水杨醛亚胺锌配合物催化环酯开环聚合的研究*
2016-08-25郭鹏峰
郭鹏峰
(广东药科大学医药化工学院,广东 广州 510006)
水杨醛亚胺锌配合物催化环酯开环聚合的研究*
郭鹏峰
(广东药科大学医药化工学院,广东广州510006)
脂肪族聚酯中的聚己内酯具有良好的生物降解性、生物相容性和可渗性等特点,在医学、药学和材料领域得到广泛的应用。聚己内酯的制备主要是通过催化ε-己内酯的开环聚合得到,催化剂的活性是影响聚合反应的一个重要因素。本论文选用毒性低、价廉易得的金属锌配合物为催化体系,设计并合成了一系列对水和空气稳定的水杨醛亚胺锌催化剂,该类催化剂对已内酯开环聚合具有高的催化活性和选择性。
ε-己内酯;环酯聚合;水杨醛亚胺锌配合物
人工合成的聚己内酯作为一种可以用作体内植入材料或药物控释材料的聚酯类高分子,在生物医用领域获得广泛应用。[1]目前聚己内酯材料的合成是由ε-己内酯单体在催化剂的作用进行环酯开环聚合得到,所用的催化剂主要有正离子型催化剂[2-3]、负离子型催化剂[4-5]和配位型催化剂[6],其中配位型催化剂催化的环酯开环聚合能产生结构明确、分子量可控的聚合物,激励着人们去发展新的高效配位型催化剂。
用于环酯开环聚合的金属催化剂有很多,其中Zn[7]、Mg[8]、Fe[9]、Ca[10]金属的配合物具有对人体低毒性、较高的催化活性和选择性等特点。配位型催化剂的催化性能除受金属本身影响外,金属的配体可以改变中心金属的电子效应和位阻效应,进而引起配位型催化剂催化活性和选择性的根本改变[11-12]。本文从合成简单、成本廉价、催化剂稳定、金属无毒性出发,设计用水杨醛和3,5-二叔丁基水杨醛分别与一系列的烷基胺反应合成水杨醛亚胺配体,进而通过与金属锌配位获得对水和空气稳定的水杨醛亚胺锌配位催化剂,考察配体空间位阻和电子效应对ε-己内酯开环聚合催化活性的影响,实现高活性与高立构选择性的环酯开环聚合。
1 实 验
1.1主要试剂和仪器
六亚甲基四胺(CP),广州市桂华化工科技有限公司;2,4-二叔丁基苯酚(AR),天津市兴复精细化工研究所;水杨醛(AR),上海凌峰化学试剂有限公司;乙酸锌(AR),天津市百世化工有限公司;乙胺、三乙胺、异丙胺、正丁胺、叔丁胺、环己胺、苄胺均购自Aladdin公司(AR);ε-己内酯(AR),Johnson Mattey 公司。
核磁共振检测是在Bruker 300 MHz上进行的,TMS为内标,CDCl3为溶剂。
聚合物的分子量和分子量分布由Waters 1515 型凝胶渗透色谱仪分析确定:温度 30 ℃,HR-1,HR-2,HR-4柱子串联,四氢呋喃作淋洗剂,淋洗液流速 1.0 mL/min,采用聚苯乙烯标样对分子量进行校正。
1.23,5-二叔丁基水杨醛的合成
以2,4-二叔丁基酚和六亚甲基四胺为原料合成3,5-二叔丁基水杨醛的方法是一种成本低廉,生产工艺也比较成熟的方法。往带有搅拌回流装置的250 mL的三口圆底烧瓶中加入2,4-二叔丁基酚(20.5 g, 0.10 mol),六亚甲基四胺(24.5 g, 0.175 mol)和50 mL冰乙酸,升温至110 ℃,在加热搅拌下使其在溶剂中溶解,反应至出现大量白色粉末(大约1 h)。再加入6 g的助催化剂多聚甲醛,升温至130~140 ℃,反应2 h后,停止加热。等温度降到80 ℃后加入82 mL质量分数为15%的稀硫酸,升温至125 ℃,搅拌回流30 min。停止加热搅拌,冷却到75 ℃,将混合液倒到一个加热到75 ℃的分液漏斗里面,在该温度下静置分层,将有机层转移到新的烧杯中,再往烧杯中加入15 mL的甲醇不断地搅拌,放置冰箱静置过夜,待粗产品过滤真空干燥,然后再次使用甲醇重结晶,最终得到黄色固体产物3,5-二叔丁基水杨醛。
1.3水杨醛亚胺锌配位催化剂的合成
将水杨醛或者3,5-二叔丁基水杨醛在烧瓶中用无水甲醇溶解,加入烷基胺、醋酸锌和少许三乙胺,68 ℃回流反应24 h,抽滤并用正己烷-二氯甲烷加热回流重结晶,即得水杨醛亚胺锌配位催化剂。
1.4ε-己内酯的开环聚合
由于ε-己内酯开环本体聚合反应要求在无水无氧的环境下进行开环聚合,各反应试剂需要进行前处理:ε-己内酯加入氢化钙常温搅拌24 h,后150 ℃减压蒸馏收集ε-己内酯并密封氮气保护,待用;苄醇在135 ℃减压蒸馏除水;甲苯常压蒸馏除水。
在氮气的保护下称取充分干燥的锌配位催化剂和苄醇的甲苯溶液一次性加入到反应瓶中,装有原料的反应瓶在常温下用真空泵抽真空1 h后,将反应瓶充满充N2(反应瓶密闭),用注射器量取前处理过的ε-己内酯并通过软胶管快速注射到反应瓶中,在60 ℃反应24 h。反应结束后,加入少量的乙醇进行淬灭,再加入适量的二氯甲烷溶解,再加入95%的乙醇搅拌,直至沉淀析出后减压抽滤得到聚己内酯,重复用二氯甲烷溶解再用水沉淀析出两次,得到的聚合物在45 ℃真空干燥24 h,计算产率。
2 结果与讨论
2.1水杨醛亚胺锌配位催化剂的合成
如图1所示,水杨醛亚胺锌配位催化剂是通过水杨醛一步法合成的。我们首先利用水杨醛和烷基胺反应合成水杨醛亚胺配体,同时醋酸锌加入配位获得水杨醛亚胺锌配位催化剂。乙胺、异丙胺、正丁胺、苄胺与水杨醛反应获得4种不同位阻的配体,随之获得了4个配位催化剂(化合物C1、C2、C3、C4)。乙胺、异丙胺、正丁胺、叔丁胺、环己胺、苄胺与3,5-二叔丁基水杨醛反应获得6种不同位阻的配体,随之获得了6个配位催化剂(化合物C5、C6、C7、C8、C9、C10)。各配位催化剂产物的核磁数据如下:

图1 水杨醛一步法设计合成水杨醛亚胺锌配位催化剂
C1:1H NMR (300 M, CDCl3, ppm) (Isomer ratio isca3)。 Major isomer, δ: 8.206 (s, 2H, CH=N), 7.306 (m, 2H, Ar-H), 7.117 (m, 2H, Ar-H), 6.863 (m, 2H, Ar-H), 6.626 (m, 2H, Ar-H), 3.756~3.611 (m, 4H, =NCH2CH3), 1.296~1.238 (m, 6H, =NCH2CH3)。13C NMR (75 M, CDCl3, ppm) δ: 170.603, 163.920, 135.513, 131.894, 123.071, 118.307, 114.363, 55.549, 16.310。 Minor isomer, δ: 8.350 (s, 2H, CH=N), 7.325 (m, 2H, Ar-H), 7.095 (m, 2H, Ar-H), 6.835 (m, 2H, Ar-H), 6.600 (m, 2H, Ar-H), 3.756~3.611 (m, 4H, =NCH2CH3), 1.296~1.238 (m, 6H, =NCH2CH3)。13C NMR (75 M, CDCl3, ppm) δ: 170.603, 163.920, 134.795, 130.939, 124.522, 116.967, 114.363, 53.826, 16.310。
C2:1H NMR (CDCl3, ppm) (Isomer ratio isca4)。 Major isomer, δ: 8.215 (s, 2H, CH=N), 7.260 (d,J=8.1 Hz, 2H, Ar-H), 7.106 (d,J=7.2 Hz, 2H, Ar-H), 6.859 (d,J=7.2 Hz, 2H, Ar-H), 6.608 (d,J=7.2 Hz, 2H, Ar-H), 3.630 (m, 2H, CH(CH3)2) 1.318 (d,J=6.6 Hz, 12H, CH(CH3)2)。13C NMR (CDCl3, ppm) δ: 170.070 (C=N), 168.832, 135.565, 134.691, 122.988, 117.773, 114.170, 62.591, 24.519。 Minor isomer, δ: 8.345 (s, 2H, CH=N), 7.260 (d,J=8.1 Hz, 2H, Ar-H), 6.883 (d,J=7.2 Hz, 2H, Ar-H), 6.859 (d,J=7.2 Hz, 2H, Ar-H), 6.608 (d,J=7.2 Hz, 2H, Ar-H), 3.561 (m, 2H, CH(CH3)2), 1.220 (d,J=6.6 Hz, 12H, CH(CH3)2)。13C NMR (CDCl3, ppm) δ: 170.070 (C=N), 168.832, 135.565, 134.691, 122.988, 117.773, 114.170, 62.591, 23.945。
C3:1H NMR (300 M, CDCl3, ppm) (Isomer ratio isca2)。 Major isomer, δ: 8.330 (s, 2H, CH=N), 7.317~6.594 (m, 8H, Ar-H), 3.607 (t,J=6.9 Hz, 4H, CH=NCH2CH2CH2CH3), 1.726~1.599(m,4H,CH=NCH2CH2CH2CH3),1.473~1.399(m,4H,CH=NCH2CH2CH2CH3), 0.972 (t,J=7.2 Hz, 6H, CH3)。 Minor isomer, δ: 8.165 (s, 2H, CH=N), 7.317~6.594 (m, 8H, Ar-H), 3.562 (t,J=6.9 Hz, 4H, CH=NCH2CH2CH2CH3), 1.473~1.399(m,4H,CH=NCH2CH2CH2CH3), 1.372~1.275(m,4H,CH=NCH2CH2CH2CH3), 0.854 (t,J=7.2 Hz, 6H, CH3)。13C NMR (75M, CDCl3, ppm) δ: 170.833(C=N), 135.414, 134.624, 122.966, 117.815, 114.231, 109.522, 60.884, 32.554, 19.962, 13.618。
C4:1H NMR (CDCl3, ppm) (Isomer ratio isca1)。 Isomer 1, δ: 8.443 (s, 2H, CH=N), 7.362~7.290 (m, 9H, Ar-H), 7.169~6.575 (m, 9H, Ar-H), 4.822 (s, 2H, CH2Ph)。13C NMR (CDCl3, ppm) δ: 170.333 (C=N), 170.005, 135.741, 135.462, 134.698, 129.104, 128.461, 127.865, 123.085, 117.832, 114.176, 64.386。 Isomer 2, δ: 8.022 (s, 2H, CH=N), 7.362~7.290 (m, 9H, Ar-H), 7.169~6.575 (m, 9H, Ar-H), 4.281 (s, 2H, CH2Ph)。13C NMR (CDCl3, ppm) δ: 170.333 (C=N), 170.005, 135.741, 135.462, 134.698, 129.104, 128.461, 127.865, 123.085, 117.832, 114.176, 64.386。
C5:1H NMR (300 M, CDCl3, ppm) (Isomer ratio isca1.8)。 Major isomer, δ: 8.354 (s, 2H, CH=N), 7.434 (s, 2H, Ar-H), 7.367 (s, 2H, Ar-H), 7.078 (s, 2H, Ar-H), 6.941 (s, 2H, Ar-H), 3.755~3.527 (m, 4H, =NCH2CH3), 1.461 (s, 18H, C(CH3)3), 1.321 (s, 18H, C(CH3)3), 1.263 (t,J=4.5 Hz, 6H, =NCH2CH3)。13C NMR (75 M, CDCl3, ppm) δ: 171.041, 168.318, 155.741, 141.357, 134.785, 129.360, 116.694, 55.608, 35.607, 33.907, 31.496, 29.398, 16.710。 Minor isomer, δ: 8.217 (s, 2H, CH=N), 7.425 (s, 2H, Ar-H), 7.358 (s, 2H, Ar-H), 7.070 (s, 2H, Ar-H), 6.932 (s, 2H, Ar-H), 3.755~3.527 (m, 4H, =NCH2CH3), 1.403 (s, 18H, C(CH3)3), 1.321 (s, 18H, C(CH3)3), 1.232 (t,J=4.5 Hz, 6H, =NCH2CH3)。13C NMR (75 M, CDCl3, ppm) δ: 171.041, 168.318, 155.741, 141.357, 134.785, 129.198, 116.694, 55.608, 35.607, 33.907, 31.496, 29.398, 16.710。
C6:1H NMR (CDCl3, ppm) (Isomer ratio isca6.3)。 Major isomer: δ: 8.243 (s, 2H, CH=N), 7.433 (s, 2H, Ar-H), 6.927 (s, 2H, Ar-H), 3.667 (m, 2H, CH(CH3)2), 1.393 (s, 18H, C(CH3)3), 1.320 (s, 18H, C(CH3)3), 1.302 (t,J=6.6 Hz, 12H, CH(CH3)2)。13C NMR (CDCl3, ppm) δ: 169.814, 168.237, 141.289, 134.485, 129.514, 129.453, 116.689, 61.954, 35.540, 33.817, 31.526, 29.457, 24.993。 Minor isomer: δ: 8.243 (s, 2H, CH=N), 7.425 (s, 2H, Ar-H), 6.918 (s, 2H, Ar-H), 3.667 (m, 2H, CH(CH3)2), 1.459 (s, 18H, C(CH3)3), 1.320 (s, 18H, C(CH3)3), 1.302 (t,J=6.6 Hz, CH(CH3)2)。13C NMR (CDCl3, ppm) δ: 169.814, 168.237, 141.289, 134.485, 129.514, 129.453, 116.689, 61.954, 35.540, 33.817, 31.526, 29.457, 24.386。
C7:1H NMR (CDCl3, ppm) δ: 8.334 (s, 2H, CH=N), 7.367 (s, 2H, Ar-H), 7.074 (s, 2H, Ar-H), 3.588 (t,J=6.6 Hz, 4H, =NCH2CH2CH2CH3), 1.699(m, 4H, =NCH2CH2CH2CH3), 1.463 (s, 18H, C(CH3)3), 1.424 (m, 4H, =NCH2CH2CH2CH3), 1.324 (s, 18H, C(CH3)3), 0.970 (t,J=7.5 Hz, 6H, =NCH2CH2CH2CH3)。13C NMR (CDCl3, ppm) δ: 171.465 (C=N), 168.445, 141.337, 134.736, 129.330, 129.191, 116.738, 61.003, 35.609, 33.916, 32.903, 31.519, 29.487, 20.063, 13.643。
C8:1H NMR (CDCl3, ppm) (Isomer ratio isca3)。 Major isomer, δ: 8.345 (s, 2H, CH=N), 7.424 (s, 2H, Ar-H), 7.095 (s, 2H, Ar-H), 1.469 (s, 18H, C(CH3)3), 1.361 (s, 18H, C(CH3)3), 1.332 (s, 18H, C(CH3)3)。13C NMR (CDCl3, ppm) δ: 168.802 (C=N), 160.474, 141.367, 136.618, 129.844, 126.385, 117.794, 58.791, 35.536, 34.188, 31.619, 30.834, 29.792。 Minor isomer, δ: 8.284 (s, 2H, CH=N), 7.426 (s, 2H, Ar-H), 6.929 (s, 2H, Ar-H), 1.398 (s, 18H, C(CH3)3), 1.361 (s, 18H, C(CH3)3), 1.332 (s, 18H, C(CH3)3)。13C NMR (CDCl3, ppm) δ: 167.866 (C=N), 158.565, 139.438, 134.411, 129.385, 125.749, 116.845, 56.756, 35.090, 33.921, 31.549, 30.834, 29.546。
C9:1H NMR (CDCl3, ppm) (Isomer ratio isca9)。 Major isomer: δ: 8.234 (s, 2H, CH=N), 7.404 (s, 2H, Ar-H), 6.908 (s, 2H, Ar-H), 3.215 (m, 2H, CH), 1.933~1.670 (m, 12H, Cy-H), 1.387 (s, 18H, C(CH3)3), 1.322 (s, 18H, C(CH3)3), 1.264~0.924 (m, 8H, Cy-H).13C NMR (CDCl3, ppm) δ: 169.982, 168.246, 141.249, 134.381, 129.376, 129.261, 116.836, 70.161, 35.506, 34.555, 33.893, 31.514, 29.537, 25.342, 25.018. Minor isomer: δ: 8.359 (s, 2H, CH=N), 7.352 (s, 2H, Ar-H), 7.063 (s, 2H, Ar-H), 3.215 (m, 2H, CH), 1.933~1.670 (m, 12H, Cy-H), 1.460 (s, 18H, C(CH3)3), 1.322 (s, 18H, C(CH3)3), 1.264~0.924 (m, 8H, Cy-H)。13C NMR (CDCl3, ppm) δ: 169.982, 168.246, 141.249, 134.381, 129.376, 129.261, 116.836, 70.161, 35.144, 34.555, 34.175, 31.514, 29.537, 25.260, 24.584。
C10:1H NMR (CDCl3, ppm) δ: 8.446 (s, 2H, CH=N), 7.381~7.097 (m, 14H, Ar-H), 6.908 (s, 2H, Ar-H), 4.794 (m, 2H, CH2), 1.445(s, 18H, C(CH3)3), 1.319 (s, 18H, C(CH3)3)。13C NMR (CDCl3, ppm) δ: 171.343, 141.321, 136.608, 134.814, 129.474, 129.289, 128.957, 128.827, 128.572, 128.469, 127.524, 63.954, 35.581, 33.913, 31.542, 29.460。
2.2锌配合物催化的ε-己内酯开环聚合
图2是以苄醇做引发剂,考察了锌配位催化剂的空间位阻和电子效应对ε-己内酯开环聚合的影响。表1列出了开环聚合24 h的聚合产物,反应物中单体、催化剂、引发剂的配比为[M]:[C]:[I]=50:1:1,产率由1H-NMR分析得到。从催化剂的结构对比可以看出,引入大位阻的3,5-二叔丁基显著提高了催化剂的催化活性,催化剂C5~10开环聚合的产率接近或超过90%,低位阻的催化剂C1~4催化活性较差。锌配位催化剂进行开环聚合时,产物的分子量分布(PDI)较窄,在1.09~1.24之间。在较低的反应温度条件下(60 ℃),催化剂C1~C4催化ε-己内酯开环聚合的效果并不理想,虽然产物的分子量分布较窄,但产率低,理论分子量[Mn(理论)]与实测分子量[Mn(GPC)]相差较大,不符合活性聚合设计上的要求。增加开环聚合反应温度到80 ℃,各个催化剂催化聚合产物的理论分子量与实测分子量数值较为接近,可以实现对聚己内酯开环聚合分子量的调控。
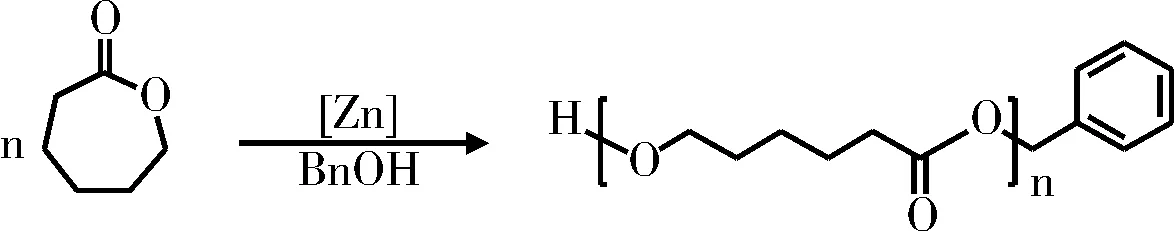
图2 ε-己内酯的开环聚合
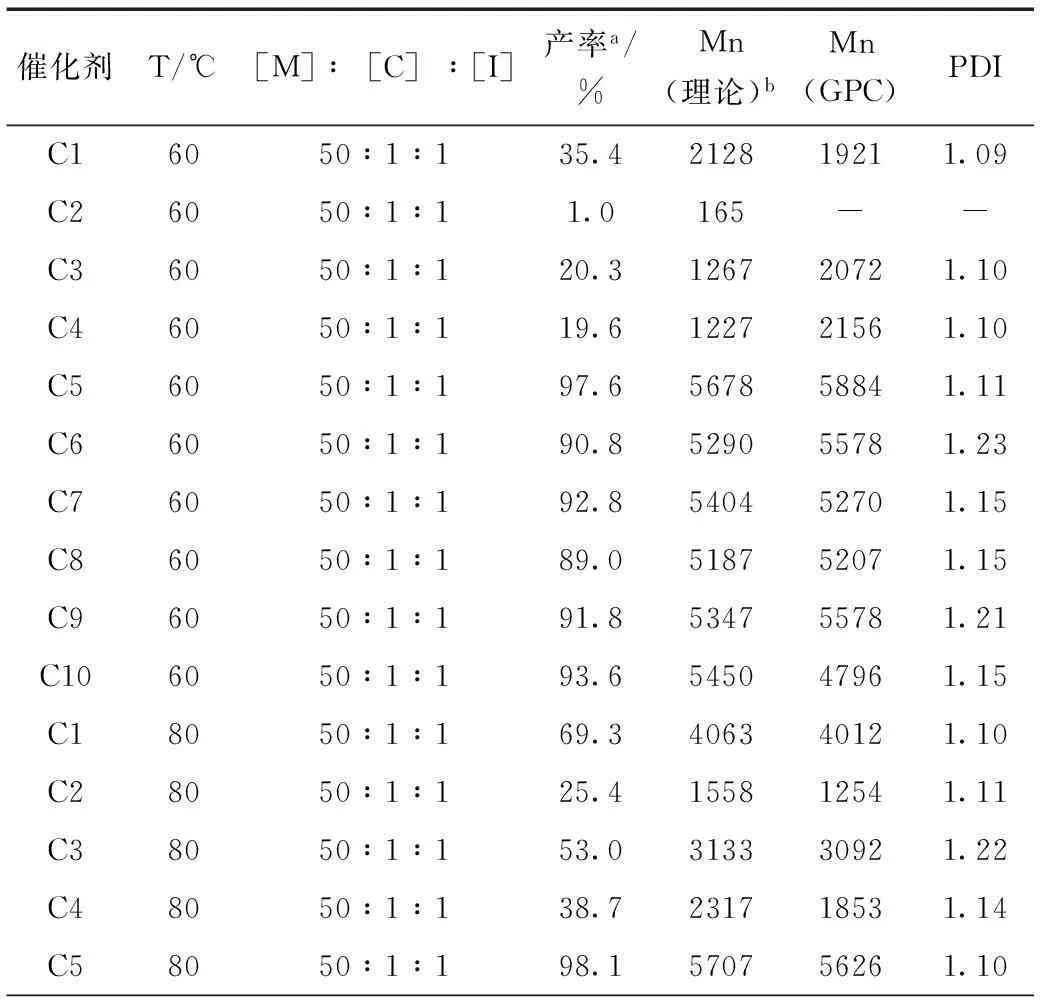
表1 锌配合物催化的ε-己内酯开环聚合Table 1 Polymerization of ε-caprolactone with salen zinc catalysts
续表1

C680501193.7545552751.12C780501194.3549049641.10C880501195.1553553791.21C980501197.2565555141.17C1080501198.7574150321.24
a反应24 h后的1H-NMR产率;b通过Mε-caprolactone×Yield×[M]/[I]+MI计算得到。
3 结 论
本论文设计并合成了一系列水杨醛亚胺锌配位催化剂,所有配合物在空气和水气中都很稳定。考察了锌配位催化剂的空间位阻和电子效应对ε-己内酯开环聚合的影响,以3,5-二叔丁基水杨醛为母核结构的锌配位催化剂催化环酯开环聚合的效果要比水杨醛为母核结构的锌配位催化剂好。水杨醛亚胺锌配位催化剂可以实现对聚己内酯开环聚合分子量的调控。
[1]Rohner D, Hutmacher DW, See P, et al. Individually CAD-CAM technique designed, bioresorbable 3-dimensional polycaprolactone framework for experimental reconstruction of craniofacial defects in the pig[J]. Mund Kiefer Gesichtschir, 2002, 6(3): 162-167.
[2]Hayakawa M, Mitani M, Yamada T, et al. Living ring-opening polymerzation of lactones using cationic zirconocene complex catalysts[J]. Macromolecular Chemistry and Physics, 1997, 198(5): 1305-1317.
[3]Nomura N, Taira A, Tomioka T, et al. A catalytic approach for cationic living polymerization: Sc(Otf)3-catalyzed ring-opening polymerization of lactones[J]. Macromolecules, 2000, 33(5): 1497-1499.
[4]Yoshida E, Osagawa Y. Synthesis of poly(epsilon-caprolactone) with a stable nitroxyl radical as an end-functional group and its application to a counter radical for living radical polymerization[J]. Macromolecules, 1998, 31(5): 1446-1453.
[5]Balsamo V, Gyldenfeldt F V, Stadler R. Synthesis of SBC, SC and BC block copolymers based on polystyrene (S), polybutadiene (B) and a crystallizable poly(e-caprolactone) (C) block[J]. Macromolecular Chemistry and Physics, 2003, 197(3): 1159-1169.
[6]王建明, 陈伟, 祝桂香. 开环聚合制备聚己内酯[J]. 石化技术与应用, 2006,24(6): 492-493.
[7]Silvernail C M, Yao L J, Hill L M R, et al. Structural and mechanistic studies of bis (phenolato) amine zinc (II) catalysts for the polymerization of ε-caprolactone[J]. Inorganic chemistry, 2007, 46(16): 6565-6574.
[8]Lian B, Thomas C M, Casagrande O L, et al. Magnesium complexes based on an amido-bis (pyrazolyl) ligand: Synthesis, crystal structures, and use in lactide polymerization[J]. Polyhedron, 2007, 26(14): 3817-3824.
[9]O’ Keefe B J, Breyfogle L E, Hillmyer M A, et al. Mechanistic comparison of cyclic ester polymerizations by novel iron (III)-alkoxide complexes: single vs multiple site catalysis[J]. Journal of the American Chemical Society, 2002, 124(16): 4384-4393.
[10]Chisholm M H, Gallucci J, Phomphrai K. Lactide polymerization by well-defined calcium coordination complexes: comparisons with related magnesium and zinc chemistry[J]. Chemical Communications, 2003 (1): 48-49.
[11]Haiyan Ma, Gianluca Melillo, Leone Oliva, et al. Living polymerization of racemic lactide by aluminum initiators supported by tetradentate OSSO ligands[J]. Dalton Trans, 2005:721-727.
[12]Chamberlain B M, Cheng M, Moore D R, et al. Polymerization of lactide with zinc and magnesium β-diiminate complexes: stereocontrol and mechanism[J]. Journal of the American Chemical Society, 2001, 123(14): 3229-3238.
Salicylaldehyde Imines Zinc Complexes Catalyzed Ring Opening Polymerization of Cyclic Esters*
GUO Peng-feng
(School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University,GuangdongGuangzhou510006,China)
Aliphatic polyester, especially polycaprolactone (PCL), is widely used in medical, pharmaceutical and material field, due to their biodegradability and biocompatibility. The main method for synthesizing PCL is the ring-opening polymerization (ROP) of ε-caprolactone (CL), and the activity of the catalyst is one of the important factors that influence the polymerization. low-toxic and accessible zinc complexes were chose as catalytic system, a series of water-and air-stable salicylaldehyde imines zinc complexes were designed and synthesized, these catalysts showed high catalytic activity and selectivity for the ring opening polymerization of CL.
ε-caprolactone; cyclic ester polymerization; salicylaldehyde imines zinc complexes
广州市科技计划项目(No.1563000282);广东药科大学“创新强校工程”医药化工省级实验教学示范中心资助项目。
郭鹏峰(1979-),男,讲师,主要从事功能性材料的制备和应用研究。
O624.11
A
1001-9677(2016)014-0059-04
