7]such as mechanical grinding[8], pyrolysis[9], oxidation in a fluidized bed[10], and chemical treatment[11,12]. Mechanical grinding through crushing and milling is relatively cheap and simple, but it only reclaim short milled fibers with poor mechanical properties, which could only be used as filler in reinforced materials[13,14]. Pyrolysis is a commercially applied technology in which the epoxy resin is thermally burned away in an oxygen deficient combustion process[15]. In addition, pyrolysis and oxidation in a fluidized bed is also investigated, in which waste material is fluidized by high-temperature gas passing through from beneath the bed, resulting in gradual separation of its constituents[16,17]. For example, Yip[18]recycled carbon fibers with the fluidized bed method, which retained 75% of their tensile strength. However, these methods can lead to fiber shortening, some degradation of fiber properties, or both. Various chemical recycling methods are developed to preserve the strength and the stiffness of the original carbon fibers[19,20]. For instance, Bai[21]used supercritical water to recycle carbon fibers from carbon fiber reinforced epoxy resin composites at 30 ± 1 MPa and 440 ± 10 ℃. On the other hand, supercritical alcohols represent an alternative for supercritical water because not only are the critical conditions less extreme but also the solubility of polar and high molecular weight solutes in supercritical alcohols is high. Therefore, Jiang[22, 23]used supercritical 1-propanol in a semi-continuous flow reactor to recycle carbon fibers, and unexpectedly found that the tensile strength and modulus of the recycled carbon fibers were almost the same as the virgin carbon fibers. Although much progress has been made in these methods for recycling CFRP waste, there is still some issues needing further investigation, such as the effects of degradation temperature, reaction time and additive on the recovery of carbon fibers with clean surface and high retention of both mechanical properties and fiber length.
The objective of this paper was to evaluate the effects of degradation temperature, reaction time and additive on the properties of the recycled carbon fibers using supercritical 1-propanol in a stirred batch reactor. The carbon fiber reinforced epoxy resin composites were placed in a sample holder made of stainless steel mesh. The direct contact between stripped carbon fibers and rotating blades was avoided, while the high mass transfer rate in the stirred batch reactor was guaranteed at the same time. The morphology, the tensile strength and surface chemical properties of the recycled carbon fibers were characterized from representative composites.
2 Experimental
2.1Materials
The commercial epoxy resin (tetraglycidyl 4,4′-diaminodiphenylmethane, TGDDM) and curing agent (4,4′-diaminodiphenylsulfone, DDS) were supplied by Shanghai Research Institute of Synthetic Resins and Sinopharm Chemical Reagent Co., Ltd, respectively. The chemical structures of the epoxy resin, curing agent and cured resin were reported in our previous investigation[24]. The reaction medium in this experiment was 1-propanol. Carbon fiber reinforced epoxy resin composites and the virgin carbon fibers were produced in National Engineering Laboratory for Carbon Fiber Technology, Institute of Coal Chemistry, Chinese Academy of Sciences. The average diameter of carbon fibers was about 7 μm, and tensile strength was about 3.53 GPa according to the standard of GB/T 26749. Carbon fiber reinforced epoxy resin composites were manufactured through autoclave processing. In a typical process, 8 layers of the carbon fiber fabric were pressed in a mold with the epoxy resin system containing TGDDM and DDS to form a composite, which was heated at 130 ℃ for 1 h before it was cured at 180 ℃ for 4 h. After curing, the composite sample was cut into bars (50 mm × 10 mm × 2 mm) for recycling experiments.
2.2Recycling of carbon fibers
The recycling was conducted in a stainless steel batch reactor. The temperature was controlled by a PID controller. The composite samples were inserted into a sample holder made of stainless steel mesh. In order to enhance the mass transfer rate, mechanical stirring was used and stirring speed was set at 200 r/min. Potassium hydroxide (KOH) was used as an additive. The recycling of carbon fiber reinforced epoxy resin composites was performed under supercritical conditions at different temperatures for different times with or without KOH additive. After the supercritical treatment, epoxy resin was decomposed, the recycled carbon fibers were removed from the sample holder, cleaned in an ultrasonic bath of acetone for 30 min, rinsed with fresh acetone several times at room temperature and dried in an oven at 80 ℃ for 24 h before characterization.
2.3Removal of sizing agent on the as-received carbon fibers
Several bundles of the as-received carbon fibers were refluxed in butanone for one day to remove sizing agent. After refluxing, the fibers were rinsed 3 times with fresh acetone and dried in an oven at 80 ℃ for 24 h before use.
2.4Characterization
Field emission scanning electron images for carbon fibers were taken using a LEO 1530VP microscope with an accelerating voltage of 10 KeV. Samples of virgin and recycled fibers were all tested using a thermogravimetric analyzer (NETZSCH STA 409 PC/PG, Germany) to analyze resin content and the thermal stability of the fibers. Each sample was heated at 10 ℃/min in argon up to a maximum temperature of 900 ℃. Single fiber tensile test was conducted with a LLY-06E tensile testing machine (Laizhou electron Instrument Co. Ltd. China). Filaments were bonded to a standard card frame with epoxy resin. The length of the fibers was 25 mm and the cross-head speed of the tensile testing machine was set to 0.5 mm/min. At least 40 filaments were tested to give an average result. The tensile test gave a load,Pas a function of extension. The diameter of the single carbon fiber,Dwas measured using a scanning electron microscope. After each tensile test, the single fiber was carefully collected for the diameter measurement by SEM. Tensile stressσwas calculated as follows:
σ= 4P/(πD2)
(1)
The fiber surface compositions and surface functional groups were analyzed by XPS (PHI Quantera SXM, Japan), operated at ultrahigh vacuum about 6.7 × 10-8Torr with an excitation from an AlKαas a single anode source. The flood gun was an Ar+scanner with an energy about 2 eV and the binding energy of C 1s (284.6 eV) was used as a reference. The contact angle between carbon fiber and epoxy resin (diglycidyl ether of bisphenol A, DGEBA) were statistically measured using the Image-Proplus 6.0 software from the images taken using an optical microscope (YS100, Nikon, Japan) equipped with a camera (A620, Canon, Japan).
3 Results and discussion
3.1Resin content
The epoxy resin content in the composites was determined by thermogravimetric analysis (TGA). As shown in Fig.1b, the epoxy resin in the composites was carbonized completely under the inert atmosphere at 515 ℃, and the mass fraction of the residual carbonized products remained 77.6% (Fig.1c). The residue contained carbon fiber and coke from the epoxy resin. Almost no weight loss is observed for the carbon fiber (Fig.1a) within the range from room temperature to 500 ℃. However, 30 wt% of coke (Fig.1d) was generated from the pure TGDDM/DDS resin at 515 ℃. Therefore, the epoxy resin content in the composites was calculated to be approximately 32 wt%.
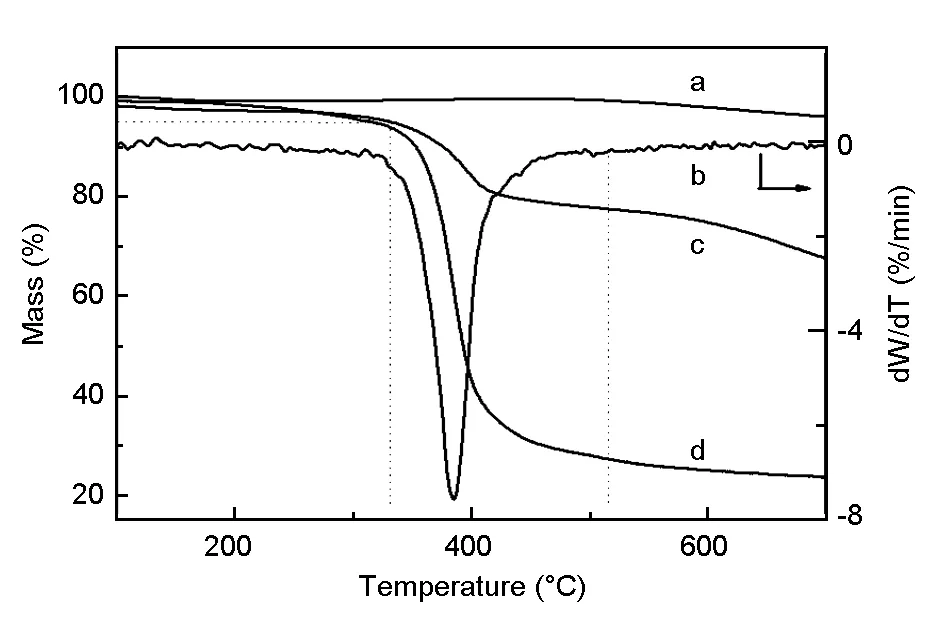
Fig. 1 TGA analysis of (a) carbon fibers,(b, c) carbon fiber/TGDDM composites and (d) pure TGDDM resin cured with DDS.
3.2Effect of recovery temperature
Carbon fibers were successfully recycled using supercritical 1-propanol in the stirred batch reactor. The recycled carbon fibers are usually in a fluffy form due to the removal of sizing agent and epoxy resin. Furthermore, the plain woven texture of the composites almost completely disappears after the mild recycling, and the recycled carbon fibers with two different lengths, about 50 mm and 10 mm, might come from the warp fibers and weft fibers of the composites, respectively.
Table 1 shows the elimination ratio of the CF/TGDDM composites under different temperatures in supercritical 1-propanol. A minor increase of sample weight was observed below 280 ℃, resulting from the swelling of the epoxy resin with 1-propanol in the composites. A 3.4 wt% of weight loss and the presence of few carbon fibers on the composite surface were observed at 300 ℃, indicating a slow decomposition reaction at this temperature. The weight loss increased to 8.2 wt% at 310 ℃ and more carbon fibers were observed on the composite surface. The weight loss the composites achieved more than 28.4 wt% above 330 ℃, which was close to the epoxy resin content in the composites. Therefore, the decomposition rate of the epoxy resin in the composites increased with the recovery temperature.
Fig. 2 shows SEM images of the recycled carbon fibers under different recovery temperatures. From the SEM images, some residual epoxy remained on the surface of the recycled carbon fibers after the recovery was carried out below 310 ℃. However, the recycled fibers appeared to be basically free from epoxy resin polymers, and clean surface and distinct groove were observed on the samples above 330 ℃. In the whole temperature range, no obvious reduction in diameter or surface scratches on the recycled fibers was observed, suggesting that the carbon fibers were recycled with little damage.

Table 1 Weight loss of the CF/TGDDM composites under different recovery temperatures in supercritical 1-propanol.
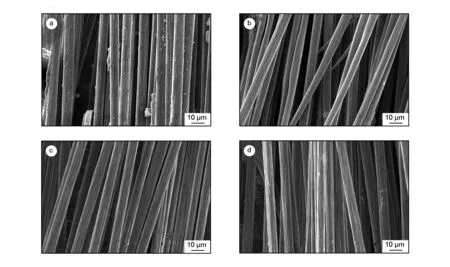
Fig. 2 SEM images of the recycled carbon fibers after the supercritical degradation at (a) 310, (b) 320, (c) 330 and (d) 340 ℃.
Fig.3 exhibits the tensile strength of the recycled carbon fibers after the degradation at different temperatures. The tensile strength of the carbon fibers was obtained using two types of mechanical testing: fiber tows (GB/T26749) and single fiber (ISO 11566). The single fiber tensile test was implemented for the recycled and the original fiber because the recycled fiber was 5 cm in length. The tensile strength of the original single fiber was identical to the tensile strength of the fiber tows provided by the laboratory. The tensile strength of the carbon fiber recycled at 310 ℃ was same as that of the original carbon fiber. Compared to the original carbon fiber, the tensile strength of the carbon fiber retained 95.2% and 94.6% when the recovery was performed at 320 ℃ and 330 ℃, respectively. However, the tensile strength of the recycled carbon fiber only possessed 88.6% of original value at recovery temperature of 340 ℃. Two effects might result in the decrease of the mechanical performance. On one hand, the reaction between KOH and carbon atoms on the fiber surface led to some defects: On the other hand, the degradation reaction within the samples resulted in a significant stress heterogeneity and subsequent damage to fiber. The fact that the carbon fiber recycled at 310 ℃ has the same tensile strength as the original carbon fiber could be accounted for by the fact that the residual epoxy resin might remedy the surface defect of the carbon fiber at 310 ℃.
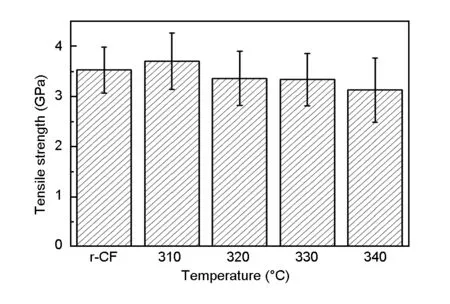
Fig. 3 Tensile strength of the recycled carbon fibers at different temperatures.
3.3Effect of recovery time
The effect of recovery time on the weight loss of the composites and the mechanical performance of the recycled carbon fibers were investigated. Fig.4 shows the weight loss of the CF/TGDDM composites in supercritical 1-propanol at 320 ℃ for different times.
The degradation of the composites proceeded from the surface to the central part. The carbon fibers released by decomposition of resin in the central part of the composites at early stage are due to the capillary effect. The thickness of the released carbon fibers increased with the time, which increased the resistance for the double diffusion of the solvent into and the degradation products out of the composites. Therefore, the decomposition rate of the resin in the composites decreased with recovery time. The residual composites had some degree of hardness after the treatment at 320 ℃ for 90 min. The needle shape of the recycled carbon fibers after the treatment for 120 min suggested that the carbon fibers were stuck together by epoxy resin. However, the carbon fibers were completely separated from each other after the treatment at 320 ℃ for 180 min.
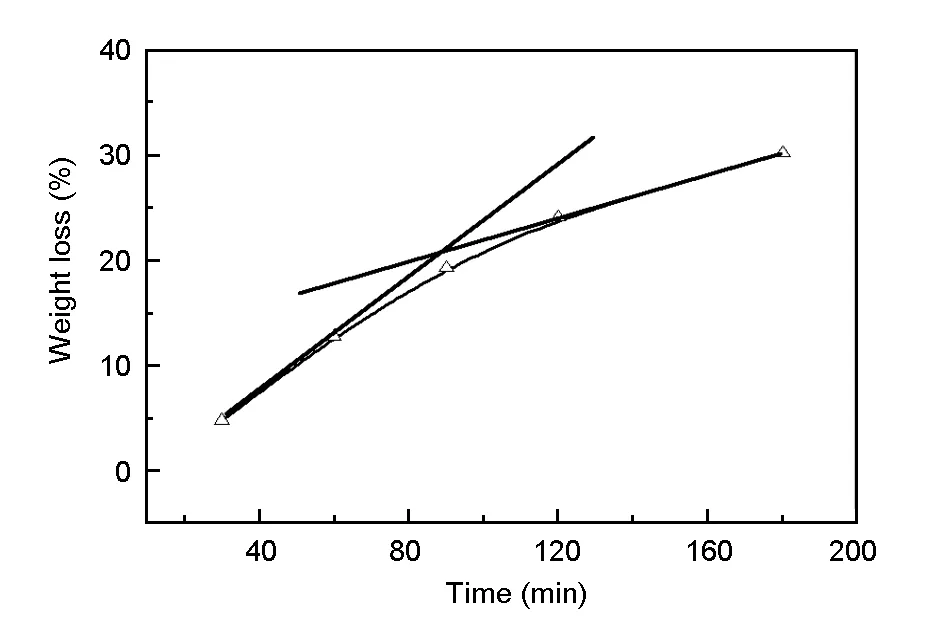
Fig. 4 Weight loss of the composites in supercritical 1-propanol at 320 ℃ for different times.
Fig.5 shows the SEM images of the recycled carbon fibers after the treatment for different times. Although the recycled carbon fibers had some solid particle on the surface after 30 min, much epoxy resin was removed from the fiber surface. No obvious harm on the surface suggested that the carbon fibers could be recycled without damage.
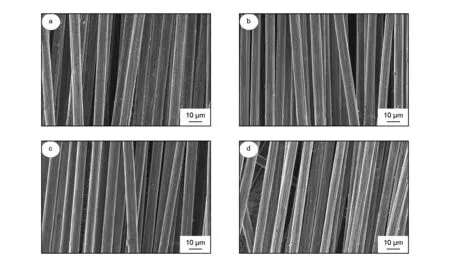
Fig. 5 SEM images of the recycled carbon fibers after the treatment at 320 ℃ for (a) 30, (b) 60, (c) 90 and (d) 120 min with 1 wt% KOH as additive.
Fig. 6 shows tensile strength of the recycled carbon fibers after the treatment for different times. The tensile strength of the recycled carbon fibers decreased with time. The tensile strength of the recycled carbon fibers retained 90% of their original value when the treatment time was shorter than 90 min, but suffered a loss of 15% after the treatment for 180 min. The quality of the recycled carbon fibers degraded and the standard deviation of the tensile strength grew bigger beyond 90 min. In order to get carbon fibers with good performance, the recovery time should be as short as possible.
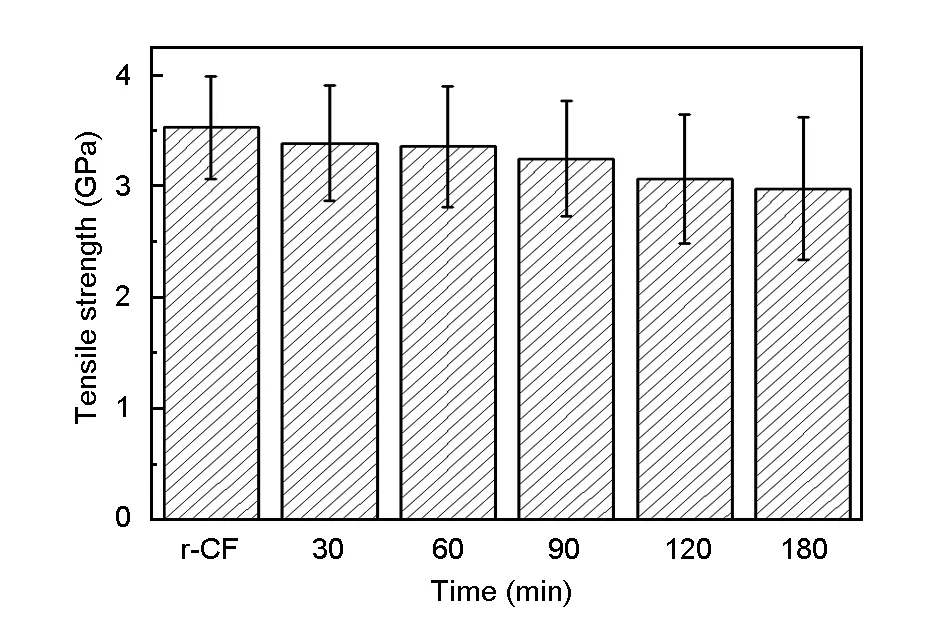
Fig. 6 Tensile strength of the recycled carbon fibers after the treatment at 320 ℃ for different times.
3.4Effect of KOH additive
The KOH additive could promote the decomposition rate of the resin in the composites, but destroy the mechanical strength of the recycled carbon fibers. Fig.7 shows the SEM images of the recycled carbon fibers with and without KOH. Without KOH, 7.6 wt% of weight loss in the composites was obtained at 320 ℃ and no carbon fibers were observed on the composite surface. The weight loss reached 21.4% when the composites were recycled at 330 ℃ for 2 h. After 2 h of treatment at 360 ℃, the weight loss reached 26.4% and most of the epoxy resin was removed. With 1wt% of KOH, carbon fibers with clean surface were obtained at 330 ℃ for 1 h. The significant promotion effect of KOH on the decomposition rate of resin resulted from the hydrogen-donating property of the 1-propanol solvent. When the KOH concentration increased from 0.5% to 2.0%, the weight loss slightly increased from 11.6% to 13.2%. A simple increase of the KOH content could not lead to an obvious improvement of the decomposition rate.
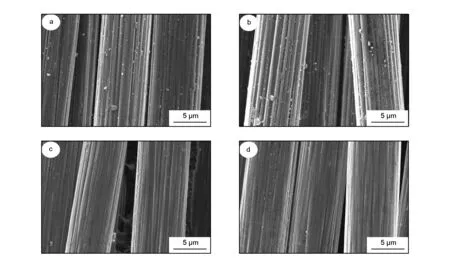
Fig. 7 SEM images of the recycled carbon fibers at different conditions:(a) 330 ℃, without KOH; (b) 360 ℃, without KOH; (c) 320 ℃, 0.5 wt% KOH and (d) 320 ℃, 2 wt% KOH.
Fig. 8 shows tensile strength of the recycled carbon fibers under different conditions. As a whole, the tensile strength of the recycled carbon fibers without KOH was higher than that with KOH. The tensile strength of the recycled fibers was identical to each other under the KOH concentrations of 0.5% and 1.0%. The tensile strength of the recycled carbon fibers decreased significantly under the KOH concentrations of 2%. Furthermore, the higher temperature and KOH content would result in a larger standard deviation and poorer quality of the fibers. The excessive amount of KOH prevented the recycling of carbon fibers with clean surface from the composites.
3.5The surface chemistry of the recycled carbon fibers
XPS was used to evaluate the surface chemistry of the recycled carbon fibers. The original carbon fibers underwent sizing removal, and the recycled carbon fibers were obtained after recovery in the presence of 1 wt% KOH at 320 ℃ for 1 h. The XPS survey scans and C1s high resolution scans for the virgin and recycled carbon fibers are shown in Fig.9. The elemental compositions of the virgin and recycled carbon fibers determined by XPS are shown in Table 2.
Three main peaks including carbon (284.6 eV), oxygen (532.0 eV), nitrogen (399.5 eV) are present in both XPS survey scans. The elemental contents of C, N and Si decreased while the O content increased. The results indicated that the recycled carbon fibers might contain some residual polymers because the C and Si content of the polymer were lower than that on carbon fiber surface. The presence of K on the surface suggested that K2CO3was absorbed on the surface after transformation from KOH. Therefore, the O/C ratio for the recycled fibers increased from 0.168 to 0.206.

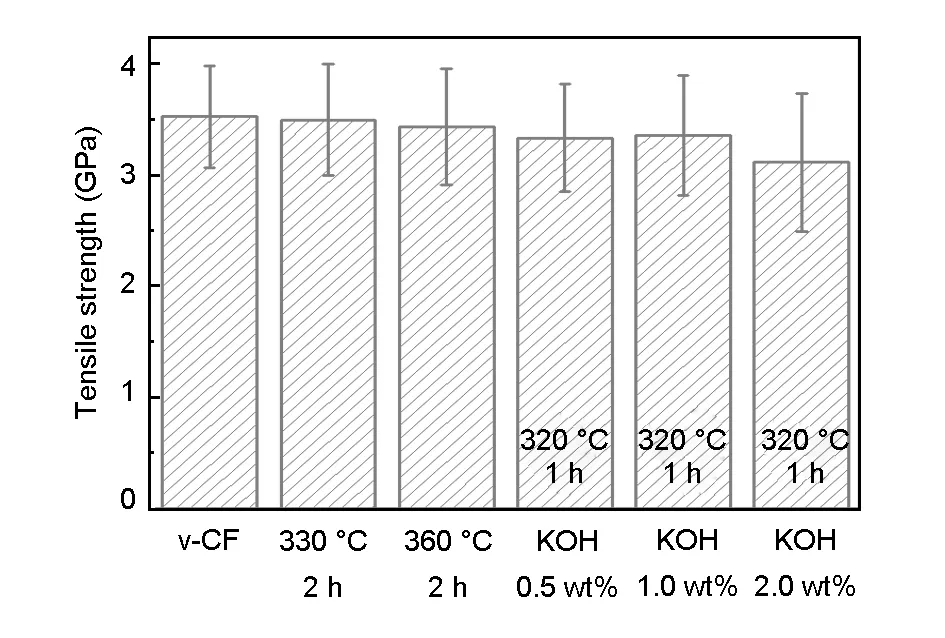
Fig. 8 Tensile strength of the recycled carbon fibers in the presence of KOH with different concentrations.
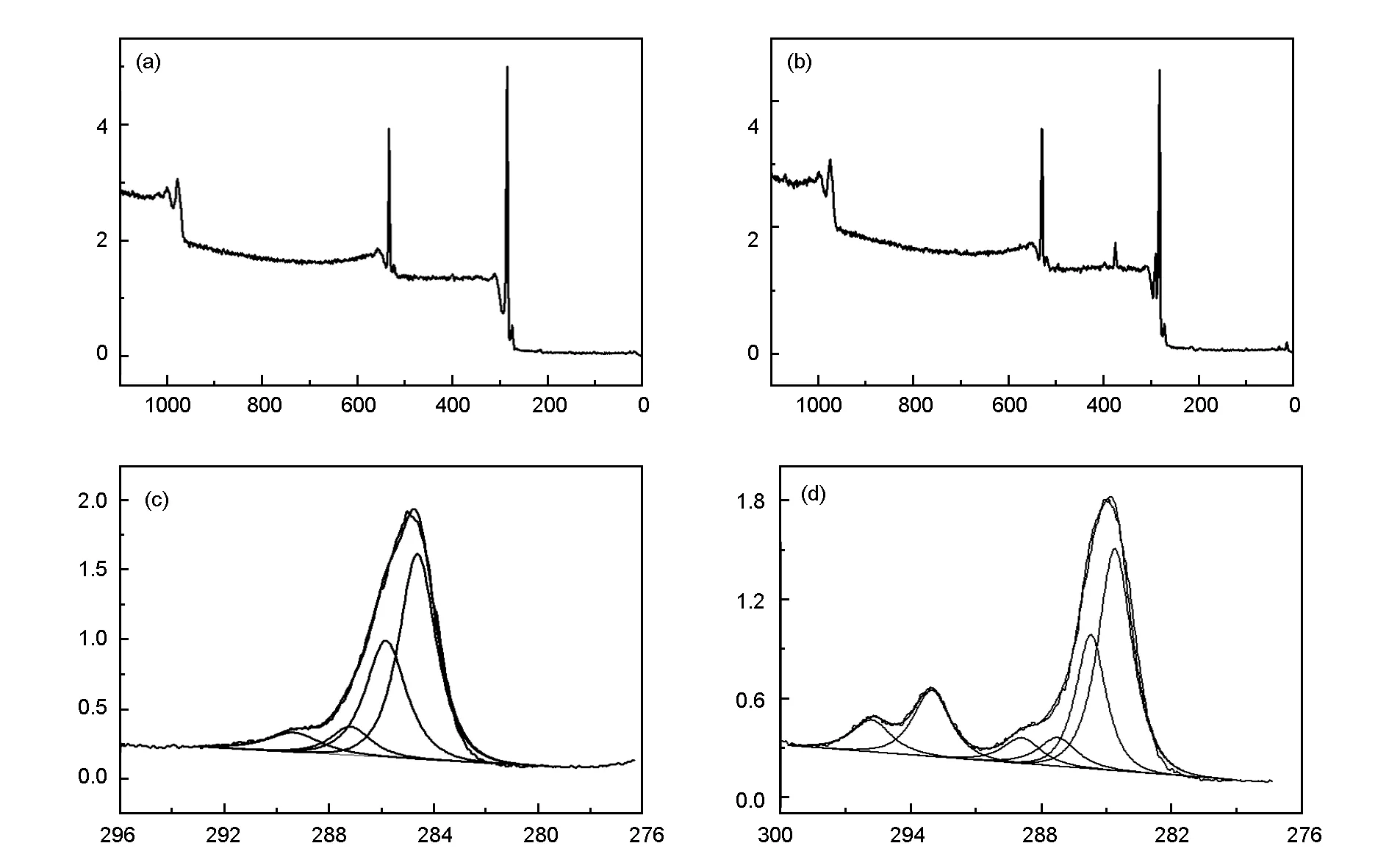
Fig. 9 XPS spectra and curve fitting of C 1s photoelectron peak of carbon fibers: (a, c) virgin carbon fibers and (b, d) the recycled carbon fibers.

SamplesC(%)O(%)N(%)Si(%)K(%)O/CVirgincarbonfibers83.8614.101.300.7400.168Recycledcarbonfibers78.1216.131.080.424.250.206

Table 3 Percentages of various oxygenated functional groups on the carbon fiber surface.
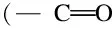
The interaction between the fiber surface and the epoxy resin was examined using contact angle measurement. Two typical images of carbon fiber which was covered symmetrically with a drop of epoxy resin are shown in Fig. 8. The average contact angle increased slightly from 34.2° to 35.4° after the treatment, probably resulting from the roughness increase of the fiber surface.
Fig. 10 Digital images for contact angle measurement: (a) virgin fiber, (b) recycled fiber.
4 Conclusions
In summary, carbon fibers with clean surface, good thermal stability and excellent mechanical properties were successfully recycled through supercritical 1-propanol from the carbon fiber reinforced epoxy resin composites. With the temperature increase, the decomposition rate of the resin in the composites increased, but the mechanical properties of the recycled fibers decreased slightly. With the extension of reaction time, the decomposition rate decreased, and obvious deterioration in the mechanical performance of the recycled carbon fibers was observed. 1 wt% of KOH additive could improve recovery efficiency of the composites significantly. With the increase of the KOH concentration, no obvious enhancement in the decomposition rate was achieved, and the mechanical properties of the recycled fibers became poor. The increase in roughness of the fiber surface resulted in an increase of contact angle with epoxy resin. Supercritical 1-propanol recycling method has many potential benefits, including the high quality of the recycled carbon fibers. Because of the high retention of mechanical properties and fiber length through this general, simple and efficient route, it is believed that the application of recycled carbon fibers in automotive industry, industrial injection moulds and sporting goods will gradually increase.
[1]Yang Y H, Pan Y X, Feng Z H, et al. Evaluation of aerospace carbon fibers[J]. New Carbon Mater, 2014, 29(3): 161-168.
[2]Liu Y, Deng T S, Lu C X, et al. Recovery of carbon fibers from carbon fiber/epoxy composites by epoxy degradation with concentrated zinc chloride in ethanol[J]. New Carbon Mater, 2014, 29(5): 363-368.
[3]Liu X Y, Song Y, Li C M, et al. Effects of carbon nanotubes grafted on a carbon fiber surface on their interfacial properties with the matrix in composites[J]. New Carbon Mater, 2012, 27(6): 455-461.
[4]Leste E, Kingman S, Wong K H, et al. Microwave heating as a means for carbon fibre recovery from polymer composites: a technical feasibility study[J]. Mater Res Bull, 2004, 39(10): 1549-1556.
[5]Liu Y Y, Meng L H, Huang Y D, et al. Recycling of carbon/epoxy composites[J]. J Appl Polym Sci, 2004, 94(5): 1912-1916.
[6]Pickering S J. Recycling technologies for thermoset composite materials-current status[J]. Composites Part A, 2006, 37(8): 1206-1215.
[7]Pimenta S, Pinho S T. Recycling carbon fibre reinforced polymers for structural applications: Technology review and market outlook[J]. Waste Manage, 2011, 31(2): 378-392.
[8]Palmer J, Ghita O R, Savage L, et al. Successful closed-loop recycling of thermoset composites[J]. Composites Part A, 2009, 40(4): 490-498.
[9]Nahil M A, Williams P T. Recycling of carbon fibre reinforced polymeric waste for the production of activated carbon fibres[J]. J Anal Appl Pyrol, 2011, 91(1): 67-75.
[10]Wong K H, Pickering S J, Rudd C D. Recycled carbon fibre reinforced polymer composite for electromagnetic interference shielding[J]. Composites Part A, 2010, 41(6): 693-702.
[11]Pinero-Hernanz R, Garcia-Serna J, Dodds C, et al. Chemical recycling of carbon fibre composites using alcohols under subcritical and supercritical conditions[J]. J Supercrit Fluids, 2008, 46(1): 83-92.
[12]Liu Y Y, Shan G H, Meng L H. Recycling of carbon fibre reinforced composites using water in subcritical conditions[J]. Mater Sci Eng A, 2009, 520(1-2): 179-183.
[13]Palmer J, Savage L, Ghita O R, et al. Sheet moulding compound (SMC) from carbon fibre recyclate[J]. Composites Part A, 2010, 41(9): 1232-1237.
[14]Turner T A, Pickering S J, Warrior N A. Development of recycled carbon fibre moulding compounds-Preparation of waste composites[J]. Composites Part B, 2011, 42(3): 517-525.
[15]Cunliffe A M, Jones N, Williams P T. Recycling of fibre-reinforced polymeric waste by pyrolysis: thermo-gravimetric and bench-scale investigations[J]. J Anal Appl Pyrol, 2003, 70(2): 315-338.
[16]Jiang G, Pickering S J, Walker G S, et al. Soft ionisation analysis of evolved gas for oxidative decomposition of an epoxy resin/carbon fibre composite[J]. Thermochim Acta, 2007, 454(2): 109-115.
[17]Jiang G, Pickering S J, Walker G S, et al. Surface characterisation of carbon fibre recycled using fluidised bed[J]. Appl Surf Sci, 2008, 254(9): 2588-2593.
[18]Yip H L H, Pickering S J, Rudd C D. Characterisation of carbon fibres recycled from scrap composites using fluidised bed process[J]. Plast Rubber Compos, 2002, 31(6): 278-282.
[19]Hyde J R, Lester E, Kingman S, et al. Supercritical propanol, a possible route to composite carbon fibre recovery: A viability study[J]. Composites Part A, 2006, 37(11): 2171-2175.
[20]Gersifi K El, Durand G, Tersac G. Solvolysis of bisphenol a diglycidyl ether/anhydride model networks[J]. Polym Degrad Stabil, 2006, 91(4): 690-702.
[21]Bai Y, Wang Z, Feng L. Chemical recycling of carbon fibers reinforced epoxy resin composites in oxygen in supercritical water[J]. Mater Des, 2010, 31(2): 999-1002.
[22]Jiang G, Pickering S J, Lester E H, et al. Characterisation of carbon fibres recycled from carbon fibre/epoxy resin composites using supercritical 1-propanol[J]. Compos Sci Technol, 2009, 69(2): 192-198.
[23]Yan H, Lu C X, Jing D Q, et al. Chemical degradation of amine-cured DGEBA epoxy resin in supercritical 1-propanol for recycling carbon fiber from composites[J]. Chinese J Polym Sci, 2014, 32(11): 1550-1563.
[24]Yan H, Lu C X, Jing D Q, et al. Chemical degradation of TGDDM/DDS epoxy resin in supercritical 1-propanol: Promotion effect of hydrogenation on thermolysis[J]. Polym Degrad Stabil, 2013, 98(12): 2571-2582.
Recycling of carbon fibers in epoxy resin composites using supercritical 1-propanol
YAN Hua1,2,LU Chun-xiang2,JING De-qi2,CHANG Chun-bao1,LIU Na-xin1,HOU Xiang-lin3
(1.ShanxiGangkeCarbonMaterialsCo.Ltd.,Taiyuan030100,China;2.NationalEngineeringLaboratoryforCarbonFiberTechnology,InstituteofCoalChemistry,ChineseAcademyofSciences,Taiyuan030001,China;3.StateKeyLaboratoryofCoalConversion,InstituteofCoalChemistry,ChineseAcademyofSciences,Taiyuan030001,China)
The effects of degradation temperature, reaction time and additive on the efficiency of the recovery of carbon fibers from their epoxy resin composites by supercritical 1-propanol were investigated. The recycled carbon fibers were characterized using SEM, TGA, XPS, contact angle measurements and single fiber tensile strength tests. Results indicated that the rate of decomposition of the resin increased, but the mechanical properties of the recycled fibers decreased slightly with temperature. The decomposition rate of the resin and tensile strength of the recycled carbon fibers decreased with reaction time. 1 wt% of KOH additive in 1-propanol improved the recovery efficiency significantly. When the KOH concentration was increased beyond 1 wt% there was no obvious increase in the decomposition rate and the mechanical properties of the recycled fibers became worse. There were slight changes in the surface chemistry of the recycled carbon fibers and their contact angle with epoxy resin. Supercritical 1-propanol is an excellent recycling technology for carbon fibers in epoxy resin composites.
Carbon fiber; Recycling; Supercritical 1-propanol; Epoxy resin; Composites
date: 2015-12-08;Reviseddate: 2016-01-07
National Natural Science Foundation of China (51103174).
LU Chun-xiang, Ph. D, Professor. E-mail: chunxl@sxicc.ac.cn
introduction:YAN Hua, Ph. D, Engineer. E-mail: yanhuachem@163.com
1007-8827(2016)01-0046-09
TQ342.+74
A
国家自然科学基金(51103174).
吕春祥,研究员. E-mail: chunxl@sxicc.ac.cn
严华,博士,工程师. E-mail: yanhuachem@163.com
10.1016/S1872-5805(16)60004-5
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).
