利用原位变温EPR研究富勒烯双体笼间的弱C―C键
2016-09-09杨佳慧朱春华王官武陈家富苏吉虎
谭 天 杨佳慧 朱春华 王官武 陈家富 苏吉虎,*
(1中国科学技术大学,合肥微尺度物质科学国家实验室,近代物理系,合肥230026;2中国科学技术大学,合肥微尺度物质科学国家实验室,化学系,合肥230026)
利用原位变温EPR研究富勒烯双体笼间的弱C―C键
谭天1杨佳慧1朱春华2王官武2陈家富2苏吉虎1,*
(1中国科学技术大学,合肥微尺度物质科学国家实验室,近代物理系,合肥230026;2中国科学技术大学,合肥微尺度物质科学国家实验室,化学系,合肥230026)
本文研究了(C60)2-[P(O)(OCH3)]2富勒烯双体内的笼间C―C键的热力学性质(该双体的结构详见文献, Chem.Commun.2011,47,6111)。原位、变温电子顺磁共振波谱实验结果表明,该C―C键的键离解能(BDE) 为72.4 kJ·mol-1(17.3 kcal·mol-1),仅约为常见氢键的两倍,或约为常见有机C―C键的五分之一。因此,该二聚体于较高温度时容易发生均裂反应,形成单体自由基;降温时又容易发生自由基聚合反应。基于该笼间C―C键所具有的这些热力学特性,我们对其可被用于制备有序的富勒烯分子元器件等材料作展开讨论。
C60富勒烯双体;笼间C―C键;热力学性质;键离解能;电子顺磁共振波谱学
www.whxb.pku.edu.cn
1 Introduction
Molecular devices have been studied and applied because of supplying high-performance analytical systems and wide applications1-4.One of them,fullerene,since its discovery in 1985 by Kroto et al.5,is of great interest for its unique chemical and physical properties6-8.A great effort has been made on fullerene molecules and their derivative,and the potential application6-11. Fullerenes used in most of these studies are powder or solution ordopant12,since the single crystals with the rather larger scale are difficult to be grown.For some applications,the aligned fullerenes are however necessary8,for example,the multilayers at Si surface13.The de novo preparation of the aligned fullerenes is difficult since they are synthesized at~2300 K6,7,14.Fullerene oligomers, i.e.the dimer and trimer,are generally produced by the chemical modifications15-18.For[C60]fullerene,an intercage C―C bond is present in the[C60]fullerene dimer,and the dimerization has been found to be promoted by the manganese(III)-catalyzed acetatemediated reaction(Scheme 1)16,17.Although the fullerene oligomers are not stable,the thermodynamics property of this intercage C―C bond is not clear when compared to those of the common organic C―C bonds19.This,however,has not been studied,which might be of importance as a very fundamental and crucial question19.Chemically,the different stability of C―C bond gives to the different active property in the designed experiments.In the present study,this question was stressed,and investigated by the X-band in situ variable-temperature electron paramagnetic resonance(EPR)spectroscopy.
2 Methods and materials
The dimeric fullerene,(C60)2-[P(O)(OCH3]2(termed dimer below, see Scheme 1)was synthesized as the method in the previous report17.The dimer was suspended in 1,2-dichlorobenzene (ODCB)with a concentration of 0.5 mmol·L-1,and the suspension solution was transferred into the 100 μL standard capillary.After sealed,the capillary was inserted into the standard 4-mm EPR tube.The in situ EPR measurements were performed on an X-band(9.087 GHz)JES-FA200 EPR spectrometer equipped with a cavity which is capable of varying the temperature from room temperature to 473 K.The sample in EPR tube was heated by the nitrogen(99.9%)gas flow with the heating rate of 1-100 K· min-1.The examined temperature varied from 293 to 413 K(20-140°C)in stepwises of 5 K.After 2 min heating per step,the spectrum was scanned in 1 min.A buildup equilibrium between the initial dimer and the monomeric·C60P(O)(OCH3)2radical product was assumed to be established after 2-min heating at certain temperature(cf.Scheme 1)when the EPR signal did not change with elongated heating time.EPR settings:microwave frequency 9.048 GHz,microwave power 1 mW,modulation amplitude 1 Gauss,room temperature to 408 K(or 135°C), sweeping time 60 s.

Scheme 1 Molecular structure and the reversible dissociation of (C60)2-[P(O)(OCH3)2]2dimer at 373 K
3 Results and discussion
The geometric structure of the dimer was depicted in Scheme 1,and the pivot intercage C―C bond was highlighted.The intercage C―C bond was located in a diamond-like framework where three carbon bonds composed the respectively C60cage and the fourth,i.e.the intercage C―C bond,bridged directly between the two cages(cf.Ref.17 for the detailed molecular structure). Although the C60dimer was previously found to be not stable17,the thermodynamic mechanism was not known.Apparently,no signal was observed in the initial sample at room temperature EPR measurement.When the sample solution was heated up to 343 K (70°C)or higher,a two-band hyperfine feature centered around the characterized isotropic g-value,giso~2.0022,was clearly resolved in the spectrum(Fig.1).The signal was assigned to the formed monomer product radicals·C60P(O)(OCH3)2induced by heating17.The isotropic hyperfine interaction between the unpaired electron and31P,Aiso,was determined from the spectrum.The value of 64.7 Gauss(or 181.4 MHz)was the splitting distance between the two hyperfine peaks.As for the contaminant C60radical,no hyperfine structure was found(marked by star).The value of Aiso(64.7 Gauss)indicated that the31P nucleus carried 1.4%of all the spin density;for 100%density,the value is 4748 Gauss in principle20.Obviously,the unpaired electron was delocalized onto the C60cage and a π-radical was formed21.The electronic structure unveiled by EPR was also consistent with the fact that the C60cage is a known π-conjugation system6-8.
The EPR spectrum in Fig.1 also showed no spectral overlapping,i.e.,only one type of radical originating from the dimer was induced thermally.At this point,only the homolysis of the intercage C―C bond can give to the identical radical product (Scheme 1).The cleavage elsewhere in the dimer will lead to the different EPR signals20,21.Therefore,the EPR signal intensity as a function of the heating temperature was examined to reveal the thermodynamics property of the intercage C―C bond.In Fig.2a, the peak-to-trough amplitudes of both hyperfine peaks increasedwith the elevated temperature from 343 to 408 K.At 343 K (70°C)or lower,the EPR signal was hardly resolved.To avoid the boiling point(453 K)of the solvent 1,2-dichlorobenzene,the temperature was stopped at 408 K(135°C).In the following reversible treatment,the EPR signal vanished after the sample was cooled down to room temperature,and the similar temperature dependence was re-appearing again when the sample was heating again.

Fig.1 Experimental(black)and simulated(red)EPR spectra of the formed monomer·C60P(O)(OCH3)2radical induced by heating
Herein,the equilibrium constant Keqbetween the dimer(D)and the monomeric radical(R)was simplified as Keq=[R]2/[D] (Scheme 1).The temperature-dependent Keqwas monitored by the corresponding EPR signal intensity(I)(Fig.2a,cf.the methods in Refs.22,23).In principle,Keqat ambient temperature can be calculated by equation(1),

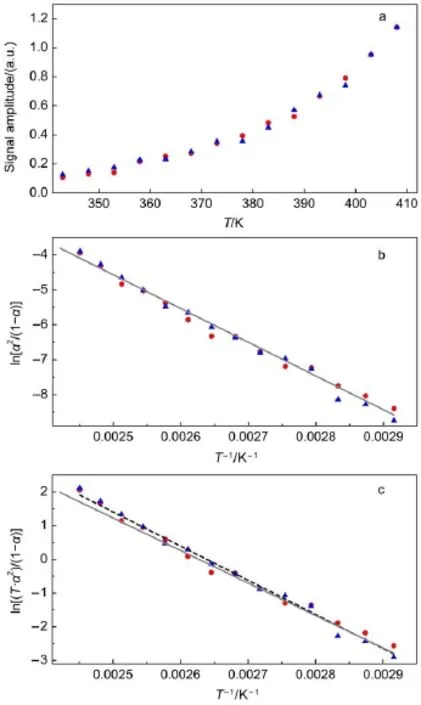
Fig.2 Temperature dependence of the EPR signal
where I is the ambient EPR signal amplitude,I0is the maximum amplitude at 408 K,and C0is the initial concentration of C60dimer. If the bonddissociationenergyor enthalpy(BDE,ΔHӨ)andentropy (ΔSӨ)were temperature independent at the range of 343 to 408 K, both were calculated from the known van′t Hoff′s equation(2),

where R(8.31 J·mol-1·K-1)and T are the universal gas constant and the experimental temperature respectively.The plot of ln[α2/ (1-α)]vs 1/T,i.e.,lnKeqvs 1/T,was fit by linear regression in Fig.2b because the corresponding slope k was equal to-ΔHӨ/R. Namely,ΔHӨ=-k·R.Herein,the experimental slope was -8704.7 K-1(Fig.2b).Therefore,ΔHӨ=8704.7×8.31=72.4 kJ· mol-1(17.3 kcal·mol-1)(1 kcal·mol-1=4.186 kJ·mol-1)was calculated,as well ΔSӨ=135.5 J·mol-1·K-1(32.4 cal·mol-1·K-1).
When the Boltzmann distribution difference was considered, the theoretical calibration can give a few percent fluctuation of the anthalpy(Fig.2c).Anyway,the experimental BDE was only twice of that of the typical hydrogen bond(~40 kJ·mol-1),or about one fifth of those in the diamond or the saturated hydrocarbons(Table 1,also cf.the monograph19).This intercage C―C bond was active and flexible giving to the unstable dimer.The small BDE also implied that the length of the intercage C―C bond was unusually long,which is consistent with the length(0.1582 nm)revealed by the X-ray crystal structure17.Similarly,we also noticed in the literature that the BDE values for the C―C bond formed during the dimerization of the phenalenyl radicals were estimated to be 11.34 kcal·mol-1in CCl4and 9.8 kcal·mol-1in toluene solvent22. These were comparable to data in present study.After scrutinized inspection,these radicals have the similar large-scale π-conjugation features which are spherical or planar,and the interaction between the big π-groups possibly weakens the resultant C―C bond. However,it is still premature to elucidate how the complicate sp orbital hybridization in the carbon leads to the different strength of the C―C bond in the diamond-like framework,even down to the level of the common hydrogen bonds.Anyway,the small BDE demonstrates that this C―C bond is an active site for the further reactions,say,dimerization,reduction or oxidation19.These data also provide the meaningful information to design the similar flexible C―C bond for the organic synthesis.
Generally,the cleavage and rearrangement reactions always occur at the site with the small value of BDE19.The thermodynamic property of the single C―C bond in the[C60]fullerene dimer may shed light onto designing the different functionalized fullerenes.So far as we know,one of bottleneck problems to apply the aligned fullerene as molecular device is how to align the fullerene at the molecular or nano level.The present study shows the possibility to produce the aligned fullerene molecule,which is based on the structural and thermal property of the fullerenedimer17.Firstly,the phosphonate ester has the advantage to stabilize the monomeric fullerene radical.Secondly,the phosphonate esters can provide either the active site for the medication/alteration,or the affinity to the anticipated supporting base,and then aligned[C60]fullerene dimer is produced on the designed base,as that in our previous study11.Thereafter,the heating treatment at around 400 K causes the readily homolysis of the intercage C―C bond,and gives the aligned fullerene radical pairs on the base.If the treatment is performed at the reductive or oxidative ambient atmosphere,the formed radical is quenched selectively.Finally the aligned monomeric[C60]fullerene as the molecular functionalization of surfaces for device applications is thus produced,which can be utilized in the potential fields.These multi-step protocol might provide the better aligned fullerenes when compared to that gowned directly as in Ref.13.For example,the interaction between the radical pair is an interesting fundamental research in quantum computation.The variety of the supporting base or matrix are also helpful to further exploit the variable applications of the fullerenes,such as in material science,condensed physics, quantum computation science,and life science6-8,24-28.

Table 1 BDE and length of common C―C bond
4 Conclusions
The bond dissociation enthalpy of a intercage C―C bond in [C60]fullerene dimer was explored thermodynamically by in situ variable-temperature EPR.The value of 72.4 kJ·mol-1(17.3 kcal· mol-1)indicated that this C―C bond was an active and flexible site in the molecule.
References
(1)Balzani,V.;Venturi,M.;Credi,A.Molecular Devices and Machines:a Journey into the Nano World;Wiley-VCH: Weinheim,2003;pp xvii,494.
(2)Balzani,V.;Credi,A.;Venturi,M.Molecular Devices and Machines:Concepts and Perspectives for the Nanoworld,2nd ed.;Wiley-VCH:Weinheim,2008.
(3)Tiwari,A.Intelligent Nanomaterials:Processes,Properties,and Applications;John Wiley&Sons,Scrivener Pub.:Hoboken,NJ, 2012;pp xxiv,838.
(4)Pignataro,B.Ideas in Chemistry and Molecular Sciences. Advances in Nanotechnology,Materials and Devices;Wiley-VCH:Weinheim,2010;pp xxii,410.
(5)Kroto,H.W.;Heath,J.R.;Obrien,S.C.;Curl,R.F.;Smalley, R.E.Nature 1985,318,162.doi:10.1038/318162a0
(6)Langa,F.;Nierengarten,J.F.Fullerenes:Principles and Applications;The Royal Society of Chemistry:Cambridge CB4 0WF,UK,2007.
(7)Martín,N.;Giacalone,F.Fullerene Polymers:Synthesis, Properties and Applications;WILEY-VCH Verlag GmbH&Co. KGaA:Weinheim,2009.
(8)Yang,S.;Wang,C.R.Endohedral Fullerenes:from Fundamentals to Applications;World Scientific Publishing Company,2014;pp xii,434.
(9)Suter,D.;Lim,K.Phys.Rev.A 2002,65,052309.doi:10.1103/ Physreva.65.052309
(10)Ju,C.Y.;Suter,D.;Du,J.F.Phys.Rev.A 2007,75,012318. doi:10.1103/Physreva.75.012318
(11)Yang,J.H.;Feng,P.B.;Sygula,A.;Harneit,W.;Su,J.H.;Du, J.F.Phys.Lett.A 2012,376,1748.doi:10.1016/j. physleta.2012.04.014
(12)Marcaccio,M.E.;Paolucci,F.E.;Bachawala,P.A.Making and Exploiting Fullerenes,Graphene,and Carbon Nanotubes(3) Springer:Heidelberg,New York,Dordrecht,London,2014.
(13)Du,X.Q.;Li,H.Q.;Zhu,Q.R.;Zou,Z.Q.;Liang,Q.Acta Phys.-Chim.Sin.2011,27,2457.[杜晓清,李慧琴,朱齐荣,邹志强,梁齐.物理化学学报,2011,27,2457.]doi:10.3866/ PKU.WHXB20111010
(14)Deng,S.;Liu,X.S.;Huang,R.B.;Zheng,L.S.Scientia Sinica Chimica 2012,42,1587.[邓顺柳,谢素原,黄荣彬,郑兰荪.中国科学:化学,2012,42,1587.]doi:10.1360/032012-239
(15)Cheng,F.Y.;Murata,Y.;Komatsu,K.Org.Lett.2002,4,2541. doi:10.1021/Ol026281s
(16)Zhang,T.H.;Lu,P.;Wang,F.;Wang,G.W.Org.Biomol.Chem. 2003,1,4403.doi:10.1039/B309939c
(17)Wang,G.W.;Wang,C.Z.;Zhu,S.E.;Murata,Y.Chem. Commun.2011,47,6111.doi:10.1039/C1cc10820d
(18)Lu,S.R.;Jin,T.N.;Kwon,E.;Bao,M.;Yamamoto,Y.Angew. Chem.Int.Edit.2012,51,802.doi:10.1002/anie.201107505
(19)Luo,Y.R.Handbook of Bond Dissociation Energies in Organic Compounds;CRC Press LLC:Boca Raton,London,New York, Washington,D.C.,2002.
(20)Weil,J.A.;Bolton,J.R.Electron Paramagnetic Resonance: Elementary Theory and Practical Applications,2nd ed.;John, A.W.,James,R.B.Eds.;Wiley:Chichester;John Wiley: Hoboken,N.J.,2007.
(21)Gerson,F.;Huber,W.Electron Spin Resonance Spectroscopy of Organic Radicals;Wiley-VCH:Weinheim,2003.
(22)Zheng,S.J.;Lan,J.;Khan,S.I.;Rubin,Y.J.Am.Chem.Soc. 2003,125,5786.doi:10.1021/Ja029263o
(23)Denisov,E.T.;Denisova,T.G.;Pokidova,T.S.Handbook of Free Radical Initiators;Wiley-Interscience:Hoboken,N.J., 2003.
(24)Guo,Z.X.;Li,Y.L.;Daoben,Z.Progress in Chemistry 1998, 10,1.[郭志新,李玉良,朱道本.化学进展,1998,10,1.] doi:10.3321/j.issn:1005-281X.1998.01.001
(25)Lyshevski,S.E.Nano and Molecular Electronics Handbook; CRC:London,Taylor&Francis,Boca Raton,Fla.,2007.
(26)Treat,N.D.;Chabinyc,M.L.Annu.Rev.Phys.Chem.2014,65, 59.doi:10.1146/annurev-physchem-040513-103712
(27)Scharber,M.C.Adv.Mater.2016,28,1994.doi:10.1002/ adma.201504914
(28)Pilehvar,S.;De Wael,K.Biosensors(Basel)2015,5,712. doi:10.3390/bios5040712
A Weak Intercage C―C Bond in a[C60]fullerene Dimer Studied by In situ Variable Temperature EPR Spectroscopy
TAN Tian1YANG Jia-Hui1ZHU Chun-Hua2WANG Guan-Wu2
CHEN Jia-Fu2SU Ji-Hu1,*
(1Hefei National Laboratory for Physical Science at Microscale,Department of Modern Physics,University of Science and Technology of China,Hefei 230026,P.R.China;2Hefei National Laboratory for Physical Science at Microscale, Department of Chemistry,University of Science and Technology of China,Hefei 230026,P.R.China)
This work studied the thermodynamic properties of a single intercage C―C bond in a[C60]fullerene dimer,(C60)2-[P(O)(OCH3)]2,previously synthesized by Wang et al.(Chem.Commun.2011,47,6111).Data obtained from in situ variable temperature electron paramagnetic resonance(EPR)indicated a relatively low bond dissociation enthalpy(BDE)for this bond of 72.4 kJ·mol-1(17.3 kcal·mol-1).This value is only approximately twice that of a typical hydrogen bond,or one fifth of the values determined for bonds in diamond or saturated hydrocarbons.The application of this pre-synthesized dimer to the formation of aligned fullerenes is discussed.
[C60]fullerene dimer;Singly intercage C―C bond;Thermodynamic property; Bond dissociation enthalpy;Electron paramagnetic resonance spectroscopy
March 22,2016;Revised:May 5,2016;Published on Web:May 9,2016.
O642
10.3866/PKU.WHXB201605092
*Corresponding author.Email:sujihu@ustc.edu.cn;Tel:+86-551-63607672.
The project was supported by the National Key Basic Research Program of China(973)(2013CB921802).国家重点基础研究发展规划项目(973)(2013CB921802)资助
©Editorial office ofActa Physico-Chimica Sinica
[Article]
