Salt-free reactive dyeing of betaine-modified cationic cotton fabrics with enhanced dye fixation☆
2016-05-29WeiMaMeiMengShuminYanShufenZhang
Wei Ma,Mei Meng,Shumin Yan,Shufen Zhang
State Key Laboratory of Fine Chemicals,Dalian University of Technology,Dalian 116023,China
1.Introduction
In recent years,environmental pollution in the dyeing industry has aroused great public concern and substantial researches were focused on solving the problem[1-5].Reactive dyes are a kind of popular dyes for cotton dyeing due to their excellent properties,such as wide range of hue,brilliancy and good w et fastness.How ever,ow ing to the low affinity between the dyes and the fibers,a large amount of salt(30-100 g·L-1),such as sodium sulfate or sodium chloride,is added in the dyebath to promotedyeadsorption in the exhaust dyeing method[6].As it is not consumed during dyeing process,the added salt is all released after dyeing.How ever,the salt-containing dye wastewater is quite difficult to deal with and does great harm to the environment.In order to solve the problem,cationization of cotton has been widely studied in recent years for effective adsorption of reactive dyes in the absence of salt[7-15].Among the cationic agents used,most of them are synthetic compounds which may present safety problem in application.Although some biopolymers or their derivatives,such as chitosan and its derivative,have been studied,the polymers on cotton may prevent dye penetration into the fibers and influence dye fixation.In addition,the existence of cationic groups on cotton surface easily leads to color staining and results in inferior color fastnesses,especially low light fastness[16-19].
In this study,a novel cationic agent—betaine is designed to be used to modify cotton fibers.Betaine(N,N,N-trimethyl glycine)is a kind of natural product.It was named after its discovery in sugar beets(Beta vulgaris)in the 19th century.Betaine show s good biodegradability and biocompatibility.It iseven ediblefor health care,and usually used asadditive in food or animal feed.Under acidic condition,betaine partially or totally turns into betaine hydrochloride which contains both a quaternary ammonium group and carboxyl group.Cationization of the fibers can berealized through reaction of thecarboxyl group of betaine hydrochloride and the hydroxyl group of cotton to form an ester bond(see Fig.1).In this study,as-prepared cationic fabrics were for the first time designed to beapplied in salt-free dyeing of reactive dyes.Another merit of the betaine-modified fabrics is that the formed ester bonds could hydrolyze under alkaline and high-temperature conditions,which just accords with the dye fixation conditions,thus the cationic groups could be removed from cotton to decrease the influence of color staining.
As know n,ester bonds are usually formed in organic solvents and the reaction conditions are severe,which are not suitable for treatment of cotton fabrics.Our previous study show ed that with the dry method and dicyandiamide as condensing agent,a kind of novel cationic cellulose—cellulose betainate was successfully prepared[20].Borrowing the synthesis idea above,a facile pad-dry-bake method was designed for pretreatment of cotton fabrics with betaine as a cationic agent.In addition,to achieve high dye fixation and effective hydrolysis of ester bonds,the steaming dye fixation process was designed for the cationic cotton[21].The objectives of this study are to characterize the betaine-modified cationic fabrics,measure their properties and investigate their dyeing performance with three commercial dyes—C.I.Reactive Red 195,C.I.Reactive Yellow 145 and C.I.Reactive Blue 19(as shown in Fig.2).

Fig.1.Preparation of cationic cotton.
2.Experimental
2.1.Materials
100%cotton,bleached,desized and mercerized,was purchased from Testfabrics,Inc.,Shanghai(China).Anhydrous betaine was purchased from Hangzhou Wan Jing New Materials Co.,Ltd.(Zhejiang,China).Dicyandiamide was purchased from Tianjin Bodi Chemical Co.,Ltd.(China)and was analytically pure.The reactive dyes in this study were obtained from Shanghai Dyestuff Co.(China)and used asreceived.The other reagents and solvents were analytically pure.
2.2.Pretreatment of cotton fabrics
Anhydrous betaine was dissolved in water to obtain an 8wt%solution and a certain amount of hydrochloric acid was added to yield a molar ratio of it to betaine of 1:1.Then dicyandiamide was added to the above solution to obtain a 5wt%concentration.Cotton fabrics were dipped into the above aqueous solution at a liquor ratio of 10:1 and padded on a mangle to give 90%pickup.The two-dip-two-pad procedure was used.The padded fabrics were dried at 80°C for 3 min,and then baked at 150°C for 40 s.In the follow ing,the pretreated fabrics were washed and then dried in vacuo.The nitrogen content of the pretreated cotton was 0.027 mol·g-1.
2.3.Dyeing procedure
Dyeing was carried out using a liquor ratio of 20:1.C.I.Reactive Red 195 applied was 2%o.w.f,C.I.Reactive Yellow 145 was 1%o.w.f and C.I.Reactive Blue 19 was 3%o.w.f.Exhaust dyeing was used for both untreated and cationic cotton fabrics,while two different dye fixation procedures—dye-bath fixation and steaming fixation were employed and compared.
Procedure 1:untreated cotton fabrics were dyed at 30°C over 40 min in dyebath with the addition of 60 g·L-1anhydrous sodium sulfate,and then the tempera turerose to 60°C with the heating-rate of 2 °C·min-1,followed by the addition of 10 g·L-1of sodium carbonate for dye fixation and kept at fixation temperature for 40 min.Procedure 2:cationic cotton fabrics were dyed at 30°C over 40 min in dyebath without the addition of sodium sulfate,and then the temperature rose to 60 °C with the heating-rate of 2 °C·min-1,follow ed by the addition of 10 g·L-1of sodium carbonate for dye fixation and kept at fixation temperature for 40 min.
Procedure 3:untreated cotton fabrics were dyed at 30°C over 40 min in dyebath with the addition of 60 g·L-1anhydrous sodium sulfate.After that,the fabrics were taken out of the dyebath and padded,then dipped in 10 g·L-1of Na2CO3aqueous solution and padded with 80%pick-up.The dyed cotton was dried and steamed for 10 min.
Fig.2.Structures of the dyes applied.
Procedure 4:cationic cotton fabrics were dyed at 30°C over 40 min in dyebath without the addition of sodium sulfate.After that,the fabrics were taken out of the dyebath and padded,then dipped in 10 g·L-1of Na2CO3aqueous solution and padded with 80%pickup.The dyed cotton was dried and steamed for 10 min.
All of the dyed cotton fabrics above were rinsed successively in cold,w arm and cold water and soaped at 95°C with anionic detergent LS(2 g·L-1,Shanghai Dyestuff Co.)using a liquor ratio of 20:1 for 15 min.Then,the bath was dropped and the fabrics were washed with water and dried.
2.4.Measurements
The nitrogen content of the cotton was obtained by the Kjeldahl method(GB12091-89).
Fourier-transform infrared spectroscopic(FT-IR)study was recorded on a NICOLET 6700 FT-IR spectrometer(Thermo Fisher,America)with a universal ATR sampling accessory to measure the FT-IR spectra of the pretreated and untreated cotton fabrics.
X-ray diffraction of cotton fibers was measured stepwise in the 2θ between 4°and 60°by a Rigaku diffractometer D/max-2400(Rigaku,Japan)and Monochromatic(Graphite monochromator)Cu-Ka1-radiation(40 kV,100 m A)was used.
Tensile strength of the cationic cotton was measured using a YG026 tensile strength machine(Changzhou Textile Apparatus Plant,China).
K/S values and whiteness and yellowness index were measured using an UltraScan XE Color Measuring and Matching Meter(Roaches Co.).
Light fastness was tested according to ISO 105-B06-1998 using a Xenotext 150s Weatherometer(Heraeus Co.,Germany).Wash fastness of the dyes was tested according to ISO 105-B01:1994 using an S-1002 tw o-bath dyeing and testing apparatus(Roaches Co.).Rub fastness was tested according to ISO 105-X12:1993 using a Y(B)571-II crockmeter(Wenzhou Darong,Co.,China).
Cross-sections of the dyed cotton fibers were prepared using a LEICA EM UC6 microtome(Leica,Germany).Images of the cross-sections were obtained at 600×magnification using an Olympus BX63 light microscope(Japan).
3.Results and Discussion
3.1.IR analysis of the cotton fabrics
In order to characterize the structure of the cationic cotton fabrics,IR spectra of the untreated fabric,the cationic one and the cationic one after treatment under alkaline steaming condition were measured and compared(see Fig.3).
In Fig.3,the principal spectral features of the untreated cotton fabric were show n as follow s:3277 cm-1(absorption peak of stretching vibration of O-H),2890 cm-1(absorption peak of stretching vibration of C-H),1637 cm-1(absorption peak of bending vibration of O-H),1425 cm-1and 1363 cm-1(absorption peaks of deformation vibration of C-H of CH2and CH),1157 cm-1(adsorption peak of bending vibration of CH2),and 1015 cm-1(absorption peak of stretching vibration of C-O-C).Compared to the untreated cotton,the cationic one presents a small new peak at 1753 cm-1,which was assigned to the stretching vibration of C═O of the ester carbonyl group,indicating the formation of ester bonds between cotton fibers and betaine hydrochloride.Evidence was also observed in references that adsorption peaks of the ester bonds of cellulose betainate and starch betainate both appeared at~1750 cm-1[20,22,23].

Fig.3.IRspectra of cotton fabric,cationic one and cationic one after treatment under alkaline steaming condition.
In addition,it showed that the peak at 1753 cm-1disappeared on the IR spectrum of the cationic cotton after treatment under alkaline steaming condition and washing,which demonstrated the hydrolysis of the ester bonds under the condition.
3.2.XRD of the cotton fibers
X-ray diffraction spectra of the untreated fibers and the cationic ones were also measured and the results were show n and compared in Fig.4(a)and(b).It show s that their X-ray spectra are almost the same with typical diffraction peaks appearing at 22.6°,16.2°and 14.8°.Fig.4(b)presents that the peak intensity of both patterns is almost the same,indicating that the facile pad-dry-bake pretreatment process showed little influence on the degree of crystallinity of the cotton fibers.
3.3.Tensile strength of the cotton fabrics
Chemical modification under acidic and high temperature conditions usually decreases the tensile strength of the cotton fabrics,which will affect their application properties[24].The tensile strength of the cationic fabrics was measured and compared with that of the untreated ones.The results were listed in Table 1.
It could be observed that through modification of cotton fabrics,tensile strength in w arp decreased by 6.1%and that in w oof decreased by 4.2%.Since modification was carried out under acidic and high-temperature conditions,the tensile strength of the fibers decreased,indicating the adverse effect of the pretreatment.While as the average decrease was only about 5%,application properties would not be affected much.
3.4.Whiteness and yellowness of the cotton fabrics
Cotton fabrics may turn yellow under acidic and high temperature conditions[25].If so,the wear ability of the fabrics will be affected.Moreover,w hen being dyed,the fabrics will exhibit darker color.The whiteness and yellowness index can be used to evaluate the color of the undyed fabrics.These values presented the consistency,which means the higher the whiteness is,the lower the yellowness is.In this study,the whiteness and the yellowness of the cotton fabrics before and after pretreatment were examined,and the results were show n in Table 2.
From Table 2,the whiteness of the untreated cotton was63.97,while that of the cationic one was 66.6,which indicated that after pretreatment,the cotton became even whiter.From the values of yellowness of the cotton in Table 2,it also gave the sameresult that the cationic fabrics were less yellow than the untreated ones.Although temperature as high as 150°C was employed for baking,as the treatment time is quite short,the fabricsdid not influence the color even under acidic condition.A little increase in whiteness may be illustrated by that some impurity was removed from the surface of the fabrics during pretreatment.
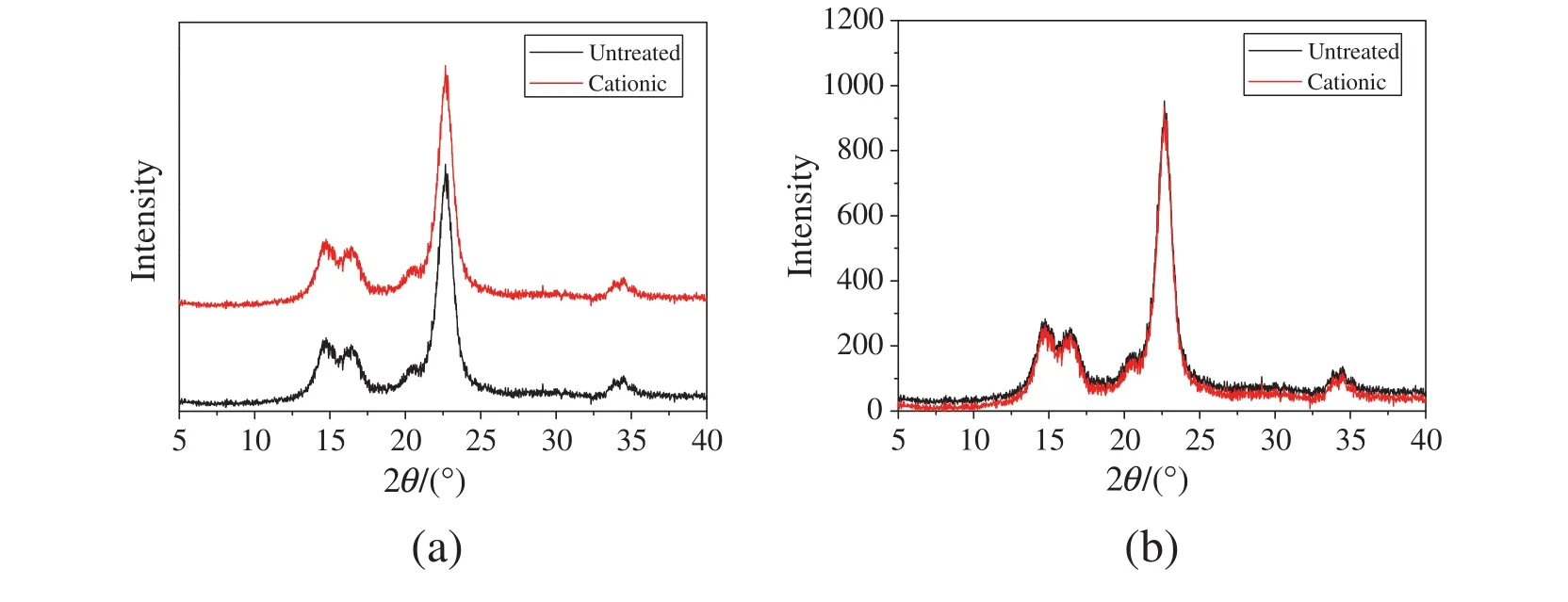
Fig.4.X-ray diffraction patterns of untreated cotton fibers and cationic one(a)separating patterns;(b)overlapping patterns.

Table 1 Tensile strength of the untreated cotton fabrics and the cationic ones

Table 2 Whiteness and yellowness of the untreated and cationic cotton fabrics
3.5.Dye penetrability
Dye penetrability commonly influences dye fixation and color fastness properties.If dyes can penetrate into fibers w ell,it is beneficial for reaction of the dyes with the fibers to achieve high dye fixation.In the study,dye penetrability into the cationic cotton fibers was tested by a light microscope and the results were show n in Fig.5(a)and(b).From cross-section photographs of the cotton fibers dyed with C.I.Reactive Red 195,it was observed that the inner parts of both the untreated(a)and cationic(b) fibers were colored,that meant that the dyes penetrated w ell in the fibers.In addition,it is clearly seen that the color is deeper in Fig.4(b)than that in(a),which should be mainly due to the much higher color yield of the cationic cotton fibers.
3.6.Dyeing test
The untreated and cationic cotton fabrics were all dyed with C.I.Reactive Red 195,C.I.Reactive Yellow 145 and C.I.Reactive Blue19.Besides the dye-bath fixation procedure,the steaming fixation procedure was designed and comparison was made between them.The dye fixation of all three dyes was listed in Table 3.The results show ed that with the conventional dye-bath fixation procedure(Proc.1 and Proc.2), fixation of C.I.Reactive Red 195,C.I.Reactive Yellow 145 and C.I.Reactive Blue 19 on the untreated cotton in the presence of 60 g·L-1sodium sulfate reached 78.2%,75.7%and 67.2%,respectively(Proc.1).How ever,with this dyeing method,the cationic cotton could not achieve good dye fixation,only about 50%of the dyes applied was utilized(Proc.2).While with the steaming fixation procedures(Proc.3 and Proc.4),dyefixation of all three dyes on the untreated cotton(Proc.3)was much lower than that obtained with Proc.1,even in the presence of salt.While for the cationic cotton fabrics with Proc.4 in the absence of sodium sulfate,dye fixation of C.I.Reactive Red 195,C.I.Reactive Yellow 145 and C.I.Reactive Blue 19 reached 84.9%,85.2%and 74.9%,respectively,which were 6.7%,9.5%and 7.7%respectively higher than that obtained with Proc.1.These results revealed that the fixation procedure influences the dye fixation on different fabrics very much.And the steaming fixation procedure is more suitable for the betaine-modified cationic cotton.The reason is now under investigation.
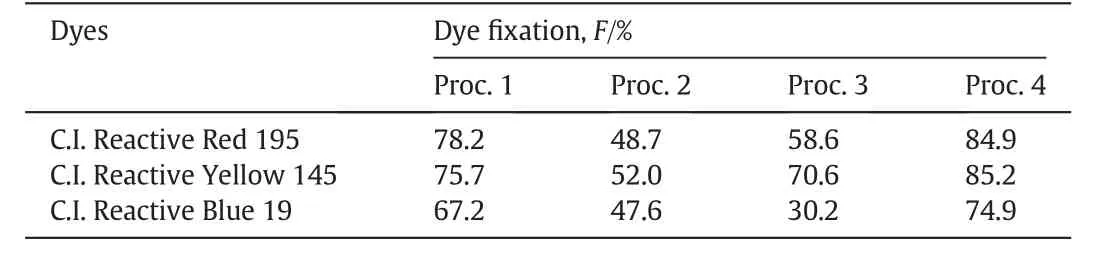
Table 3 Comparison of dye fixation of three reactive dyes with different dye fixation procedures

Fig.5.Cross-sections of cotton fibers dyed with C.I.Reactive Red 195(a)untreated and(b)cationic fibers.
The color fastness of C.I.Reactive Red 195,C.I.Reactive Yellow 145 and C.I.Reactive Blue 19 on both the cationic and untreated cotton was also measured and compared.The results were listed in Table 4.As can be seen from Table 4,light fastness of C.I.Reactive Red 195,C.I.Reactive Yellow 145 and C.I.Reactive Blue 19 on cationic cotton fabrics was4,5 and 7 grade,respectively,which was the same with that on the untreated ones.These results were inspiring as some reports presented that cationization of the fabrics decreased light fastness of the dyes on them[15-17].However,in this study,the light fastness was still satisfactory,this was presumably due to that the dye penetrability was good in the cationic fibers with the designed dye fixation method,which benefited obtaining high light fastness.Besides,much higher dye fixation on the fabrics also favored maintaining good dye light fastness.As for wash fastness,most result data reached 3-4 grade except that wool staining of C.I.Reactive Blue 19 was 3 grade.For rub fastness,dry rub fastness of the dyes on cationic cotton was excellent,which was not lower than 4 grade;while wet rub fastness on the cationic fabrics was all half grade lower than that on the untreated ones.The color fastness results show ed that even with the steaming fixation process,the influence of cationization of cotton on dyeing still existed.It was analyzed that although ester bonds could break under the steaming fixation condition and the cationic groups be easily removed without the addition of dyes,the washing off of the unfixed dyes together with the cationic groups could not be as easy as expected after dyeing.Since all of the fastnesses obtained could reach 3 grade or higher and dye fixation increased distinctly without the addition of salt,this dyeing method still show ed good application prospect.
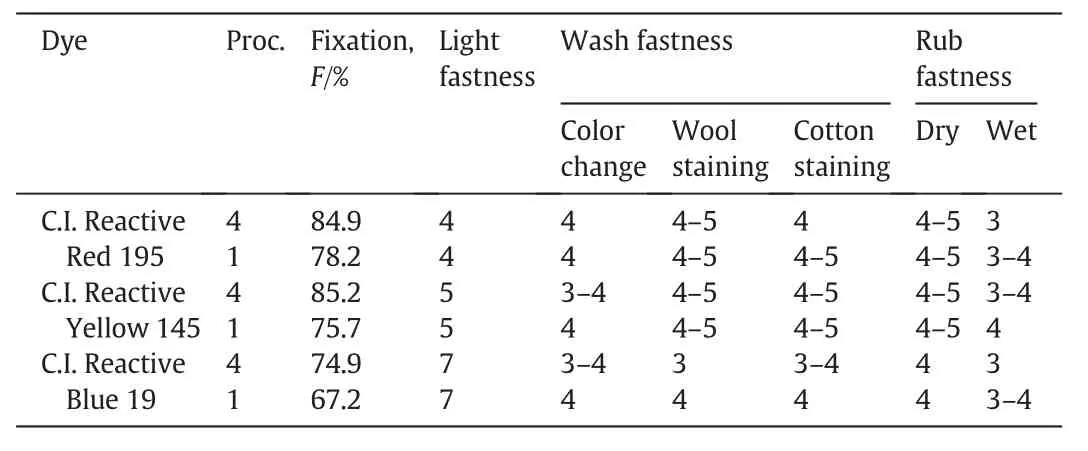
Table 4 K/S and fastness properties of the dyes on cationic and untreated cotton
4.Conclusions
Novel cationic cotton fabrics were prepared by pretreating the fabrics with betaine acidic solution with a pad-dry-bake method.FTIRART analysis of the cotton fabrics with different treatment procedures show ed that ester bonds formed under pretreatment conditions and could break under steaming fixation conditions.Tensile strength of the cationic fabrics decreased by about 5%compared with that of the untreated ones,while the whiteness of the cationic ones increased a little bit.It show ed that with the salt-free dyeing method,higher dye fixation was obtained on the cationic fabrics and more dyes could penetrate into the fibers.Compared with that on the untreated ones,the increase in dye fixation of C.I.Reactive Red 195,C.I.Reactive Yellow 145 and C.I.Reactive Blue 19 was 6.7%,9.5%and 7.7%,respectively,on cationic fabrics.Color fastness tests showed that all light and dry rub fastnesses of the dyes on the cationic cotton were excellent.How ever,some wash and w et rub fastnesses could not remain as good as that of the conventional dyeing,which was probably due to partial color staining.Further study should be carried out on improving color fastness.Anyway,in consideration of the high dye fixation,applicable color fastness,and the most important environmental concern of eliminating salt usage,this dyeing method is promising in application.
[1]M.M.Fernando,P.B.Carlos,H.M.Thais,T.H.Fernandes,C.L.Eder,R.Betina,C.Tatiana,B.F.Solange,Adsorption of Reactive Red M-2BE dye from water solutions by multi walled carbon nanotubes and activated carbon,J.Hazard.Mater.192(2011)1122-1131.
[2]H.Ma,Y.P.Huang,M.W.Shen,D.M.Hu,H.Yang,M.F.Zhu,S.P.Yang,X.Y.Shi,Enhanced decoloration efficacy of electrospun polymer nano fibers immobilized with Fe/Ni bimetallic nanoparticles,RSC Adv.3(2013)6455-6465.
[3]W.Zhang,H.Li,X.Kan,L.Dong,H.Yan,Z.W.Jiang,H.Yang,A.M.Li,R.S.Cheng,Adsorption of anionic dyes from aqueous solutions using chemically modified straw,Bioresour.Technol.117(2012)40-47.
[4]V.Janaki,K.Vijayaraghavan,B.T.Oh,K.J.Lee,K.Muthuchelian,A.K.Ramasamy,S.Kamala-Kannan,Starch/polyaniline nanocomposite for enhanced removal of reactive dyes from synthetic effluent,Carbohydr.Polym.90(2012)1437-1444.
[5]X.Fang,S.L.Xiao,M.W.Shen,R.Guo,S.Y.Wang,X.Y.Shi,Fabrication and characterization of water-stable electrospun polyethyleneimine/polyvinyl alcohol nano fibers with super dye sorption capability,New J.Chem.35(2011)360-368.
[6]R.S.Blackburn,S.M.Burkinshaw,A greener approach to cotton dyeings with excellent wash fastness,Green Chem.4(2002)47-52.
[7]S.H.Seong,S.W.Ko,Synthesis,application and evaluation of cationising agents for cellulosic fibers,J.Soc.Dye.Colour.114(1998)124-129.
[8]L.L.Wang,W.Ma,S.F.Zhang,X.X.Teng,J.Z.Yang,Preparation of cationic cotton with two-bath pad-bake process and its application in salt-free dyeing,Carbohydr.Polym.78(2009)602-608.
[9]P.J.Hauser,A.H.Tabba,Dyeing cationic cotton with fiber reactive dyes—effect of reactive chemistries,AATCC Rev.5(2002)36-39.
[10]W.Huitu,D.M.Lew is,Chemical modification of cotton to improve fiber dyeability,Color.Technol.118(2002)159-168.
[11]K.L.Xie,A.Q.Hou,X.Wang,Dyeing and diffusion properties of modified novel cellulose with triazine derivatives containing cationic and anionic groups,Carbohydr.Polym.72(2008)646-651.
[12]X.X.Teng,W.Ma,S.F.Zhang,Application of tertiary amine cationic polyacrylamide with high cationic degree in salt-free dyeing of reactive dyes,Chin.J.Chem.Eng.18(2010)1023-1028.
[13]R.S.Blackburn,S.M.Burkinshaw,Treatment of cotton with cationic,nucleophilic polymers to enable reactive dyeing at neutral p H without electrolyte addition,J.Appl.Polym.Sci.89(2003)1026-1031.
[14]S.Acharya,N.Abidi,R.Rajbhandari,F.Meulewaeter,Chemical cationization of cotton fabric for improved dye uptake,Cellulose 21(2014)4693-4706.
[15]G.W.Wang,L.H.Zhuang,J.Sun,C.L.Zheng,Salt-free dyeing of ramie fabric with an amino-terminated hyperbranched polymer,Cellulose 21(2014)3725-3736.
[16]W.Ma,S.F.Zhang,B.T.Tang,J.Z.Yang,Pretreatment of cotton with poly(vinylamine chloride)for salt-free dyeing with reactive dyes,Color.Technol.121(2005)193-197.
[17]S.H.Lim,S.M.Hudson,Application of a fiber-reactive chitosan derivative to cotton fabric as a zero-salt dyeing auxiliary,Color.Technol.120(2004)108-113.
[18]V.A.Dehabadi,H.J.Buschmann,J.S.Gutmann,Pretreatment of cotton fabrics with polyamino carboxylic acids for salt-free dyeing of cotton with reactive dyes,Color.Technol.129(2013)155-158.
[19]R.S.Blackburn,S.M.Burkinshaw,A greener approach to cotton dyeings.Part 2:application of 1:2 metal complex acid dyes,Green Chem.4(2002)261-265.
[20]W.Ma,S.M.Yan,M.Meng,S.F.Zhang,Preparation of betaine-modified cationic cellulose and its application in the treatment of reactive dye wastewater,J.Appl.Polym.Sci.131(2014)http://dx.doi.org/10.1002/APP.40522.
[21]X.X.Teng,S.F.Zhang,W.Ma,Application of a hydrolyzable cationic agent,poly(acryloxyethyl trimethylammonium chloride),in salt-free reactive dyeing for good dyeing properties,J.Appl.Polym.Sci.122(2011)2741-2748.
[22]H.Grano,J.Y.Kauhaluoma,T.Suortti,J.Käki,K.Nurmi,Preparation of starch betainate:a novel cationic starch derivative,Carbohydr.Polym.41(2000)277-283.
[23]T.Heinze,T.F.Liebert,K.S.Pfeiffer,M.A.Hussain,Unconventional cellulose ester:synthesis,characterization and structure-property relations,Cellulose 10(2003)283-296.
[24]I.S.Kang,C.Q.Yang,W.S.Wei,G.C.Lick field,Mechanical strength of durable press finished cotton fabrics part I:effects of acid degradation and crosslinking of cellulose by polycarboxylic acids,Text.Res.J.68(1998)865-870.
[25]V.A.Dehabadi,H.J.Buschmann,J.S.Gutmann,Durable press finishing of cotton fabrics with polyamino carboxylic acids,Carbohydr.Polym.89(2012)558-563.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scoping biology-inspired chemical engineering☆
- Review on the nanoparticle fluidization science and technology☆
- Multi-functional forward osmosis draw solutes for seawater desalination☆
- Bio-inspired enantioseparation for chiral compounds☆
- Process engineering in electrochemical energy devices innovation☆
- In-situ design and construction of lithium-ion battery electrodeson metal substrates with enhanced performances:A brief review☆
