钽掺杂六方相氧化钨对Sr2+的吸附:Zeta电位的测量及吸附机理的研究
2016-09-09牟婉君彭述明
蹇 源 牟婉君 刘 宁 彭述明
(1四川大学原子核科学技术研究所,辐射物理及技术教育部重点实验室,成都610064;2中国工程物理研究院核物理与化学研究所,四川绵阳621900)
钽掺杂六方相氧化钨对Sr2+的吸附:Zeta电位的测量及吸附机理的研究
蹇源1,2,*牟婉君2刘宁1彭述明1,2,*
(1四川大学原子核科学技术研究所,辐射物理及技术教育部重点实验室,成都610064;2中国工程物理研究院核物理与化学研究所,四川绵阳621900)
研究Ta掺杂六方相氧化钨(hex-WO3)材料在吸附Sr2+过程中其表面zeta电位的变化情况,并进一步探讨了吸附过程的热力学及吸附机理。结果表明:(1)在实验pH值范围内,Ta掺杂hex-WO3悬浮液的zeta电位值随溶液中电解质的价态增大而增大;(2)且zeta电位随体系中离子强度的增加而增大;(3)Ta掺杂hex-WO3对Sr2+的吸附容量随着温度降低而增大,随着离子强度的增加而减少;(4)吸附过程的吸附焓为-47 kJ· mol-1,且Sr2+离子与材料表面之间主要为化学相互作用;(5)Ta掺杂hex-WO3对Sr2+吸附过程主要为材料表面吸附及材料孔道内离子交换共同作用。
Ta;hex-WO3;Zeta电位;Sr2+;吸附机理
www.whxb.pku.edu.cn
1 Introduction
With the development of nuclear industry and technology,much radioactive liquid waste is released into the environment,which leads to a direct threat to human health1.For example,90Sr,with a half-life of 30 years,is an important source of radioactivity in liquid waste,and can cause detrimental effects to animals and humans because it can substitute for the calcium in bones,leading to an increased risk of leukemia and other diseases2.Hence,re-duction of strontium migration is an actively pursued goal in the treatment of radioactive liquid waste.With growing attention to this issue,a number of technologies and materials have been investigated for reducing strontium concentration,such as adsorption,adsorption/ion exchange,chemical precipitation,and biochemical reductive precipitation.Compared to other technologies,adsorption technologies are inexpensive,effective,swift, and environmentally friendly ways to dispose of radioactive liquid waste3-5.Many excellent materials have been adopted for the removal of90Sr ions from nuclear wastewater,such as clay minerals6,zeolites7,silicotitanates8,and carbon nanotubes9.In fact, some outstanding results have been achieved in this regard. However,the fairly narrow pH operating range and/or slow ion exchange kinetics of nanomaterials have restricted their practical application.The pH of wastewater is a limiting factor that affects the use of available exchangers because most radioactive liquid waste is highly acidic.Thus,to design and fabricate adsorbents that possess excellent chemical stability and high selectivity for fission nuclides in acidic solutions,at low cost,and in a simple and environmentally friendly way,is a significant challenge for radioactive liquid waste treatment in real applications.
Hexagonal tungstate oxide(hex-WO3)has received much attention because of its well-known tunnel structure in which WO6octahedra share their corners with each other to form hexagonal tunnels along the c-axis10,11.Guest ions(e.g.,Na+,NH4+,etc.)reside in the“hexagonal window”tunnels between layers,and ion exchange can replace them by K+,Cs+,and other ions.Moreover,it has been reported that hex-WO3can act as an adsorbent for removing radionuclides(Cs+,Sr2+,etc.)from acidic solution.It has also been reported that the adsorption capacity of Sr2+on hex-WO3may be increased by inserting heteroatoms at W6+sites,which can potentially affect tunnel dimensions and adsorption site acidity. This is one strategy for modulating cation selectivity4,12,13.
The electrokinetic behavior of nanomaterials is used to explain charge formation,charge density,and changes in the adsorption process,such as the isoelectric point(iep)and potential-determining ions(pdi),of fine particles in an aqueous solution.These play a significant role in allowing understanding of the adsorption mechanism of inorganic and organic species at the solid/solution interface14-16.The zeta-potential is defined as the shear plane potential of the particle when it moves in a liquid.Determination of the ζ-potential gives an indication of the magnitude of the potential at the edges of the diffuse double layer around the particle17.
In our previous work,we successfully prepared Ta-doped hex-WO3nanomaterials and found that they exhibited excellent adsorption ability for Sr2+in acidic solution4.The purpose of the present paper is to investigate the surface electrokinetic properties of Ta-doped hex-WO3nanomaterials,as well as their adsorption mechanism for Sr2+ions.
2 Material and methods
2.1Synthesis of Ta-doped WO3nanomaterials
The method of synthesis Ta-doped WO3was as follows:1 g

whereζis the zeta potential(mV),μMis the electrophoretic mobility(m2·V-1·s-1),ηis the dynamic viscosity of the liquid(Pa·s), ε0is the permittivity of the vacuum(F·m-1),andεris the relative permittivity of the liquid.Asample of 0.1 g Ta-doped hex-WO3in 50 mL distilled water containing the desired electrolytes was added to a thermostatic shaker bath and rinsed for 12 h at(25.0± 0.1)°C.An aliquot taken from the supernatant was used to measure the zeta potential.The average of 5 measurements was taken to represent the measured potential.The applied voltage during the measurements was generally varied in the range of 50-150 mV.
2.4Adsorption experiment
Studies on the Sr2+sorption behavior were performed on Tadoped hex-WO3samples in batch experiments using 200 mL volume flasks.Aqueous solutions of non-radioactive Sr(NO3)2were used instead of radioactive isotopes to avoid radiation damage.Solution pH was adjusted and measured using a digital pH-meter(PHS-4CT,China),using HNO3and NaOH solutions. To study the adsorption ability of Ta-doped WO3samples in acidic solutions,the pH of the initial solution did not exceed 8.Adsoranalytical grade Na2WO4·2H2O(99.9%,Aladdin)was dissolved in 45 mLdeionized water by stirring at room temperature,then 0.1 mol·L-1TaCl5(99.99%,Aladdin)solution(dissolved in ethanol) and 5 mL of 3 mol·L-1HCl solution was added to the above solution with continuous stirring until tungstenic acid was precipitated thoroughly.Finally 35 mL of 0.5 mol·L-1ammonium sulfate(NH4)2SO4solution was added to this solution,which was then transferred into a Teflon-lined autoclave with a capacity of 100 mL.Hydrothermal treatment was carried out at 170°C for 48 h.After that,the autoclave was allowed to cool down naturally. The final products were collected,then washed with deionized water and ethanol several times,and dried in air at 80°C to give the Ta-doped hex-WO3samples.
2.2Material characterization
The crystalline structure of the prepared samples was characterized using X-ray diffraction(PXRD,X′Pert PRO,PANalytical,Almelo,Netherlands)with Cu-Kαradiation(λ=0.15406 nm at 40 kV and 45 mA).The composition and chemical state were determined by X-ray photoelectron spectroscopy(XPS) using an RBD upgraded PHI-5000C ESCAsystem(Perkin-Elmer) using an Mg-monochromatic X-ray at a power of 25 Wwith an X-ray-beam diameter of 10 mm,and a pass energy of 29.35 eV.The binding energy was calibrated by the C 1s hydrocarbon peak at 284.8 eV.Fourier transform infrared(FT-IR)spectra of the samples were obtained on a Perkin-Elmer1730 infrared spectrometer in the range of 400-4000 cm-1.
2.3Zeta potential measurements
The zeta potential of the Ta-doped hex-WO3suspension was measured using a zeta Meter(zetaPALS,BLK,USA).The zeta potential was calculated from the measured electrophoretic mobility using the Smoluchowski equation18:bent(0.2 g)was added to Sr(NO3)2solution(50 mL)at different acidities(pH=1-7).The suspension was agitated at 298 K for 12 h.Equilibrium studies were conducted within a Sr2+concentration range of 20-200 mg·L-1.Detailed experimental conditions are presented in the related figure captions for clear identification.The solutions were withdrawn from the flasks and separated from the solids by centrifugation,then the initial and the residual concentration of tested ion(s)in supernatants were determined by atomic absorption spectroscopy(AAalyst800,PerkinElmer,USA). The sorption amount qe(mg·g-1)of adsorbed Sr2+ions is calculated as:

where C0(mg·L-1)is the initial concentration of the metal ion,Ce(mg·L-1)is the equilibrium concentration,V(L)is the volume of the test solution,and m(g)is the sorbent dose.
3 Results and discussion
3.1FT-IR characterization
FT-IR spectra of hex-WO3and Ta-doped hex-WO3are shown in Fig.1.Both samples display similar spectra.The broad peak observed at 3141 cm-1can be attributed to the stretching vibration of surface hydroxyls and adsorbed water molecules19.The peaks appearing at 1623 and 1400 cm-1relate to the bending vibration of adsorbed water molecules and―OH groups,respectively.This indicates that―OH groups have strong bonds to either water molecules or surface oxygen atoms20.The strong peaks at 823 cm-1can be attributed to the stretching vibration of W―Ointer―O,and the relatively weak intensity peaks at 657 and 588 cm-1can be assigned to the bending vibration of W―O.Peaks at 1189 and 1112 cm-1belong to shortened W―O bonds of WO3,and these degrade in the Ta-doped hex-WO3sample21.Furthermore,a broad peak at 3415 cm-1is observed following incorporation of Ta into the hex-WO3framework,indicative of the presence of a high hydroxyl group content and the adsorbed water on the surface of Ta-doped hex-WO3that is beneficial for adsorption of Sr2+.
3.2Zeta potential of Ta-doped hex-WO3
3.2.1Effect of solid concentration
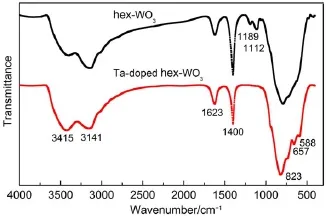
Fig.1 FT-IR spectra of hex-WO3and Ta-doped hex-WO3
Solid concentration in solution is a major parameter governing surface charge generation,which has an important effect on zeta potential.This means that the ionic species produced at the solidliquid interface increase with increase in solid concentration,and that using inadequate solids concentration can lead to erroneous conclusions in the interpretation of zeta potential measurements. As shown in Fig.2,the effect of solid concentration in solution on zeta potential is minor.Therefore,in the subsequent zeta potentials measurements the solid-to-liquid ratio was kept constant at 10 g· L-1.
3.2.2Effect of pH
Fig.3 shows the zeta-potential values of Ta-doped hex-WO3nanomaterials as a function of pH of the buffer solution used for treatment.As shown,the Ta-doped hex-WO3has no point of zero charge and exhibits negative zeta-potential values in the chosen pH range,indicating that the sample has a highly negatively charged surface.The surface charge of nanomaterials is attributed to the edge surface and structural charge sites.The edge surface charge comes from proton adsorption by the hydroxyl groups(―W―OH at octahedral layers);whereas the structural charge sites are the permanent negative charges resulting from isomorphic substitutions taking place in the octahedral layers(W5+substituted by Ta5+)22.The hex-WO3possesses greater negative charge because it has been doped with Ta5+,which provides a favorable environment for adsorbing the positively charged Sr2+through electrostatic interactions.Incorporating Ta5+can increase the content of hydroxyl groups on the hex-WO3surface,thus leading to agreater negative charge.

Fig.2 Effect of solid concentration on the zeta potential of Ta-doped hex-WO3
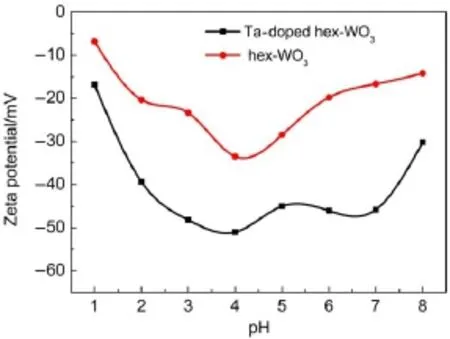
Fig.3 Zeta potential value as a function of pH for hex-WO3and Ta-doped hex-WO3
The electrical charge at the oxide surface/aqueous phase to protonation/deprotonation of the surface hydroxyl can be ascribed as23:
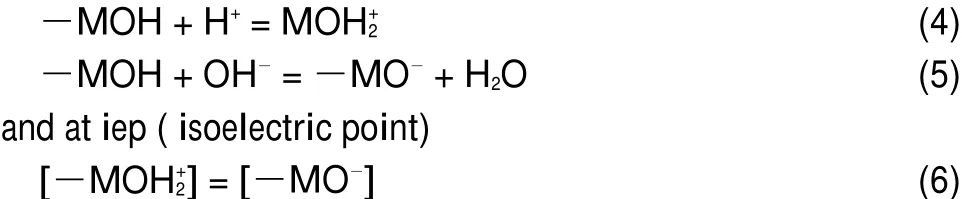
The fact that there is no iep which shows that the reaction responsible for the surface charge of the solid is mainly the reaction in Eq.(5).
3.2.3Effect of electrolyte
Discussion of the source of surface charges on the oxide surfaces may be useful before investigating the change in zetapotential with equilibrium pH of the Ta-doped hex-WO3suspensions in various electrolyte media24.Fig.4(a,b)shows the change in zeta-potential of the Ta-doped hex-WO3suspensions as a function of equilibrium pH value in 10-3mol·L-1monovalent and divalent electrolytes.Ta-doped hex-WO3suspensions have a negative zeta-potential in the experimental equilibrium pH range, and the negative charges decrease with increase in equilibrium pH value.Moreover,the zeta-potential of Ta-doped hex-WO3in divalent electrolyte solution was much higher than in the monovalent electrolyte solution having the same ionic strength.
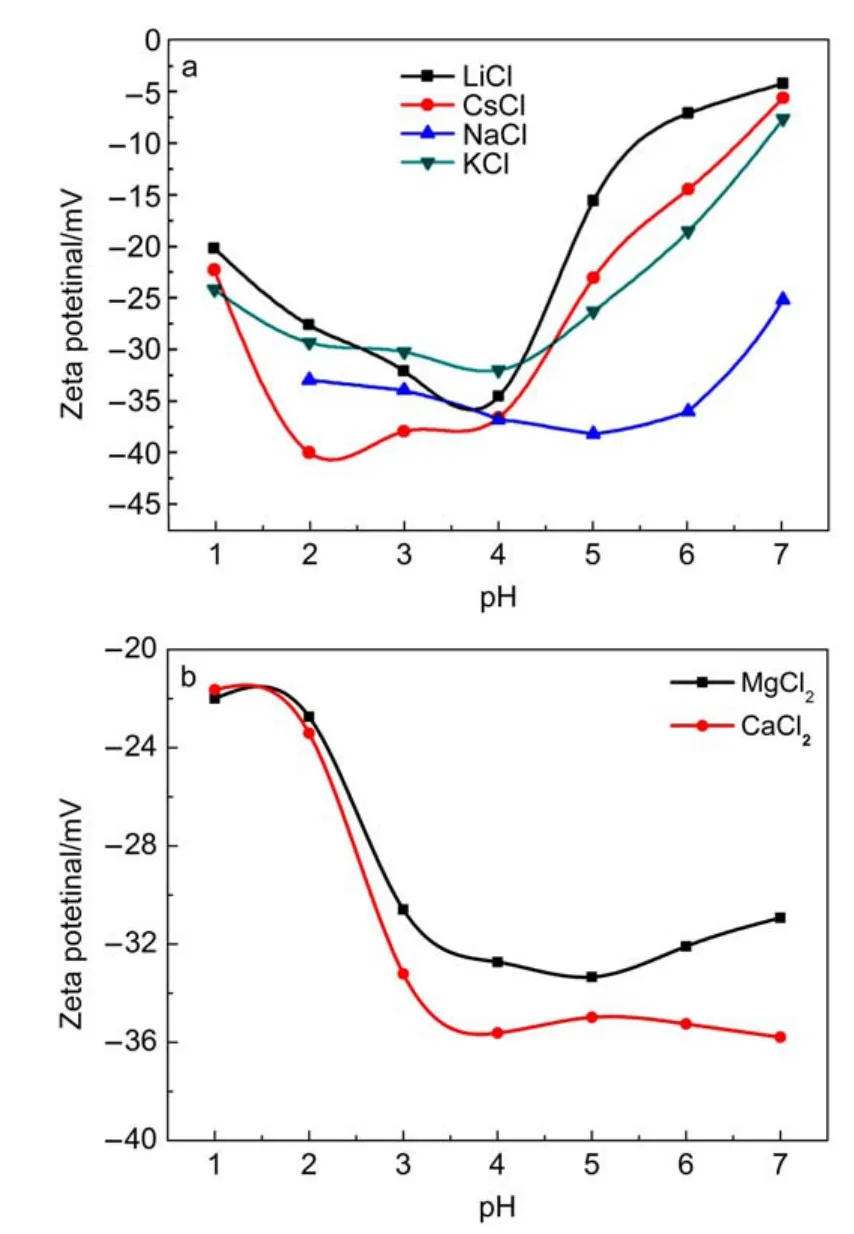
Fig.4 Variation of zeta potential with equilibrium pH of Ta-doped hex-WO3suspensions in the presence of different monovalent(a)and divalent(b)electrolytes at constant concentration
Changes in the zeta-potential of minerals with background electrolytes of different valences follow the Hardy-Schulze rule25. It is believed that divalent cations can be specifically adsorbed onto the surface of minerals(in the inner Helmholtz plane)causing charge reversal.Additionally,hydrated ions present in the outer Helmholtz plane can also increase the zeta-potential by compressing the electrical double layer26.One can deduce,therefore, that divalent ions will reduce the double layer thickness more than will monovalent ions(given that they are both present in the same concentration),causing a sharper potential drop across the Stern layer27,28.
3.2.4Effect of ionic strength
Investigations carried out into adsorption revealed that the extent of waste uptake was strongly influenced by the concentration and nature of the electrolyte ionic species added to the aqueous media29.Electrolyte concentration,along with pH,influences the development of positive and negative surface charges, which directly affect the surface adsorption.KCl was chosen as a salt for investigating the effect of ionic strength on the adsorption of Sr2+ions onto the Ta-doped hex-WO3surface.This was carried out in no salt,and salt concentrations of 10-5,10-4,and 10-3mol·L-1.As shown in Fig.5a,increasing the ionic strength significantly decreased the adsorption of Sr2+ions onto the Ta-doped hex-WO3.The electrostatic interaction between opposite charges of the oxide surface and the Sr2+ions is screened by the salt,and increases with salt concentration,leading to a decrease in the amount adsorbed30,31.This result is consistent with the changes in zeta-potential of the Ta-doped hex-WO3suspension at differentconcentrations of KCl electrolyte(Fig.5b),where an increase in electrolyte concentration causes an increase in surface potential, which is unfavorable for Sr2+adsorption.

Fig.5 (a)Effect of ionic strength on the adsorption of Sr2+ions onto Ta-doped hex-WO3;(b)variation of zeta potential with equilibrium pH of Ta-doped hex-WO3at different KCl concentrations
3.2.5Effect of temperature
The temperature dependence of adsorption reactions has an important effect on the adsorption process.Fig.6 shows that the adsorption process for Sr2+ions on the Ta-doped hex-WO3is exothermic,and is more favorable at low temperatures.The adsorption amount decreases as the temperature increases.Two possible reasons for this are:(1)A decrease in the number of active surface sites for adsorption onto the adsorbents;(2)an increase in thickness of the boundary layer surrounding the adsorbent with rising temperature.Moreover,increasing temperature is favorable for reaching equilibrium quickly.The enthalpy change for Sr2+ion adsorption can be estimated by the van′t Hoff equation32,33:

where the subscript θ refers to the equilibrium;Rgis the gas constant;and qmis the ultimate constant at each temperature measured at constant coverage.Under these conditions,from the Langmuir equation at θ=qe/qm=0.5;K=1/Ce:

where qeis the equilibrium loading of adsorbate onto adsorbent (mol·g-1);Ceis the equilibrium concentration of adsorbate in solution(mol·L-1);and K is the relative energy of adsorption(L· mol-1).
This value of ΔH is termed the isosteric heat of adsorption, referring to the fact that it applies to a certain coverage value.The Langmuir model implies that ΔH should be constant,but it is more likely to be a function of coverage(θ=qe/qm).From Eq.(8),the value of ΔH was calculated as-47 kJ·mol-1from the data given in Fig.7.The results show that the interaction between surface and adsorbate ions is chemical in nature.
3.2.6Effect of coexisting ions
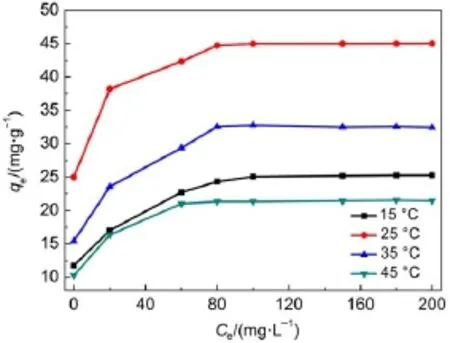
Fig.6 Effect of temperature on the adsorption of Sr2+ions onto Ta-doped hex-WO3
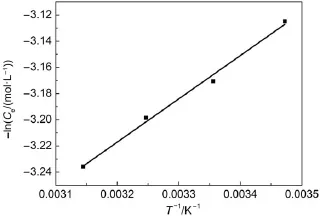
Fig.7 Plot of-lnCevs 1/T for Sr2+adsorption onto Ta-doped hex-WO3
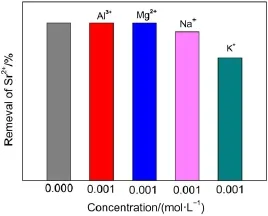
Fig.8 Effect of competitive ions on the adsorption of Sr2+
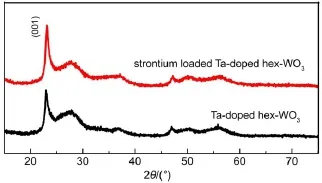
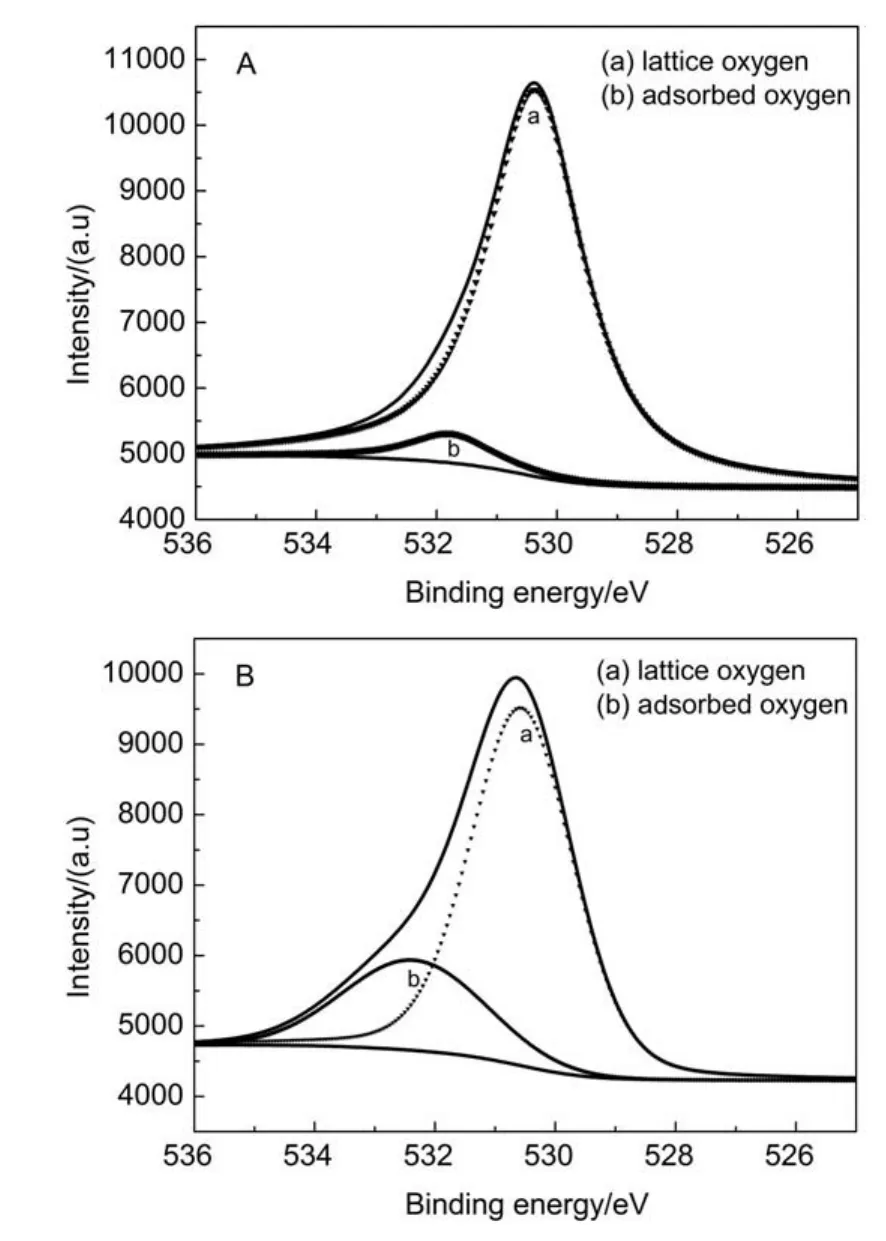
Fig.8 shows the selective uptake of Sr2+ions on Ta-doped hex-WO3in the presence of various cations.It can be found that the removal of Sr2+was slightly influenced by the cations of K+, whereas in the presence of Mg2+,Al3+,Na+,one can see that they had no obvious effect on the Sr2+absorption onto Ta-doped hex-WO3.These results are attributed to the configuration of the hydrated metal ions in aqueous solution.The alkali metal ions are highly hydrated and smaller ions have a greater degree of hydration.The ionic radius increases order as follows:Al3+≤Mg2+< Na+ 3.3Adsorption mechanism Previous studies have shown adsorption of Sr2+ions onto Tadoped hex-WO3samples mainly takes place through chemisorption (covalent bonding and ion exchange).Fig.9 schematically illustrates the adsorption of Sr2+ions onto Ta-doped hex-WO3. The―OH groups on the adsorbent surface and particular tunnels of the adsorbent structure are favorable for improved adsorption capacity of Sr2+. Fig.9 Schematic diagram of the adsorption of Sr2+by Ta-doped hex-WO3nanomaterials Fig.10 XRD patterns of Ta-doped hex-WO3before and after Sr2+adsorption Fig.11 FT-IR spectra of Ta-doped hex-WO3before and after Sr2+adsorption To further understand the interaction mechanism between Sr2+and the Ta-doped hex-WO3samples,XRD patterns for Ta-doped hex-WO3before and after Sr2+adsorption were analyzed.As shown Fig.10,the XRD patterns for Ta-doped hex-WO3before and after Sr2+adsorption are similar,but the position of the(001)peak moves to a higher angle,indicating that the Ta-doped hex-WO3structure contracted after adsorption of Sr2+,and that a forceful interaction exists between Sr2+and the material framework.This result demonstrates that some Sr2+ions entered into the tunnels of the Ta-doped hex-WO3structure. The FT-IR spectra of Ta-doped hex-WO3before and after Sr2+adsorption are shown in Fig.11.The peaks at 3415 and 1400 cm-1can be attributed to the stretching and bending vibrations,respectively,of―OH groups(see Fig.2),where the intensities of these peaks weakened after Sr2+adsorption.Moreover,the broad peak at 3141 cm-1disappeared after absorption of Sr2+,demonstrating that the―OH groups on the Ta-doped hex-WO3surface were bound to Sr. Fig.12 O 1s XPS spectra of Ta-doped hex-WO3before(a)and after(b)Sr2+adsorption To obtain an in-depth understanding of the composition of the Ta-doped hex-WO3samples before and after adsorption of Sr2+, O 1s XPS spectra of these samples are shown in Fig.12.Two peaks,at binding energies of 530.0 and 531.5 eV,can be attributed to the surface lattice oxygen and adsorbed hydroxyl species,respectively.Clearly,the surface―OH concentration in the Tadoped hex-WO3sample before Sr2+adsorption was higher than that in the Ta-doped hex-WO3sample after Sr2+adsorption,further indicating that the hydroxyl groups have coordinated with Sr2+ions. The electrokinetic properties of Ta-doped hex-WO3suspensions were investigated using microelectrophoresis as a function of pH in the presence of various electrolytes,such as LiCl,NaCl,KCl, CsCl,CaCl2,and MgCl2.The adsorption of Sr2+ions onto Ta-doped hex-WO3from aqueous solutions were then studied as a function of pH,ionic strength,temperature,and coexisting ions. The zeta-potential values of the studied Ta-doped hex-WO3suspensions in divalent electrolytes were much higher than in monovalent electrolytes.Increasing the ion strength in solution caused the zeta potential of the Ta-doped hex-WO3suspensions to increase accordingly.Sr2+ion adsorption increased with a decrease in temperature and ionic strength.The interaction between the Tadoped hex-WO3surface and Sr2+ions was concluded to be chemical in nature. Zeta-potential measurements coupled with adsorption experiments clearly indicate that the adsorption of Sr2+onto Ta-doped hex-WO3nanoparticles involves two mechanisms:(i)simple superficial adsorption via electrostatic interactions between Sr2+ions and negatively charged sites on the sample surfaces;and(ii)intercalation of Sr2+into the Ta-doped hex-WO3framework through displacement of water molecules,Na+,and NH4+that reside in the tunnels along the c-axis. Results obtained here suggest that measuring the zeta-potential of sorbents in suspension can play a major role in investigating the adsorption mechanism in the absorption process,and further demonstrates that Ta-doped hex-WO3is a suitable candidate for sorption of Sr2+in acidic radioactive liquid waste treatment. References (1)Buesseler,K.;Aoyama,M.;Fukasawa,M.Environ.Sci. Technol.2011,45,9931.doi:10.1021/es202816c (2)Nielsen,S.Bone 2004,35,583.doi:10.1016/j.bone.2004.04.026 (3)Wen,T.;Wu,X.L.;Liu,M.C.;Xing,Z.H.;Wang,X.K.;Xu, A.W.Dalton Trans.2014,43,7464.doi:10.1039/c3dt53591f (4)Li,X.L.;Mu,W.J.;Xie,X.;Liu,B.J.;Tang,H.;Zhou,G.H.; Wei,H.Y.;Jian,Y.;Luo,S.Z.J.Hazard.Mater.2014,264,386. doi:10.1016/j.jhazmat.2013.11.032 (5)Anthony,R.G.;Philip,C.V.;Dosch,R.G.Water Manage. 1993,13,503.doi:10.1016/0956-053X(93)90080-G (6)Manos,M.;Kanatzidis,M.J.Am.Chem.Soc.2012,134,16441. doi:10.1021/ja308028n (7)El-Kamash,A.M.J.Hazard.Mater.2008,151,432. doi:10.1016/j.jhazmat.2007.06.009 (8)Latheef,I.M.;Huckman,M.E.;Anthony,R.G.Ind.Eng. Chem.Res.2000,39,1356.doi:10.1021/ie990748u (9)Lu,S.;Xu,J.;Zhang,C.;Niu,Z.J.Radioanal.Nucl.Chem. 2011,287,893.doi:10.1007/s10967-010-0849-1 (10)Pang,H.F.;Xiang,X.;Li,Z.J.;Fu,Y.Q.;Zu,X.T.Phys. Status Solidi A 2012,209,537.doi:10.1002/pssa.201127456 (11)Phuruangrat,A.;Ham,D.J.;Hong,S.J.;Thongtema,S.;Lee,J. S.J.Mater.Chem.2010,20,1683.doi:10.1039/B918783A (12)Griffith,C.S.;Luca,V.Chem.Mater.2004,16,4992.doi: 10.1021/cm049335w (13)Griffith,C.S.;Luca,V.;Hanna,J.V.;Pike,K.J.;Smith,M.E.; Thorogood,G.S.Inorg.Chem.2009,48,5648.doi:10.1021/ ic801294x (14)Alkan,M.;Karadaş,M.;Doğan,M.;Demirbaş,Ö.Coll.Surf.A: Physicochem.Eng.Aspects 2005,291,309.doi:10.1016/j. colsurfa.2005.02.024 (15)Leroy,P.;Revil,A.J.Colliod Interface Sci.2004,270,371.doi: 10.1016/j.jcis.2003.08.007 (16)Ersoy,B.;Çelik,M.S.Micropor.Mater.2002,55,305. doi:10.1016/S1387-1811(02)00433-X (17)Alkan,M.;Demirbaş,Ö.;Doğan,M.J.Colliod Interface Sci. 2005,259,155.doi:10.1016/j.jcis.2005.05.027 (18)Doğan,M.;Alkan,M.;Türkyilmaz,A.;Özdemir,Y.J.Hazard. Mater.2004,192,141.doi:10.1016/j.jhazmat.2004.03.003 (19)Liu,Z.Y.;Sun,D.D.;Guo,P.;Leckie,J.O.Chem.Eur.J.2007, 13,1851.doi:10.1002/chem.200601092 (20)Rougier,A.;Portemer,F.;Quédé,A.;Marssi,A.E.Appl.Surf. Sci.1999,153,1.doi:10.1016/S0169-4332(99)00335-9 (21)Szilágyi,I.M.;Madarász,J.;Hange,F.;Pokol,G.J.Therm. Anal.Calorim.2007,88,139.doi:10.1007/s10973-006-8078-0 (22)Wang,T.H.;Liu,T.Y.;Wu,D.C.;Li,M.H.;Chen,J.R.;Teng, S.P.J.Hazard.Mater.2010,173,335.doi:10.1016/j. jhazmat.2009.08.091 (23)Laskowski,J.S.J.Colliod Interface Sci.1993,159,349. doi:10.1006/jcis.1993.1333 (24)Moreira,W.C.;Gushikem,Y.;Nascimento,O.R.J.Colliod Interface Sci.1992,150,115.doi:10.1016/0021-9797(92) 90272-N (25)Mpandou,A.;Siffert,B.J.J.Colliod Interface Sci.1984,102, 138.doi:10.1016/0021-9797(84)90207-8 (26)Duman,O.;Tunç,S.Microporous Mesoporous Mat.2009,117, 331.doi:10.1016/j.micromeso.2008.07.007 (27)Kaya,A.;Yukselen,Y.Can.Geotech.J.2005,42,1280. doi:10.1139/t05-048 (28)Yukselen,Y.;Kaya,A.Environ.Earth.Sci.2011,62,697. doi:10.1007/s12665-010-0556-9 (29)Alkan,M.;Doğan,M.J.Colliod Interface Sci.1998,207,90. doi:10.1006/jcis.1998.5694 (30)Tekin,N.;Demirbaş,Ö.;Alkan,M.Microporous Mesoporous Mat.2005,85,340.doi:10.1016/j.micromeso.2005.07.004 (31)Blockhaus,F.;Sequaris,J.M.;Narres,H.D.;Schwuger,M.J. J.Colliod Interface Sci.1997,186,234.doi:10.1006/ jcis.1996.4639 (32)Vermöhlen,K.;Lewandowski,H.;Narres,H.D.;Schwuger,M. J.Surf.A 2000,163,45.doi:10.1016/S0927-7757(99)00429-X (33)Nassem,R.;Tahir,S.Water Res.2001,35,3982.doi:10.1016/ S0043-1354(01)00130-0 Removal of Sr2+Ions by Ta-Doped Hexagonal WO3:Zeta Potential Measurements and Adsorption Mechanism Determination JIAN Yuan1,2,*MU Wan-Jun2LIU Ning1PENG Shu-Ming1,2,* The adsorption of Sr2+ions onto Ta-doped hex-WO3nanomaterials was studied by measuring the zeta potentials of the powder nanoparticles and by determining the adsorption isotherm and adsorption mechanism.Five important results were obtained:(1)the zeta potential values of the Ta-doped hex-WO3suspensions in different electrolyte solutions,within the studied pH ranges,increased with an increase in electrolyte valence;and(2)the zeta potential of the Ta-doped hex-WO3suspensions increased with an increase in ionic strength.(3)Sr2+ion adsorption increased with a decrease in temperature and ionic strength.(4)The adsorption enthalpy was calculated as-47 kJ·mol-1,and the interaction between the Ta-doped hex-WO3surface and Sr2+ions was concluded to be chemical in nature.(5)The adsorption of Sr2+ions onto the Ta-doped hex-WO3was attributed to surface chemical adsorption and ion exchange(in tunnels). Ta;hex-WO3;Zeta potential;Sr2+;Adsorption mechanism February 29,2016;Revised:April 20,2016;Published on Web:April 21,2016. O647 10.3866/PKU.WHXB201604213 *Corresponding authors.PENG Shu-Ming,Email:pengsm01@163.com;Tel:+86-816-2495481.JIAN Yuan,Email:inpc207@163.com; Tel:+86-816-2492703. The project was supported by the National Natural Science Foundation of China(21501159). 国家自然科学基金(21501159)资助项目 ©Editorial office ofActa Physico-Chimica Sinica [Article]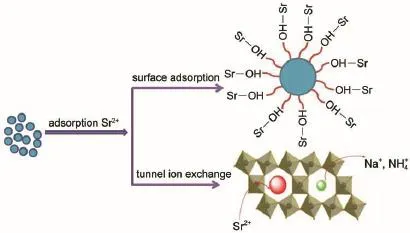

4 Conclusions
(1Key Laboratory of Radiation Physics and Technology,Ministry of Education,Institute of Nuclear Science and Technology, Sichuan University,Chengdu 610064,P.R.China;2Institute of Nuclear Physics and Chemistry, China Academy of Engineering Physics,Mianyang 621900,Sichuan Province,P.R.China)
