Review on the nanoparticle fluidization science and technology☆
2016-05-29XiaolinZhuQiangZhangYaoWangFeiWei
Xiaolin Zhu,Qiang Zhang,Yao Wang,Fei Wei
Beijing Key Laboratory of Green Chemical Reaction Engineering and Technology,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
1.Introduction
Powder is a dry,bulk solid composed of a large number of very fine solid particles.Nano-structured particulate materials consisted of variousspheres, flakes,tubes,and rodspossessunique mechanical,electrical,optical,electrochemical,and thermal properties.Recently,novel advanced nano-structured materials for energy conversion and storage,environmental,catalysis,materials engineering,biology,drug,sensors,devices,information technology are highly concerned.This emerges the opportunity for extending the know ledge base of chemical engineering[1].Usually,these pristine nanoparticles belong to group C particles according to Geldart's classification,which were extremely fine powders and therefore the most cohesive particles[2].Fluidization of most Group C particles was difficult to achieve,which may require the introduction of external forces(e.g.mechanical agitation).Accordingly,the nanomaterials with tunable structures and extraordinary properties are strongly considered by both scientific communities and industrial engineers,and the related nanomaterial processing becomes an emerging research area.
Fluidization has an ability to turn static particles to fluid-like dense flow,which greatly makes improved heat transfer among porous powders and highly efficient solid processing become reality.Many excellent review publications have been appeared to w ell illustrate the importance of fluidization science and fluidized bed technology[3-12].How ever,with the rise of nano research,the cutting edge knowledge between chemical engineering,physics,materials,as well as nanoscience is grow ing rapidly.
In this contribution,the recent progress on the science and technology of nanomaterials fluidization has been review ed.The nanoparticle building blocks belong to group C particles and their fluidization is extremely difficult.How ever,some nanoparticles can be smoothly fluidized in the form of agglomerates.Therefore,w e tried to review the essence of nanoparticle agglomeration,their fluidization phenomena,and the assisted techniques to enhance the fluidization.In addition,the applications of fluidization for mass production of nanomaterials and novel chemical engineering process were also included.
2.The Fluidization Science of Nanomaterials
Nanomaterials with morphological features on the nanoscale(smaller than 100 nm in at least one dimension)have been strongly considered.Their controllable synthesis by novel processes such as high gravity controlled precipitation[13],gas combustion[14],micro fluid[15,16],hydrothermal reaction[17],and fluidized bed chemical vapor deposition[18,19],has been w ell documented.Usually,the nanomaterials prefer to aggregate into multi-scale agglomerates[20-22],and the agglomerates behave similar to porous particles.When the gas passes through the nanoparticles,their agglomeration,fluidization behavior and related characterization are quite different from the routine Group A and C pow ders.New insight into nanoagglomerate fluidization is the first step to understand the unique fluidization behavior and develop nanomaterial process.
2.1.Nanoparticle agglomeration
When the size of powder became small,the interactive forces between nanoparticles significantly increase.Therefore,the nanoparticles prefer to be coalesced into aggregates.The properties of the primary nanoparticles determine the architectures of the agglomerates that control the behavior of the two-phase flow in a fluidized bed reactor.As show n in Fig.1(a,b),the SiO2aerogels with a size of 7 to 16 nm form micro-agglomerates by several steps with different bonding mechanisms[20].The cohesive force between nanoparticles and the gravity force on the agglomerates are effectively diminished by the porous hierarchical multi-stage agglomerate structure,thus,even with a fairly high bed expansion,these SiO2agglomerates still form bubbleless fluidized beds that have a texture much closer to the particulate fluidization of a liquid-solid system.This system obeys the Richardson-Zaki relation,as opposed to the aggregative bubbling regime in many gassolid beds.Moreover,the structure of the agglomerates as w ell as their size,apparent w eight,and the interactive forcessig nificantly influence the hydrodynamic and their group behavior of nanoparticle agglomeration based on Geldart's classification.For instance,the multi-stage agglomerates consisted of Group C primary nanoparticles exhibited the fluidization behavior that was similar to Group A particles(Fig.1(c)).Recently,de Martin and co-workers[23,24]studied the multidimensional structure of nanoparticle agglomerate with combined neutron scattering and image analysis.They indicated that the fluidized agglomerates had three fractal dimensions:the first was sintered aggregates from synthesis,the second was agglomerates formed during storage,and the last was large agglomerates broken under fluidization.
The particle densities,shapes,surface roughness,sizes,and size distributions are important parameters affecting the operating range of fluidized beds as well as the gas-solid mixing and heat/mass transfer.Among these solid properties,particle size and size distribution are of the primary elements,which significantly affect the interaction between the individual particle and its neighboring particles,the movement of particles in a group,and the fluid-particle mixing and contacting during fluidization.A force analysis show n in Fig.2(a)is presented to reveal the relationship of the ratio between the gravity and cohesive force and the drag force,(Fg+Fc)/Fd,vs.particle size range[25].With the decrease of particlesize,the gravity forcedecreases continuously and the cohesive force increases sharply.The cohesive force becomes nearly the same as the drag force at a certain particle size dp*.Thus,the particles with a size of less than dp*afford strong interparticle van der Waals force between fine particles that induces strong aggregation[25,26].Consequently,the elutriation rate constant and other hydrodynamic behaviors differ from those particles w here the gravity and drag forces are in dominance as the particle size varies.
From the theoretical consideration,Johnson-Kendall-Roberts model[28],Deryaguin-Muller-Toporov model[29],and their extended/modified models,have been developed to calculate the adhesion force between smooth surfaces undergoing elastic deformation.How ever,the measured adhesion force for particles between 10 and 100 μm is always less than the theoretical results due to the surface roughness which reduces the contact area between two contiguous bodies with respect to the case when contacting surfaces are perfectly smooth[30].The adhesion force between two rough spheres is composed of the attraction between tw o parent spheres representing the large scale curvature of the surfaces and the adhesion between an asperity and the parent spheres[30].And the asperities can dramatically reduce the interparticle adhesion force by surface coverage with nanosized particles(Fig.2(b)).Recently,Chen and his co-workers proposed a three-dimensional adhesion force model to predict the force required to separate two contacting particles,by taking into account the fully plastic deformation on the contact area and surface area coverage of nanosized guest particles[30].The flow ability and fluidization behavior of parent fine powder were significantly improved by coating with proper surface coverage of ultra- fine nanoparticles[27,31-33].

Fig.1.(a)Chain-structure and(b)multi-stage of SiO2 nanoparticles[20];(c)group transition behavior of nanoparticle agglomeration based on Geldart classification.

Fig.2.(a)The relationship among gravity force,van der Waals force and drag force as a function of particle size[25];(b)the relationship between the cohesive force and the number of small particles added,indicating that the interaction among particles can be mediated by surface coating[27].
2.2.Nanomaterial agglomerate fluidization
Since the famous work by Wilhelm and Kw auk on the modes of particulate and aggregative fluidization in 1948[34],the ‘Fluidization’emerged into academia as a new realm of chemical engineering[6,8].The nanoparticles were thought to be Geldart group C particles and were very difficult to be fluidized because of strong cohesive forces.Therefore,new know ledge on the fluidization science of nanomaterials is highly required to w ell handle large quantities of nanoparticles,such as mixing,transporting,coating,and downstream processing of nanoparticles for nanomaterials industry.
Cohesive nanoparticles aggregate in a gas- fluidized bed driven by the dynamic equilibrium between interparticle attractive force F0and flow shear,which supports the particle w eight in the gravity field.Because of the strong interparticle forces(e.g.van der Waals,electrostatic,and moisture induced surface tension forces)among nanoparticles,they are always in the form of large-sized agglomerates,rather than as individual nanosized particles w hen packed together in a gaseous medium.Thus,gas fluidization of nanoparticles actually refers to the fluidization of nanoparticle agglomerates.
Various kinds of ultra fine powders,or nanoparticles,such as SiO2,TiO2,Al2O3,Fe3O4,Fe2O3,Zn O,CeO2,and Zr O2have been put into fluidized bed.Some of these fine nanoparticles follow agglomerate particulate fluidization(APF),in which very smooth fluidization occurs with extremely high bed expansion and bubbleless behavior,and the gas velocity as a function of voidage around the fluidized agglomerates obeys the Richardson-Zaki equation[20,35,36].The agglomeration of nanoparticles is governed by the balance between hydrodynamic shear forces and interparticle attractive forces.From this balance,the theoretical scaling law Bog~kD+2has been derived,w here Bog,the granular Bond number is the ratio of the interparticle attractive force to particle w eight,k is the ratio of agglomerate to particle size,and D is the fractal dimension[37].The agglomerate size exhibits a multiscale characteristic and the size distribution is subjected to the Gaussian distribution[38].The relatively small increment of gas viscosity opens up a new operation window of highly expanded APF behavior with a delayed onset of bubbling.
In contrast,some nanoparticles exhibit agglomerate bubbling fluidization(ABF)behavior,w here bed expansion is small,and bubbling occurs soon after minimum fluidization.In ABF state,a very limited bed expansion is observed,large bubbles rise up very quickly through the bed[20],and athree-separate-peak pattern is detected in the frequency spectra of the bed pressure signals[39].Recently,Zhu and co-workers[36]proposed a classification criterion based on the value of a combination of dimensionless groups to differentiate between particulate and bubbling fluidization for classical solid fluidized particles.The criterion was very effective to predict remarkably well whether nanoparticles behave as APF or ABF.The size of the nanoparticle agglomerates can be estimated by an equation obtained from the balance between the local shear force on the particles attached at the outer layer of the agglomerates and the interparticle adhesion force[40].Recently,Valverde and Castellanos[41]proposed an important approach to treat gas- fluidized beds of fine cohesive particles with the consideration of aggregates grow n to a size limited by the balance between interparticleforce and flow shear.Adiagram(Fig.3)was proposed to predict the type of fluidization exhibited by cohesive pow ders based on simple phenomenological equation.Geldart B behavior for dp> 70 μm,Geldart A behavior for 20 μm < dp< 70 μm,solidlike-to- fluidlike-to-bubbling(SFB)behavior for 6.7 μm < dp< 20 μm,and solidlike regime to a nonbubbling fluidlike regime and from the fluidlike regime to elutriation(SFE)behavior for dp< 6.7 μm are show n in Fig.3.

Fig.3.Phase diagram determining the transition between the types of fluidization as a function of particle size;left axis:particle volume fraction at the jamming transition φJ and at the transition to bubbling φb;right axis:ratio of the maximum stable size(D m)of a fluid pocket to particle size(d*)in the fluid-like regime[41].
The fluidization behavior of nanomaterials is also dependent on their agglomeration behavior.For instance,carbon nanotubes(CNTs)as one of typical 1-D nanomaterials were selected as the model to demonstrate their unique fluidization behavior.The dependence of bed expansion and pressure drop on gas velocity in a CNT nanoagglomerate(Fig.4(a)) fluidized bed is show n in Fig.4(b)[42].A smooth and highly expanded fluidization was achieved,but a strong hysteresis exists in the CNT fluidization curve.On the de fluidization branch,similar to Geldart-A particles,particulate fluidization,bubbling,turbulent,and fast fluidizations can be successively observed.How ever,Ucand Useof the CNTs were relatively low er than that of Geldart-A particles,due to the weak interaction among the CNT agglomerates and their highly porous structure.This indicates that good fluidization can be achieved only with strong turbulence of the fluidized bed,because of the interaction between agglomerated CNTs.
The fluidization of nanoparticles and nanotubes has been highly investigated recently[43].The formation of fluidized nanoparticle agglomerates is attributed to the dynamic equilibrium between formation and breakage of the agglomerates in a fluidized bed,which is sensitive to the pow der property and the operation parameter(such as the type of the fluidizing gas,its humidity and velocity).The intrinsic property of the nanoparticles renders the complex interactions and unique fluidization behavior,but it is still not fully understood yet.New insights into the basic nanoparticle fluidization should also be strongly considered.Attributing from the dynamic nature of nanoparticleagglomeration in fluidized beds,the characterization,modeling,and general w ay for interaction mediation of nanoparticle fluidization are still highly required.
3.Enhancing the Fluidization of Nanomaterials
Aiming to improve the fluidization quality of ultra fine/nanoparticle pow ders,various fluidization aids,such as mechanical vibration[44],magnetic field disturbance[45],sound w ave vibration[46],alternating electric field[47],secondary gas flow via microject[48],and addition of fine particles as flow conditioners[49],have been proposed.In essence,all these methods improve the fluidization of nanoparticles by breaking the very large agglomerates,therefore decreasing the minimum fluidization velocity and increasing the bed expansion.In the follow ing subsections,these assisted fluidization techniques have been presented in three different categories respectively.
3.1.Mechanical measures
The most direct pathway to break the agglomerates of nanoparticles in fluidized bed is the mechanical method.Both mechanical vibration and magnetically induced stirring are included in this category.
3.1.1.Vibration
The oscillations occur about an equilibrium point may break large agglomerates and render good fluidization quality.Therefore,a vibrator operated either vertically or horizontally,has been installed at the base of the fluidized bed to oscillate the nanoparticle agglomerates.For instance,a smooth fluidization of silica nanoparticles was achieved under the assistance of vertical sinusoidal vibration by Nam and coworkers[50].After the turn off of vibration,the bed would remain fluidized for a considerable amount of time(~30 h)w ith only aeration.The agglomerate size determined by the fractal analysis combined with amodi fied Richardson-Zakiapproach was around 160μm,which was in good agreement with the result of a high-speed CCD camera.The decreased agglomerate sizes and improved fluidization quality were ow ing to the introduction of vibration energy[51].The agglomerate size under vibro fluidization was further predicted by Zhou and his coworkers[52]with the combined Richardson-Zaki equation with Stokes law.A model on the basis of energy balance of the vibro- fluidized system was also proposed[53].
The agglomerating fluidization of three different kinds of nanoparticles(silica,titania and zinc oxide)in a vertically vibrofluidized bed(Fig.5(a))was reported by Yang and co-workers[54].The bed expansion increased with the increase of vibration frequency and amplitude,and the particulate fluidization degree was greatly enhanced by vibration force.How ever,compared with the obvious reduction in minimum fluidization velocity with increasing vibration frequency,no significant influence of vibration amplitude was detected(Fig.5(b)).The separate effect of vibration frequency and amplitude on minimum fluidization velocity was also studied by Kaliyaperumal and co-workers[44],and the roles of vibration on breaking agglomerates and enhancing nanoparticle fluidization were further con firmed.
The couple of mechanical vibration with some other assistance was also an efficient method to improve the nanoparticle fluidization.Levy and Celeste[55]integrated mechanical and acoustic vibrations together to enhance the fluidization of cohesive powders.The minimum fluidization velocity decreased obviously after the introduction of horizontal mechanical vibration for silica nanoparticles,and it further reduced w hen adding vertical acoustic vibration.The horizontal vibrofluidzation of nanoparticles was as effective as vertical con figuration in terms of fluidization improvement,which has also been w ell proved by Zhang and Zhao[56].Furthermore,the combined effects of mechanical vibration and electrostatic field on the fluidization of silica nanoparticles were explored by Quintanilla and co-workers[57].The vertical vibration induced bed expansion by reducing agglomerate size,while the horizontal electrostatic field caused bed collapse due to a drift of agglomerates toward the w all.When both techniques were simultaneously applied,the opposed effects could be practically compensated.

Fig.4.(a)Agglomerate structure of CNTs[22];(b)dependence of bed expansion and pressure drop on gas velocity in a CNT nano-agglomerate fluidized bed[42].

Fig.5.(a)Schematic of the vibro- fluidized bed;(b)minimum fluidization velocity under different vibration frequency and amplitude[54].
3.1.2.Stirring
By adding and exciting coarse magnets with an oscillating magnetic field,mechanical stirring has been introduced into the fluidized bed to improve the fluidization of nanoparticles.The agitating magnetic particles directly act on the fluidized agglomerates and influence their fluidization behavior.
For instance,an oscillating magnetic field generated by coils at the bottom of fluidization column was employed to stimulate the micron-size magnetic particles(barium ferrite)premixed with APF-type silica nanoparticles(Fig.6(a))[58].Due to high-density characteristic,the magnetic particles translated and rotated just above the gas distribution and stirred the bed.Under such assistance,the nanoparticle agglomerates could be smoothly fluidized without bubbles.Although the effect of stirred fluidization on smaller agglomerates(<500 μm)was less obvious,both greatly decreasing minimum fluidization velocity and increasing bed expansion were observed for larger agglomerates(>500 μm)hardly fluidized in conventional fluidization(Fig.6(b)).Moreover,after separation from the magnetic particles,the agglomerates could still be easily fluidized with no magnetic assistance,indicating a possible reduced agglomerate size after magnetic processing,which was verified by in-situ agglomerate size measurement with a CCD camera.The roles of coarse magnets in breaking agglomerates and improving macroscopic fluidization quality were further con firmed by Zhou and co-workers[59],and an energy-balance model was also proposed to estimate the agglomerate size under such stirring fluidization[60].
How ever,the above magnetically assisted stirring fluidization was not that effective for some hard-to- fluidize(HTF)nanoparticles,such as titania and alumina nanopowders,since their large bulk densities and inter-particle forces made the shearing force of moving magnetic particles insufficient to break up the agglomerates.To cope with this point,a combined approach was proposed by Zeng and co-workers[61,62],namely:blending HTF nanoparticles with APF-type silica nanopowders to improve the flowability,meanwhile applying magnetically induced stirring to cease the channeling and slugging.Although increasing mass fraction of HTF nanoparticles reduced the minimum bubbling velocity,bed expansion and stable fluidization region,an increase in the magnetic field intensity could compensate the negative effects,and the mixture of nanoparticles could be co- fluidized stably and almost homogeneously.Magnetic Fe3O4nanoparticles could also be smoothly fluidized[63]in a magnetically assisted fluidized bed,by adding either coarse magnetic particles or other non-magnetic nanopowders,which acted as agglomerate breakers and glidants improving the flow ability respectively.

Fig.6.(a)Schematic of the magnetic assisted fluidized bed;(b)the bed expansion ratio and bed pressure drop of large agglomerates with and without magnetic excitation[58].
The effects of these two different mechanical methods(i.e.,vibration and stirring)were further compared by Quevedo and co-workers[64],through monitoring the gas moisture during humidification and drying of fluidized nanoparticles.Compared with the conventional fluidized bed,the mass transfer between gas and nanoparticles was greatly enhanced under both assisted fluidization.Moreover,vibration assistance was found to be more effective for APF-type nanoparticles.How ever,magnetically induced stirring was more preferred for ABF-type nanoparticles to break the large agglomerates.Given the superiority of magnetically assisted fluidization,Hao and co-workers[45]applied it in CH4-CO2reforming to fluidize the aerogel Co/Al2O3catalyst,w here both enhanced CH4conversion and suppressed carbon deposition were obtained.
In general,both vertical and horizontal vibrations,as well as the stirring induced by added coarse magnets,are effective in enhancing the particulate fluidization behavior of nanoparticle agglomerates.However,the introduced shear force to break up the nanoagglomerates by these two mechanical techniques is still limited.Although combined process with some other assistance(e.g.,acoustic field or adding APF-type nanoparticles)can further reduce the agglomerate size and improve the fluidization,a higher dif ficulty in scale-up and operation is brought about for future industrial applications.
3.2.Introduction of external fields
To break up the nanoparticle agglomerates and improve their fluidization behaviors,some external fields,such as acoustic,electric and centrifugal fields,have been introduced into the fluidized system.
3.2.1.Acoustic field
Acoustic fieldscharacteristic of different sound frequencies,pressure levels and w ave con figurations have been used to induce sound vibration,break large agglomerate and enhance nano fluidization.The sound-assisted fluidization of silica nanoparticles with a loudspeaker placed at the top of the bed was investigated by Zhu and co-workers[65].Under the acoustic excitation(at sound frequency of 100 Hz and pressure level about 125 d B),the bed could be uniformly fluidized,and both channeling and slugging disappeared.Meanwhile,due to the breakup of large agglomerates,the minimum fluidization velocity decreased and the bed height expanded.How ever,bubbles appeared within a higher range of sound frequency(from 200 to 600 Hz).A more detailed investigation into the effects of sound w ave characteristics was conducted by Guo and co-workers[66,67](apparatus see Fig.7(a)).It was show n that,the minimum fluidization velocity firstly decreased and then increased with increasing sound frequency,which was related to the resonant frequency of the particles/agglomerates(Fig.7(b)).In contrast,the minimum fluidization velocity decreased continuously with increasing sound pressure level.Besides,sine and triangle sound w aves were found to be more effective in enhancing nanoparticle fluidization than square and rampup waves.In addition,the surface properties of nanopowders also influenced their fluidization behavior in the acoustic field[68].Recently,the acoustic field has been further applied in a fluidized bed of fine activated carbon to enhance CO2desorption,w here higher desorption rates,CO2recovery and purity were obtained under the sound assistance,making it possible to realize a cyclic adsorption/desorption process[69].
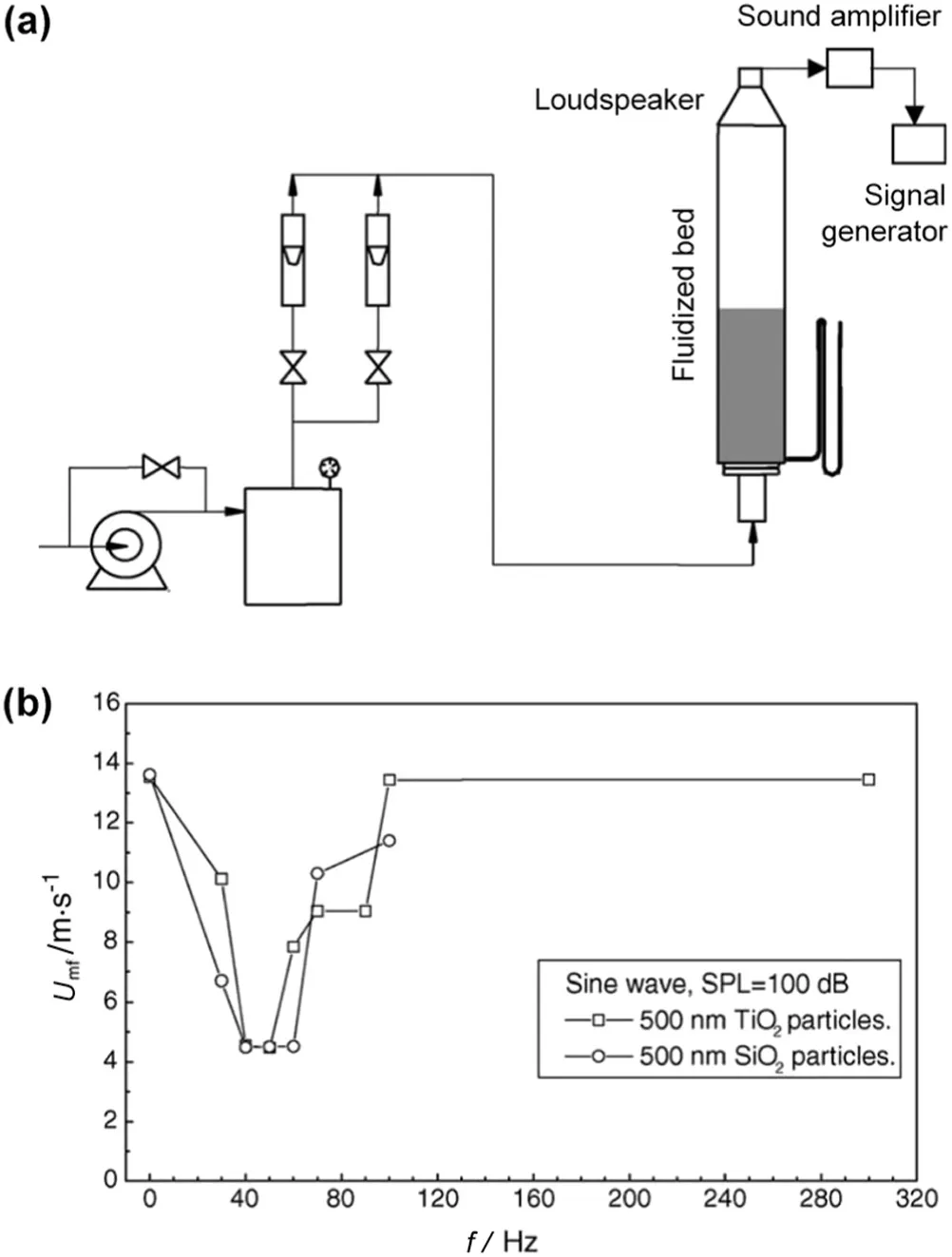
Fig.7.(a)Schematic of the sound-assisted fluidized bed;(b)plot of minimum fluidization velocity vs.acoustic frequency for 500 nm TiO2 and SiO2 particles[66].
The effects of sound vibration and pre-mixing with group A particles on enhancing the fluidization of silica nanopowders were compared by Ajar and co-workers[70].Although addition of small portions of group A particles was somew hat superior than sound assistance(given the extent of particle elutriation),the introduction of foreign particles has its limitation,especially for processes in which the contamination should be avoided.
(2)健壮性。健壮性又称鲁棒性,是指软件对于规范要求以外的输入能够判断出这个输入不符合规范要求,并能有合理的处理方式。软件健壮性是一个比较模糊的概念,却是非常重要的软件外部量度标准。
All of the aforementioned researches placed the sound w ave generator in the freeboard of the fluidization column.How ever,the large agglomerates tended to concentrate at the bottom of the bed,and the sound w aves that reached there were limited.Therefore,Kaliyaperumal and co-workers[46]designed a new sound-assisted fluidized bed,with the loudspeaker located under gas distributor.It was demonstrated that,such a design was also effective to improve the fluidization of nanopowders.
3.2.2.Electric field
Given that the nanoparticles are naturally charged[71],both DC and AC electric fields have been introduced to adjust the fluidization behavior of nanoagglomerates.The effects of electric field on the hydrodynamics of silica nanoparticles in a rectangular fluidized bed were investigated by Kashyap and co-workers[72].Tw o electrodes with opposite polarities attached to parallel w alls were used.Because of the downward electrical forcegenerated by the DCelectric field to nanoparticles,the solid volume fraction increased and the bed expansion decreased.Valverde and co-workers[73]further observed that,under such cross- flow DC electric field,the agglomerates were horizontally deflected toward the w all,consequently forming a solid layer adhered to the w all and a dilute core region of high gas velocity.Subsequently,vertical vibration was applied to compensate the decreasing bed expansion resulted from the application of horizontal electric field[57].
Considering that the DC electric field actually worsened the nanofluidization,a horizontal ACelectric field was introduced to further fluidize silica nanoparticles by Valverde and co-workers[74]and Espin and co-workers[75,76](Fig.8(a)).
As shown in Fig.8(b),under an alternating electric field with oscillation frequency around 20 Hz,the solid volume fraction decreased obviously due to electric-forced flow,consequently resulting in an increasing bed expansion and more homogeneous flow structure.How ever,at lower oscillation frequencies,the bed expansion was hindered due to electrophoretic deposition of agglomerates onto the w all.Whereas under higher frequencies,the agglomerates could not follow the rapid oscillations of the field and the bed expansion was also limited.Afterwards,a noninvasive visualization technique was applied to track the trajectories of nanoparticle agglomerates automatically by Quintanilla and co-workers[77].This enabled the estimation of agglomerate properties such as the size,charge,and density.
Recently,a more systematic investigation into the effects of AC electric field on nanofluidization was carried out by Lepek and co-workers[47].Three different field con figurations had been tested:cross- flow field(with two electrodes placed vertically),co- flow field(with an upper horizontal electrode and the bottom plate serving as the other electrode)and variable field(with two vertical electrodes held at the same high voltage and the bottom plate grounded).Due to the induced agitation of nanoparticle agglomerates by alternating electric field,the fluidization quality was improved for all three field con figurations.How ever,the best results were available for the variable field con figuration.In the variable con figuration,the higher field strength at bed bottom strongly enhanced the agitation of larger agglomerates tending to sink,which further destroyed the gas channels close to bottom distributor and homogenized the gas distribution.Meanwhile,the smaller agglomerates near the free surface were weakly excited by the lower field strength.Therefore,the fluidization was improved and the elutriation was limited.

Fig.8.(a)Cross section of the experimental system showing the electric field and equipo-tential lines obtained from numerical calculations[75];(b)relative variation of the particle volume fraction vs.electric field frequency for different values of the fixed gasvelocity,and photographs in the inset illustrate electrophoretic deposition,enhanced expansion and minor field effect respectively[77].
3.2.3.Centrifugal field
The effects of centrifugal field on nanoparticle fluidization have also been studied by Quevedo and co-workers[79].Both the bed pressure drop and minimum fluidization velocity increased with increasing centrifugal acceleration,whereas the bed expansion reduced.The advantages of rotating fluidized bed were summarized as:high operating gasvelocity with littlesolid elutriation,high gasthroughput per unit area of distributor,bubble less fluidization resulted from thin bed,little gas bypassing,and short processing time.The roles of rotating fluidized bed in breaking nanoparticle agglomerates and fluidizing them at high gas velocity with negligible entrainment were further con firmed by Nakamura and Watano[80].Given the superiorities of rotating fluidized bed,Watano and co-workers[81]have applied it for wet granulation of nanoparticles.

Fig.9.(a)Outline of the rotating fluidized bed;(b)agglomerate size evaluated from experimental data under each centrifugal acceleration condition[78].
In summary,smooth fluidization with increasing bed expansion and decreasing minimum fluidization velocity was achieved in both sound assisted and AC electric fields due to the effective reduction in nanoagglomerate size.In contrast,although the bed expansion reduced and the minimum fluidization velocity increased for a rotating fluidized bed,it allow s the bed to be operated at a higher gas throughput with little gas bypassing and solid elutriation.How ever,the energy introduced by these external fields to break the large agglomerates is still limited,and these assistance techniques usually require high investment and are also difficult to be scaled up.
3.3.Adjustment of gas injection mode
In gas-olid fluidized bed,gas injection mode generates significant influence on local flow field,and can remarkably improve the fluidization behavior.Therefore,the gas injection pattern was rationally adjusted.Both the secondary flow through microjet and pulsatile gas flow were proposed to enhance the fluidization of nanoparticle agglomerates.
3.3.1.Secondary flow through microjet
To improve the fluidization of nanoparticle agglomerates,a secondary air injection technique through micrometer-scale nozzles with an outlet velocity up to hundreds of meters per second,has been proposed and patented by Quevedo and co-workers[48]and Pfeffer and coworkers[82].In order to fluidize the particles at the base of the fluidization column,the micronozzles were recommended to be pointed downwards at close distance to the bottom air distributor(Fig.10(a)).Silica,alumina,and titania nanoparticles were respectively filled in the bed,and the bed expansion ratio was used as an indicator for the quality of fluidization[48].The maximum bed expansion increased from 6 to 50 with microjet assistance for APF-type Aerosil R974 nanoparticles(see Fig.10(b),w here the square symbols represent the increase in bed height during 30 min jet processing).While for ABF-type nanoparticles,the bed expansion more than doubled and their fluidization even converted to APF behavior.Moreover,with regard to both types of nanopowders,their bed pressure drop increased,minimum fluidization velocity decreased and onset of bubbling delayed,fully demonstrating the roles of microjet on breaking large nanoagglomerates,preventing channeling,curtailing bubbling and promoting liquid-like fluidization.In addition,microjet assisted fluidization was found to prevent powder packing in an internal,and it could even mix agglomerates on a nanoscale.
Using a focused beam re flectancemethod probe,the size of fluidized nanoparticle agglomerates was in-situ measured by Quevedo and Pfeffer[83]in conventional and microjet assisted fluidized beds.The obtained agglomerate size distributions indicated that,the mean size of both APF and ABF-type nanoparticle agglomerates decreased obviously under jet assisted fluidization,which well explained the higher quality of fluidization and larger bed expansion observed with microjet assistance.

Fig.10.(a)Schematic of the fluidized bed with secondary gas flow through micronozzle;(b)nondimensional bed height as a function of gas velocity for conventional and microjet assisted fluidization of Aerosil R974[48].
3.3.2.Pulsatile gas flow
Another approach to adjust gas injection and enhance nanoparticle fluidization is the pulsation of the inlet flow.Using a solenoid valve connected to an electronic circuit,pulsatile gas flow(induced by square-w ave pulse durations of 2,5 and 10 s,see Fig.11(a))was introduced into a fluidized bed filled with silica nanopowders by Ali and Asif[84].The flow pulsation led to agreater homogeneity of the bed,a lower minimum fluidization velocity(Fig.11(b))and a reduced agglomerate size.The collapse behavior of the pulsed fluidized bed was also investigated,and the average agglomerate size was deduced from the bed collapse data by Ali and co-workers[85].This further verified the role of flow pulsation in breaking agglomerates.
Not only for nano-sized particles,the pulsed gas flow was also found to be effective in promoting the fluidization of micron-sized cohesive particles[86],such as decreasing minimum fluidization velocity and inhibiting bubble formation.Considering the advantages of flow pulsation,Akhavan and co-worker[87]have applied this technique to enhance the drying of porous pharmaceutical granules in fluidized bed.In general,it improved the bed homogeneity and shortened the total drying time effectively.
In contrast with the aforementioned assisted fluidization techniques,including mechanical measures(e.g.,vibration and stirring)and external fields(e.g.,acoustic,electric and centrifugal fields),adjusting gas injection pattern is more easy to scale-up and cost effective.Consequently,it is very promising for industrial applications.Compared with the pulsatile gas flow which restricts the gas handling capacity to some extent,the secondary flow through microjet are more attractive given its extremely high bed expansion and nanoscale solid mixing ability.How ever,it is not only necessary to improve the macroscopic(e.g., flow homogeneity,bed expansion and minimum fluidization velocity)and microscopic(both gas and solid mixing)behaviors for industrially applied assisted fluidization technology,but also can be practically applied and operated with low investment and cost.

Fig.11.(a)Pulse generation with respect to time;(b)effect of the flow pulsation on the incipient fluidization[84].
4.The Mixing Behavior of Nanomaterials in a Fluidized bed
Nanocomposite produced from two or more kinds of nanomaterials,may possesssome uniquefeatures different from the individual components[36].To optimize the desirable properties,each component should be dispersed homogeneously inside the composite[88,89].Although good particle mixing can be achieved using solvents,some inevitable environmental issues are brought about.In contrast,gas fluidization with an efficient solid mixing characteristic is a promising alternative.Accordingly,a number of researches have been devoted to explore the mixing behavior of fluidized nanoparticles,including both among and inside the agglomerates.
4.1.Nanoparticle mixing under conventional fluidization
The dynamic breakage and reformation of the nanoparticle agglomerates during fluidization of Zn Onanoparticles in a 2-D conventional bed were observed by Gundogdu et al.[90]with a high speed X-ray imaging technique.Later on,using the nanoparticle coated phosphor tracer method,the mixing behavior of silica nanoparticles were explore by Wei and his co-workers[91,92]in a conventional fluidized bed.The axial and radial solids dispersion coefficients were two orders of magnitude lower than those in fluid catalytic cracking(FCC)catalyst systems.In the tested system,the axial solids dispersion coefficient(ranging from 9.1 × 10-4to 2.6 × 10-3m2·s-1)increased with increasing superficial gas velocity,and there was a step increase in the axial solids dispersion coefficient between the particulate fluidization regime and bubbling/turbulent fluidization regimes.Meanwhile,the radial solids dispersion coefficient also increased gradually from 1.2×10-4to 4.5 × 10-4m2·s-1with increasing gas velocity.In general,the solid dispersion coefficients are much lower than in regular fluidization systems due to the reduced density difference between fluidized particles and fluidizing medium.
4.2.Nanoparticle mixing under assisted fluidization
To enhance the solid mixing behavior of nanoparticles,a number of assisted fluidization techniques have been applied.Nam and cow orkers[50]reported some preliminary results of nanoparticle mixing in a vibrated fluidized bed.A small amount of silica nanoparticles were dyed blue as the tracer,and placed them at the top of the bed.After fluidization for a few minutes,the entire bed turned blue and appeared w ell mixed.Subsequent element analysis of the mixture of different nanomaterials indicated that,the nanoparticles could be well mixed at least on an agglomerate level under vibro fluidization.Afterwards,Hakim and co-workers[93]dyed two batches of agglomerates of silica nanoparticles with red and green colorants separately,and fed them into a fluidized bed together with the originally white agglomerates.Light microscope images of the resulting sample after 1 h vibrofluidization show ed that,the agglomerates appeared as a composite of three different colors of particles.This indicated that the initial agglomerates broke and reformed dynamically during fluidization.Recently,the fluidization behavior of different mixed SiO2,TiO2and/or Zn O nanoparticles under the application of vibrated field was explored by Zhou and his co-workers[94].The particulate fluidization degree of mixed nanoparticles was significantly improved under agglomerate particulate fluidization[94].The Richardson-Zaki exponents are employed to correlate the super ficial gas velocity with the voidage of single or binary mixed nanoparticles and verify the particulate fluidization degree of cohesive nanoparticles[95].Moreover,the adding coarse particles are better to improve the fluidization behavior of mixed nanoparticles[95].
Magnetically induced stirring has also been introduced into the fluidized bed to promote nanoparticle mixing by Scicolone and coworkers[96].Although the bed expansion decreased with time due to increasing density of nanoagglomerates,the homogeneity of solid mixing computed from statistical analysis was improved with prolonged processing time and elevated magnet-to-powder ratio.The magnetic fluidization not only broke up the agglomerates,but also led to mixing of both constituents down to a subagglomerate scale(Fig.12).
Using copper oxide nanoparticles as the tracer,the mixing behavior of alumina nanoparticles was investigated by Ammendola and Chirone[89]in a sound assisted fluidized bed.Visual observation of the bed demonstrated that,channeling was present and no mixing occurred without sound assistance.In contrast,the application of acoustic field promoted solid mixing and the entirebed turned to be well mixed globally in only few minutes.Moreover,element analysis of the fluidized samples obtained by a non-destructive method indicated that,mixing also occurred inside the agglomerates,but took a much longer time(80-150 min).Subsequently,the effects of acoustic assistance on fluidization of binary mixtures were explored in detail[97].And the effects of mixture composition,primary particle density,and sound intensity on the mixing quality of nanopowders were further clarified[88].
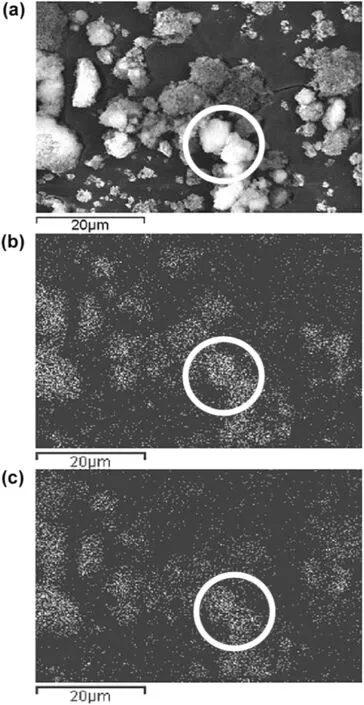
Fig.12.(a)SEM image,(b)aluminum elemental and(c)silicon elemental maps for 4:1 magnet-to-powder ratio of a 1:1 alumina to silica mixture fluidized for 30 min[96].
A rotating fluidized bed has been further applied to mix silica and alumina nanoparticles by Nakamura and Watano[80]under various processing times and centrifugal accelerations.A more homogeneous mixture was obtained with increasing centrifugal acceleration due to a higher force to break up the agglomerates.And a micron-scale mixing of nanoagglomerates was achieved in the centrifugal field.
How ever,both the conventional fluidization and aforementioned assisted techniques only mixed nanoparticles at a microscale.In contrast,a secondary gas flow through microject(w ith outlet velocity up to hundreds of meters per second)was not only efficient in breaking nanoagglomerates,preventing channeling,curtailing bubbles and promoting APF-type behavior,but also could achieve a good solid mixing down to nanoscale[48].This demonstrated a more promising future for industrial nanomaterial and nanocomposite production processes.
In summary,the mixing ability of conventional fluidization is limited,while the solid mixing is greatly enhanced under assisted techniques,and occurs on a microscale at least.How ever,more detailed investigation into the nanoparticle mixing behavior of both conventional and assisted fluidization,as w ell as advanced characterization methods,are still required to further explore the mixing mechanism.
5.The Application of Nanomaterials Fluidization
5.1.Nanostructured material production based on fluidized bed process
Nanoparticles can be deposited from the gasphase in a fluidized bed if the agglomerated nanoparticles can be fluidized,thus, fluidization technique has been an efficient route for advanced material(such as chemical vapor deposition of CNTs[18,22,98-100],WS2[101],Si[102-105],SiN[106],polypropylene[107],grapheme[108,109],and atomic layer deposition of Al2O3[110,111],SiO2[112],Sn O2[113],TiO2[114],Fe2O3[115],polysilicon[116,117],nanosilica supported CO2sorbent[118],as w ell as reduction process for producing Ni particles[119])production.How ever,how to produce tunable nanostructured materials in a fluidized bed is still an open area.We selected CNTs as a typical nanostructured material to describe their mass production via fluidized bed chemical vapor deposition(CVD)[120,121].
As show n in Fig.13,CNTs were cylinders formed by w rapping graphene sheets.CNTs possess extremely high tensile strength,high modulus,large aspect ratio,low density,good chemical and environmental stability,and high thermal and electrical conductivity.Therefore,CNTs with extraordinary performance are in demand for bulk applications in electrodes,electronic additives,conductive and high-strength composites.Recently,CNTs have been used as fillers in battery electrodes at a scale of hundreds of tons[122].For the extensive application,mass production of CNTs with desired structure at a low cost is the first step.

Fig.13.(a)Single walled CNT(SWCNT);(b)double walled CNT(DWCNT);(c)multi walled CNT(MWCNT).
In CVD methods,the carbon source is deposited with the assistance of a catalyst at temperatures lower than 1200°C.Tubular CNTs are deposited at the catalyst sites,Fe,Co or Ni particles always afford high activity for CNT grow th.With sustainable growth of CNTs,the as grown tubes will cluster into agglomerated or aligned CNTs.The agglomerated CNTs are 3-dimensional network structure composed with large amounts of CNTs(Fig.4(a)).The CNTs are prone to entangle with each other during the grow th on pow der catalysts[123].If thew all number of CNTs decreases and becomes double/single-walled,then the as-obtained CNTs will be very flexible.S/DWCNTs were con fined grow n in the porous catalyst[124],and their grow th requires not only a good dispersion of the active metal components on the catalyst supports,but also a rational designed porous nanoarchitectures.Layered double hydroxides(LDHs)have been employed as a novel catalyst for SWCNT grow th with a huge BET surface area of 1289 m2·g-1[125,126].Based on catalyst design concept,multiphase flow of CNTs and process scale up rules,a pilot plant for producing high quality and purity MWCNTs was designed and constructed[22].Eventually,a commercial production of MWCNTs with a capacity of 560 tons per year,and SWCNTs with a capacity of 8 tons per year has been developed in the middle of 2009 by Tsinghua University[18].
The aligned structures of CNTs were always obtained via a bottomup self-assemble process during thermal/ floating CVD,and a synchronous grow th of aligned CNTs induced by stress was found[127,128].It is commonly reported that aligned CNTs can be synthesized on a flat surface.However,the surface area of the flat substrate is often limited and its mobility is poor.Only 1.0 g·h-1CNT arrays can be obtained with flat silica as substrate.If a substrate with a larger surface area is used,such as spheres,more CNT arrays can be produced.We have recently synthesized CNT arrays on spheres[129],quartz fibers[130],and flakes[131-133]at large scale.Left or right handed CNT arrays were twisted into a double helix on a LDH flake through a selforganization process during grow th by catalytic CVD with a moving catalyst head through space confinement and rotation stress induced self-organization[131,134].The w all number and diameter of CNT in the double helix can be tuned by chemical precursor mediated process[132].To avoid the damage caused by the collisions among CNT arrays during the transport or fluidization process,a strategy of intercalating CNT arrays into layered compounds and directly constructing a layered hybrid nanocomposite composed of alternate CNT films and inorganic sheets was proposed[135].It has been proven that this is a successful way for the mass production of CNT arrays.A 3.0 kg·h-1CNT array productivity was realized in a fluidized bed reactor with a diameter 500 mm[136,137].The as-grow n CNT arrays were with good alignment,and could be easily purified.
Graphene,the one-atom-thick two dimensional crystal with excellent electronic conductivity and huge theoretical surface area,has been considered to be a novel nanomaterial for fabricating electronic devices and serving as a bulk material( film,foam,sponge form for capacitor electrodes and composite fillers).There are a number of methods for generating graphene and chemically modified graphene from graphite and derivatives of graphite[138,139].Epitaxial grow th on electrically insulating surfaces,such as SiC and CVD grow th of graphene on metal and metal oxides,is another effective w ay to get high quality graphene.Recently,by using porous MgO layers with polygonal shapes as a template,the nanomesh graphene with only one to two graphene layers,polygonal morphologies,large specific surface areas up to 1654 m2·g-1and good structural stability has been produced in a gas-solid fluidized bed reactor[109].Intrinsically unstacked double-layer templated graphene with a surface area of 1300-1700 m2·g-1has been grow n on catalyst template in a fluidized bed reactor[108].Graphene microspheres with a large diameter of ca.11 μm and a high surface area of 1275 m2·g-1were grow n on layered double oxide spherical templates[140].Controllable bulk grow th of graphene/CNT hybrids was obtained on LDH based catalyst in a fluidized-bed reactor through chemical vapor deposition of methane at 950°C[141].With proper catalyst selection and process design,fluidized bed CVD is a promising w ay for large scale production of nanocarbon.The fluidized bed not only offers the advantages of enough grow th space,excellent mass,and heat transfer,but also provides the easiness in scaling up and continuous operation for the massproduction of nanomaterials[18].
Based on particuological concept design,the agglomerated CNTs,aligned CNTs,and graphene have been successfully mass produced in ton scale via fluidized bed process,and are available in the market.Other important inorganic nanoparticles,such as WS2has been realized by Li and co-workers at Institute of Process Engineering,Chinese Academy of Sciences by sulfurization of WO3nanoparticles in a particulate fluidized bed reactor[142].A decoupling reduction-sulfurization process was further proposed for efficient and cost-effective production of WS2through particulate fluidization of the reduced WO3nanoparticles[143].In general,the fluidized bed based route promotes the bulk production and application of nanomaterials,and provides a sustainable pathway for the development of the novel advanced functional nanomaterials.
5.2.Fluidization of catalyst nanoparticles for sustainable chemical industry
Gas-solid fluidized bed reactors are w idely applied in diverse chemical processes,such as fuel,commodity and specialty chemical production,coal combustion and gasification,as w ell as polymer production[144-147].The solid phase always appears in the form of catalyst for a gas-solid catalytic process.The use of nanostructured catalytic materials,which offers hierarchical pores for rapid diffusion of the feedstock and products,is an effective route for novel process.
For instance,both methanol to ole fins(MTO)process and dimethyl ether to ole fins(DTO)process were important alternative ways for the production of light ole fins.Because of the strong exothermicity of MTO/DTO reactions and fast deactivation of SAPO-34 zeolite catalyst,a fluidized bed was the preferred reactor for industrial application[148].Consequently,the MTO/DTO catalysts needed to be prepared for facile fluidization.Recently,a hierarchical SAPO-34 zeolite with high crystallinity and excellent hydrothermal stability was directly synthesized in the nanoscale con fined environment provided by the natural layered material kaolin(Fig.14)[149].Activity test results in a fixed bed indicated that,the hierarchical sample show ed a much higher dimethyl ether(DME)conversion than the conventional zeolite,and the overall ole fin selectivity was as much as 96.7 w t%,which was 4 w t%-7 w t%higher than that of the conventional zeolite.In essence,the presence of the mesopore structure shortened the diffusion path,which facilitated the diffusion of primary products out of the zeolites and avoided secondary reactions.Therefore,higher propylene selectivity and overall ole fin selectivity were obtained.This procedure was expected to be a facile and economically feasible way to prepare more effective catalyst for fluidized MTO/DTO process[150].Accordingly,the SAPO-34 zeolite with a nanoscale substructure was used to prepare the catalyst by spray granulation,and a fluidized bed methanol to propylene(FMTP)industrial technology wasdeveloped[150].Lately,an industrial practice of FMTP process at Huainan Chemical Group Company with a 470 h continuous operation at full capacity was carried out,w here a high yield of propylene was obtained.
The use of nanoparticle catalysts in a fluidized bed has also been strongly considered in many other chemical processes.Methanation is widely employed to produce synthetic natural gas(i.e.,methane)and remove the trace of poison CO from inlet feed streams.The pure Ni/Al2O3nanocatalyst failed to be fluidized,but the fluidization could be significantly improved by adding large Al2O3particles[151].CO methanation reaction over a nanosized NiCo catalyst for methane production was investigated by Li and his co-workers in a magnetic fluidized bed reactor[152],w here the particle agglomeration was effectively inhibited.The use of La2O3as the support and promoter for Ni/γ-Al2O3catalysts in dry reforming of methane was further reported in a fluidzed bed reactor[153].Other nanostructured catalysts,such as hierarchical ZSM-5 zeolite with a bimodal meso-microporous system,high crystallinity,and a large surface area exhibited a remarkably enhanced activity for propylene-boosting performance in heavy oil cracking[154].The nanostructured ZSM-5 zeolite prepared by desilication possessed excellent catalytic properties in catalytic fast pyrolysis of lignocellulosic biomass in a fluidized bed reactor[155].The chemical-looping processes with different functions of looping nanoparticles have also been highly concerned for effective and viable carbonaceous fuel conversion into clean energy carriers[156-158].In general,the w ell combination of nanostructured catalysts and fluidized bed reactor technology afford new impetusfor sustainablechemical and energy production.

Fig.14.SEM images of as synthesized hierarchical SAPO-34 zeolite at different magni fications(a)and(b)[149].
6.Conclusions and Outlook
Development of fluidization technology has spanned several decades,attesting to the importance of this technique in chemical engineering,thermal engineering,and metallurgy.When nanostructured granular materials were fluidized by gas,some kinds of nanoparticles(such as SiO2,CNTs)are agglomerated into multi-stage agglomerates for stable fluidization.The structure of the agglomerates as w ell as their size,apparent w eight and the interactive forces between them significantly influence the hydrodynamic behavior of agglomerating fluidization.
APF-type nanoparticles can be smoothly fluidized in conventional fluidization with high bed expansion and bubblelessbehavior.Whereas,ABF-type nanopowders exhibited little bed expansion,and the gas phase mainly passed through the bed by bubbling and channeling when fluidized with only aeration.Therefore,some mechanical measures(e.g.,vibration and stirring),external acoustic,electric and centrifugal fields,and improved gas injection techniques have been introduced to enhance the fluidization and mixing behaviors of nanoparticles.Most of these methods are effective in breaking large agglomerates,and consequently decreasing minimum fluidization velocity,increasing bed expansion,and promoting fluid-like behaviors.Moreover,a good mixing down to microscale is also obtained.Among all these assisted-techniques,the secondary flow through microjet,which can achieve a nanoscale solid mixing and be easily scaled up,is most promising for future bulk applications.
Based on the scientific understanding of nanomaterials fluidization,the fluidization process was successfully employed for nanomaterial production.Multi-functional nanostructure,such as hierarchical SAPO-34 zeolite,was also synthesized and used to prepare advanced catalysts for catalytically fluidized bed route for methanol to ole fins.In general,the fluidization technique is a powerful tool to novel process design and process intensification for materials,energy,and chemical industry.
The scientific understanding of the coupled process based on chemical conversion,multi-phase flow,nanoscale particulate design,transport phenomenaand their scaleupisal waysacore part of successful nanomaterial production and structured catalyst design for energy and chemical industry.In the future,a number of novel chemical,energy,materials processes and techniques will be proposed and developed.Therefore,the combination of the nanoparticles and their fluidization processshould be further explored to bring novel reactivity,catalytic performance,transfer behaviors,and multi-functional properties,and extend their widely application in nanotechnology,information technology,advanced materials production,energy conversion and storage,and chemical industry.
Acknowledgements
We would like to thank Prof.Yong Jin,Prof.Zhanwen Wang,and our collaborators for their insightful discussion and industrial trial.
[1]M.Kw auk,Extending the know ledge base of chemical engineering,China Particuology 3(2005)151-164.
[2]D.Geldart,Types of gas fluidization,Powder Technol.7(1973)285-292.
[3]J.X.Zhu,Z.Q.Yu,Y.Jin,J.R.Grace,A.Issangya,Cocurrent dow n flow circulating fluidized-bed(downer)reactors-A state-of-the-art review,Can.J.Chem.Eng.73(1995)662-677.
[4]H.T.Bi,N.Ellis,I.A.Abba,J.R.Grace,A state-of-the-art review of gas-solid turbulent lf uidization,Chem.Eng.Sci.55(2000)4789-4825.
[5]H.Z.Li,X.S.Lu,M.Kwauk,Particulatization of gas-solids fluidization,Powder Technol.137(2003)54-62.
[6]M.Horio,Fluidization science,its development and future,Particuology 8(2010)514-524.
[7]Y.Cheng,C.N.Wu,J.X.Zhu,F.Wei,Y.Jin,Downer reactor:from fundamental study to industrial application,Powder Technol.183(2008)364-384.
[8]M.Kw auk,J.H.Li,D.J.Liu,Particulate and aggregative fluidization—50 years in retrospect,Powder Technol.111(2000)3-18.
[9]W.Zhang,A review of techniques for the process intensification of fluidized bed reactors,Chin.J.Chem.Eng.17(2009)688-702.
[10]Q.S.Zhu,H.Z.Li,Status quo and development prospect of magnetizing roasting via fluidized bed for low grade iron ore,CIESC J.65(2014)2437-2442(in Chinese).
[11]J.R.van Ommen,J.M.Valverde,R.Pfeffer,Fluidization of nanopowders:a review,J.Nanoparticle Res.14(2012)737.
[12]T.Zhou,Agglomerating Fluidization of Cohesive Particles,China Chemical Industry Press,Beijing,2009.(in Chinese).
[13]T.T.Hu,J.X.Wang,Z.G.Shen,J.F.Chen,Engineering of drug nanoparticles by HGCP for pharmaceutical applications,Particuology 6(2008)239-251.
[14]C.Z.Li,Y.J.Hu,W.K.Yuan,Nanomaterials synthesized by gas combustion flames:morphology and structure,Particuology 8(2010)556-562.
[15]G.S.Luo,L.Du,Y.J.Wang,Y.C.Lu,J.H.Xu,Controllable preparation of particles with micro fluidics,Particuology 9(2011)545-558.
[16]W.Wang,R.Xie,X.J.Ju,L.Y.Chu,Recent progress of micro fluidic fabrication of noel functional microparticles,CIESC J.65(2014)2555-2562(In Chinese).
[17]W.C.Zhu,G.D.Li,Q.Zhang,L.Xiang,S.L.Zhu,Hydrothermal mass production of MgBO2(OH)nanowhiskers and subsequent thermal conversion to Mg2B2O5nanorods for biaxially oriented polypropylene resins reinforcement,Powder Technol.203(2010)265-271.
[18]Q.Zhang,J.Q.Huang,M.Q.Zhao,W.Z.Qian,F.Wei,Carbon nanotube mass production:principles and processes,ChemSusChem 4(2011)864-889.
[19]M.Q.Zhao,Q.Zhang,J.Q.Huang,F.Wei,Hierarchical nanocomposites derived from nanocarbons and layered double hydroxides—properties,synthesis,and applications,Adv.Funct.Mater.22(2012)675-694.
[20]Y.Wang,G.S.Gu,F.Wei,J.Wu,Fluidization and agglomerate structure of SiO2nanoparticles,Powder Technol.124(2002)152-159.
[21]H.Z.Li,Multi-scale aggregation of particles in gas-solid fluidized beds,China Particuology 2(2004)101-106.
[22]F.Wei,Q.Zhang,W.Z.Qian,H.Yu,Y.Wang,G.H.Luo,G.H.Xu,D.Z.Wang,The mass production of carbon nanotubes using a nano-agglomerate fluidized bed reactor:a multiscale space-time analysis,Powder Technol.183(2008)10-20.
[23]L.de Martín,W.G.Bouw man,J.R.van Ommen,Multidimensional nature of fluidized nanoparticle agglomerates,Langmuir 30(2014)12696-12702.
[24]L.de Martín,A.Fabre,J.R.van Ommen,The fractal scaling of fluidized nanoparticle agglomerates,Chem.Eng.Sci.112(2014)79-86.
[25]Y.Wang,Y.Cheng,Y.Jin,H.T.T.Bi,On impacts of solid properties and operating conditions on the performance of gas-solid fluidization systems,Powder Technol.172(2007)167-176.
[26]A.Castellanos,J.M.Valverde,M.A.S.Quintanilla,Aggregation and sedimentation in gas- fluidized beds of cohesive powders,Phys.Rev.E 64(2001)041304.
[27]C.Huang,Study on the Characteristics of Nano-agglomerate Fluidization(Doctor Dissertation)Tsinghua University,2008.(In Chinese).
[28]K.L.Johnson,K.Kendall,A.D.Roberts,Surface energy and contact of elastic solids,Proc.R.Soc.A 324(1971)301-313.
[29]B.V.Derjaguin,V.M.Muller,Y.P.Toporov,Effect of contact deformations on adhesion of particles,J.Colloid Interface Sci.53(1975)314-326.
[30]Y.H.Chen,M.A.S.Quintanilla,J.Yang,J.M.Valverde,R.N.Dave,Pull-off force of coated fine powders under small consolidation,Phys.Rev.E 79(2009)041305.
[31]Q.Huang,H.Zhang,J.Zhu,Flow properties of fine powders in powder coating,Particuology 8(2010)19-27.
[32]H.Liu,Y.Li,Q.J.Guo,Fluidization quality improvement for cohesive particles by fine powder coating,Ind.Eng.Chem.Res.45(2006)1805-1810.
[33]L.Y.Song,T.Zhou,J.S.Yang,Fluidization behavior of nano-particles by adding coarse particles,Adv.Powder Technol.20(2009)366-370.
[34]R.H.Wilhelm,M.Kw auk,Fluidization of solid particles,Chem.Eng.Prog.44(1948)201-218.
[35]Z.L.Wang,M.Kwauk,H.Z.Li,Fluidization of fine particles,Chem.Eng.Sci.53(1998)377-395.
[36]C.Zhu,Q.Yu,R.N.Dave,R.Pfeffer,Gas fluidization characteristics of nanoparticle agglomerates,AICHE J.51(2005)426-439.
[37]J.M.Valverde,M.A.S.Quintanilla,A.Castellanos,D.Lepek,J.Quevedo,R.N.Dave,R.Pfeffer,Fluidization of fine and ultra fine particles using nitrogen and neon as fluidizing gases,AICHE J.54(2008)86-103.
[38]H.Liu,L.Zhang,T.Chen,S.Wang,Z.Han,S.Wu,Experimental study on the fluidization behaviors of the super fine particles,Chem.Eng.J.262(2015)579-587.
[39]M.R.Tamadondar,R.Zarghami,M.Tahmasebpoor,N.Mostou fi,Characterization of the bubbling fluidization of nanoparticles,Particuology 16(2014)75-83.
[40]J.M.Valverde,A.Castellanos,Fluidization,bubbling and jamming of nanoparticle agglomerates,Chem.Eng.Sci.62(2007)6947-6956.
[41]J.M.Valverde,A.Castellanos,Types of gas fluidization of cohesive granular materials,Phys.Rev.E 75(2007)031306.
[42]H.Yu,Q.F.Zhang,G.S.Gu,Y.Wang,G.H.Luo,F.Wei,Hydrodynamics and gas mixing in a carbon nanotube agglomerate fluidized bed,AICHE J.52(2006)4110-4123.
[43]K.Dasgupta,J.B.Joshi,S.Banerjee,Fluidized bed synthesis of carbon nanotubes—a review,Chem.Eng.J.171(2011)841-869.
[44]S.Kaliyaperumal,S.Barghi,L.Briens,S.Rohani,J.Zhu,Fluidization of nano and submicron pow ders using mechanical vibration,Particuology 9(2011)279-287.
[45]Z.G.Hao,Q.S.Zhu,Z.Jiang,H.Z.Li,Fluidization characteristics of aerogel Co/Al2O3catalyst in a magnetic fluidized bed and its application to CH4-CO2reforming,Powder Technol.183(2008)46-52.
[46]S.Kaliyaperumal,S.Barghi,J.Zhu,L.Briens,S.Rohani,Effects of acoustic vibration on nano and sub-micron powders fluidization,Powder Technol.210(2011)143-149.
[47]D.Lepek,J.M.Valverde,R.Pfeffer,R.N.Dave,Enhanced nanofluidization by alternating electric fields,AICHE J.56(2010)54-65.
[48]J.A.Quevedo,A.Omosebi,R.Pfeffer,Fluidization enhancement of agglomerates of metal oxide nanopowders by microjets,AICHE J.56(2010)1456-1468.
[49]T.Zhou,H.Z.Li,Effects of adding different size particles on fluidization of cohesive particles,Powder Technol.102(1999)215-220.
[50]C.H.Nam,R.Pfeffer,R.N.Dave,S.Sundaresan,Aerated vibro fluidization of silica nanoparticles,AICHE J.50(2004)1776-1785.
[51]X.Z.Liang,H.Duan,J.Wang,T.Zhou,Agglomerate sizes of binary nanoparticle mixtures in a vibro- fluidized bed,Chem.Eng.Technol.37(2014)20-26.
[52]H.Wang,T.Zhou,J.S.Yang,J.J.Wang,H.Kage,Y.Mawatari,Model for calculation of agglomerate sizes of nanoparticles in a vibro- fluidized bed,Chem.Eng.Technol.33(2010)388-394.
[53]L.Zhou,H.Wang,T.Zhou,K.Li,H.Kage,Y.Mawatari,Model of estimating nanoparticle agglomerate sizes in a vibro- fluidized bed,Adv.Powder Technol.24(2013)311-316.
[54]J.S.Yang,T.Zhou,L.Y.Song,Agglomerating vibro- fluidization behavior of nanoparticles,Adv.Pow der Technol.20(2009)158-163.
[55]E.K.Levy,B.Celeste,Combined effects of mechanical and acoustic vibrations on fluidization of cohesive powders,Powder Technol.163(2006)41-50.
[56]W.Zhang,M.Zhao,Fluidisation behaviour of silica nanoparticles under horizontal vibration,J.Exp.Nanosci.5(2010)69-82.
[57]M.A.S.Quintanilla,J.M.Valverde,A.Castellanos,D.Lepek,R.Pfeffer,R.N.Dave,Nano fluidization as affected by vibration and electrostatic fields,Chem.Eng.Sci.63(2008)5559-5569.
[58]Q.Yu,R.N.Dave,C.Zhu,J.A.Quevedo,R.Pfeffer,Enhanced fluidization of nanoparticles in an oscillating magnetic field,AICHE J.51(2005)1971-1979.
[59]L.Zhou,R.L.Diao,T.Zhou,H.Kage,Y.Mawatari,Characteristics of non-magnetic nanoparticles in magnetically fluidized bed by adding coarse magnets,J.Cent.S.Univ.Technol.18(2011)1383-1388(In Chinese).
[60]L.Zhou,F.Zhang,T.Zhou,H.Kage,Y.Mawatari,A model for estimating agglomerate sizes of non-magnetic nanoparticles in magnetic fluidized beds,Korean J.Chem.Eng.30(2013)501-507.
[61]P.Zeng,T.Zhou,J.S.Yang,Behavior of mixtures of nano-particles in magnetically assisted fluidized bed,Chem.Eng.Process.47(2008)101-108.
[62]P.Zeng,T.Zhou,G.Q.Chen,Q.S.Zhu,Behavior of mixed ZnOand SiO2nano-particles in magnetic field assisted fluidization,China Particuology 5(2007)169-173.
[63]L.Zhou,R.L.Diao,T.Zhou,H.Wang,H.Kage,Y.Maw atari,Behavior of magnetic Fe3O4nano-particles in magnetically assisted gas- fluidized beds,Adv.Powder Technol.22(2011)427-432.
[64]J.A.Quevedo,J.Flesch,R.Pfeffer,R.Dave,Evaluation of assisting methods on fluidization of hydrophilic nanoagglornerates by monitoring moisture in the gas phase,Chem.Eng.Sci.62(2007)2608-2622.
[65]C.Zhu,G.L.Liu,Q.Yu,R.Pfeffer,R.N.Dave,C.H.Nam,Sound assisted fluidization of nanoparticle agglomerates,Powder Technol.141(2004)119-123.
[66]Q.J.Guo,H.Liu,W.Z.Shen,X.H.Yan,R.G.Jia,In fluence of sound w ave characteristics on fluidization behaviors of ultra fine particles,Chem.Eng.J.119(2006)1-9.
[67]Q.J.Guo,Y.Li,M.H.Wang,W.Z.Shen,C.H.Yang,Fluidization characteristics of SiO2nanoparticles in an acoustic fluidized bed,Chem.Eng.Technol.29(2006)78-86.
[68]H.Liu,Q.J.Guo,S.A.Chen,Sound-assisted fluidization of SiO2nanoparticles with different surface properties,Ind.Eng.Chem.Res.46(2007)1345-1349.
[69]F.Raganati,P.Ammendola,R.Chirone,Effect of acoustic field on CO2desorption in a fluidized bed of fine activated carbon,Particuology(2015).http://dx.doi.org/10.1016/j.partic.2015.02.001.
[70]A.Ajbar,Y.Bakhbakhi,S.Ali,M.Asif,Fluidization of nano-powders:effect of sound vibration and pre-mixing with group a particles,Powder Technol.206(2011)327-337.
[71]J.W.Jung,D.Gidaspow,Fluidization of nano-size particles,J.Nanoparticle Res.4(2002)483-497.
[72]M.Kashyap,D.Gidaspow,M.Driscoll,Effect of electric field on the hydrodynamics of fluidized nanoparticles,Powder Technol.183(2008)441-453.
[73]J.M.Valverd e,M.A.S.Quintanilla,M.J.Esp in,A.Castellanos,Nano fluid ization electrostatics,Phys.Rev.E 77(2008)031301.
[74]J.M.Valverde,M.J.Espin,M.A.S.Quintanilla,A.Castellanos,Electro fluidized bed of silica nanoparticles,J.Electrost.67(2009)439-444.
[75]M.J.Espin,J.M.Valverde,M.A.S.Quintanilla,A.Castellanos,Electromechanics of fluidized beds of nanoparticles,Phys.Rev.E 79(2009)011304.
[76]M.J.Espin,J.M.Valverde,M.A.S.Quintanilla,A.Castellanos,Alternating field electronano fluidization,Powders Grains 2009(1145)(2009)97-100.
[77]M.A.S.Quintanilla,J.M.Valverde,M.J.Espin,A.Castellanos,Electro fluidization of silica nanoparticle agglomerates,Ind.Eng.Chem.Res.51(2012)531-538.
[78]S.Matsuda,H.Hatano,T.Muramoto,A.Tsutsumi,Modeling for size reduction of agglomerates in nanoparticle fluidization,AICHE J.50(2004)2763-2771.
[79]J.Quevedo,R.Pfeffer,Y.Y.Shen,R.Dave,H.Nakamura,S.Watano,Fluidization of nanoagglomerates in a rotating fluidized bed,AICHE J.52(2006)2401-2412.
[80]H.Nakamura,S.Watano,Fundamental particle fluidization behavior and handling of nano-particles in a rotating fluidized bed,Powder Technol.183(2008)324-332.
[81]S.Watano,T.Tokuda,H.Nakamura,Wet granulation of nano-particles in a rotating fluidized bed,Stud.Surf.Sci.Catal.159(2006)485-488.
[82]R.Pfeffer,J.A.Quevedo,J.Flesch,Fluidized bed systems and methods including micro-jet flow,USA patent 8439283(2013).
[83]J.A.Quevedo,R.Pfeffer,In situ seasurements of gas gluidized nanoagglomerates,Ind.Eng.Chem.Res.49(2010)5263-5269.
[84]S.S.Ali,M.Asif,Fluidization of nano-powders:effect of flow pulsation,Powder Technol.225(2012)86-92.
[85]S.S.Ali,M.Asif,A.Ajbar,Bed collapse behavior of pulsed fluidized beds of nanopow der,Adv.Powder Technol.25(2014)331-337.
[86]H.K.Bizhaem,H.B.Tabrizi,Experimental study on hydrodynamic characteristics of gas-solid pulsed fluidized bed,Powder Technol.237(2013)14-23.
[87]A.Akhavan,J.R.van Ommen,J.Nijenhuis,X.S.Wang,M.O.Coppens,M.J.Rhodes,Imp roved drying in a pulsation-assisted fluidized bed,Ind.Eng.Chem.Res.48(2009)302-309.
[88]P.Ammendola,R.Chirone,F.Raganati,Effect of mixture composition,nanoparticle density and sound intensity on mixing quality of nanopow ders,Chem.Eng.Process.50(2011)885-891.
[89]P.Ammendola,R.Chirone,Aeration and mixing behaviours of nano-sized pow ders under sound vibration,Powder Technol.201(2010)49-56.
[90]O.Gundogdu,P.M.Jenneson,U.Tuzun,Nano particle fluidisation in model 2D and 3D beds using high speed x-ray imaging and microtomography,J.Nanoparticle Res.9(2007)215-223.
[91]C.Huang,Z.Qian,M.H.Zhang,F.Wei,Solids mixing in a dow n- flow circulating fluidized bed of 0.418-m in diameter,Powder Technol.161(2006)48-52.
[92]C.Huang,Y.Wang,F.Wei,Solids mixing behavior in a nano-agglomerate fluidized bed,Powder Technol.182(2008)334-341.
[93]L.F.Hakim,J.L.Portman,M.D.Casper,A.W.Weimer,Aggregation behavior of nanoparticles in fluidized beds,Powder Technol.160(2005)149-160.
[94]X.Z.Liang,H.Duan,T.Zhou,J.R.Kong,Fluidization behavior of binary mixtures of nanoparticles in vibro- fluidized bed,Adv.Powder Technol.25(2014)236-243.
[95]H.Duan,X.Liang,T.Zhou,J.Wang,W.Tang,Fluidization of mixed SiO2and Zn O nanoparticles by adding coarse particles,Powder Technol.267(2014)315-321.
[96]J.V.Scicolone,D.Lepek,L.Louie,R.N.Dave,Fluidization and mixing of nanoparticle agglomerates assisted via magnetic impaction,J.Nanoparticle Res.15(2013)1434.
[97]P.Ammendola,R.Chirone,F.Raganati,Fluidization of binary mixtures of nanoparticles under the effect of acoustic fields,Adv.Powder Technol.22(2011)174-183.
[98]F.Danafar,A.Fakhru'l-Razi,M.A.M.Salleh,D.R.A.Biak,Fluidized bed catalytic chemical vapor deposition synthesis of carbon nanotubes-a review,Chem.Eng.J.155(2009)37-48.
[99]R.Philippe,A.Moranqais,M.Corrias,B.Caussat,Y.Kihn,P.Kalck,D.Plee,P.Gaillard,D.Bernard,P.Serp,Catalytic production of carbon nanotubes by lfuidized-bed CVD,Chem.Vap.Depos.13(2007)447-457.
[100]C.H.See,A.T.Harris,A review of carbon nanotube synthesis via fluidized-bed chemical vapor deposition,Ind.Eng.Chem.Res.46(2007)997-1012.
[101]A.Margolin,R.Rosentsveig,A.Albu-Yaron,R.Popovitz-Biro,R.Tenne,Study of the grow th mechanism of WS2nanotubesproduced by a fluidized bed reactor,J.Mater.Chem.14(2004)617-624.
[102]S.Morooka,T.Okubo,K.Kusakabe,Recent work on fluidized-bed processing of fine particles as advanced materials,Powder Technol.63(1990)105-112.
[103]L.Cadoret,N.Reuge,S.Pannala,M.Syamlal,C.Rossignol,J.Dexp ert-Ghys,C.Coufort,B.Caussat,Silicon chemical vapor deposition on macro and submicron powders in a fluidized bed,Powder Technol.190(2009)185-191.
[104]S.Balaji,J.Du,C.M.White,B.E.Ydstie,Multi-scale modeling and control of fluidized beds for the production of solar grade silicon,Pow der Technol.199(2010)23-31.
[105]W.O.Filtvedt,M.Javidi,A.Holt,M.C.Melaaen,E.Marstein,H.Tathgar,P.A.Ramachandran,Development of fluidized bed reactors for silicon production,Sol.Energy Mater.Sol.Cells 94(2010)1980-1995.
[106]Y.Wang,Y.H.Wo,K.H.Yao,H.L.Zhu,N.Y.Wang,Cvd synthesis of silicon nitride(sin)nanopow ders in a novel two-stage fluidized bed reactor,J.Inorg.Mater.21(2006)41-45.
[107]P.Cai,L.F.Chen,J.van Egmond,M.Tilston,Some recent advances in fluidized-bed polymerization technology,Particuology 8(2010)578-581.
[108]M.Q.Zhao,Q.Zhang,J.Q.Huang,G.L.Tian,J.Q.Nie,H.J.Peng,F.Wei,Unstacked double-layer templated graphene for high-rate lithium-sulphur batteries,Nat.Commun.5(2014)3410.
[109]G.Q.Ning,Z.J.Fan,G.Wang,J.S.Gao,W.Z.Qian,F.Wei,Gram-scale synthesis of nanomesh graphene with high surface area and its application in supercapacitor electrodes,Chem.Commun.47(2011)5976-5978.
[110]X.H.Liang,G.D.Zhan,D.M.King,J.A.Mc Cormick,J.Zhang,S.M.George,A.W.Weimer,Alumina atomic layer deposition nanocoatings on primary d iamond particles using a fluidized bed reactor,Diam.Relat.Mater.17(2008)185-189.
[111]F.Grillo,M.T.Kreutzer,J.R.van Ommen,Modeling the precursor utilization in atomic layer deposition on nanostructured materials in fluidized bed reactors,Chem.Eng.J.268(2015)384-398.
[112]L.F.Hakim,J.Blackson,S.M.George,A.W.Weimer,Nanocoating individual silica nanoparticles by atomic layer deposition in a fluidized bed reactor,Chem.Vap.Depos.11(2005)420-425.
[113]J.D.Ferguson,K.J.Buechler,A.W.Weimer,S.M.George,Sn O2atomic layer deposition on Zr O2and Al nanop articles:pathway to enhanced thermite materials,Powder Technol.156(2005)154-163.
[114]D.M.King,X.H.Liang,Y.Zhou,C.S.Carney,L.F.Hakim,P.Li,A.W.Weimer,Atomic layer deposition of TiO2films on particles in a fluidized bed reactor,Powder Technol.183(2008)356-363.
[115]J.R.Scheffe,A.Frances,D.M.King,X.H.Liang,B.A.Branch,A.S.Cavanagh,S.M.George,A.W.Weimer,Atomic layer deposition of iron(iii)oxide on zirconia nanoparticles in a fluidized bed reactor using ferrocene and oxygen,Thin Solid Films 517(2009)1874-1879.
[116]J.L.Li,G.H.Chen,P.Zhang,W.W.Wang,J.H.Duan,Technical challenges and progress in fluidized bed chemical vapor deposition of polysilicon,Chin.J.Chem.Eng.19(2011)747-753.
[117]C.J.Wang,T.F.Wang,P.L.Li,Z.W.Wang,Recycling of SiCl4in the manufacture of granular polysilicon in a fluidized bed reactor,Chem.Eng.J.220(2013)81-88.
[118]C.Soria-Hoyo,J.Valverde,J.van Ommen,P.Sánchez-Jiménez,L.Pérez-Maqueda,M.Sayagués,Synthesis of a nanosilica supported CO2sorbent in a fluidized bed reactor,Appl.Surf.Sci.328(2015)548-553.
[119]J.Li,X.Liu,L.Zhou,Q.Zhu,H.Li,A two-stage reduction process for the production of high-purity ultra fine Ni particles in a micro- fluidized bed reactor,Particuology 19(2014)27-34.
[120]Y.Wang,F.Wei,G.H.Luo,H.Yu,G.S.Gu,The large-scale production of carbon nanotubes in a nano-agglomerate fluidized-bed reactor,Chem.Phys.Lett.364(2002)568-572.
[121]J.Q.Huang,Q.Zhang,M.Q.Zhao,F.Wei,A review of the large-scale production of carbon nanotubes:the practice of nanoscale process engineering,Chin.Sci.Bull.57(2012)157-166.
[122]Q.Zhang,J.Q.Huang,W.Z.Qian,Y.Y.Zhang,F.Wei,The road for nanomaterials industry:a review of carbon nanotube production,post-treatment,and bulk applications for composites and energy storage,Small 9(2013)1237-1265.
[123]Y.Hao,Q.F.Zhang,F.Wei,W.Z.Qian,G.H.Luo,Agglomerated CNTs synthesized in a fluidized bed reactor:agglomerate structure and formation mechanism,Carbon 41(2003)2855-2863.
[124]Y.Liu,W.Z.Qian,Q.Zhang,G.Q.Ning,Q.Wen,G.H.Luo,F.Wei,The con fined grow th of double-walled carbon nanotubes in porous catalysts by chemical vapor deposition,Carbon 46(2008)1860-1868.
[125]M.Q.Zhao,Q.Zhang,J.Q.Huang,J.Q.Nie,F.Wei,Layered double hydroxides as catalysts for the efficient growth of high quality single-walled carbon nanotubes in a fluidized bed reactor,Carbon 48(2010)3260-3270.
[126]M.Q.Zhao,Q.Zhang,X.L.Jia,J.Q.Huang,Y.H.Zhang,F.Wei,Hierarchical composites of single/double-walled carbon nanotubes interlinked flakes from direct carbon deposition on layered double hydroxides,Adv.Funct.Mater.20(2010)677-685.
[127]Q.Zhang,W.P.Zhou,W.Z.Qian,R.Xiang,J.Q.Huang,D.Z.Wang,F.Wei,Synchronous grow th of vertically aligned carbon nanotubes with pristine stress in the heterogeneous catalysis process,J.Phys.Chem.C 111(2007)14638-14643.
[128]J.Q.Huang,Q.Zhang,G.H.Xu,W.Z.Qian,F.Wei,Substrate morphology induced self-organization into carbon nanotube arrays,ropes,and agglomerates,Nanotechnology 19(2008)435602.
[129]Q.Zhang,J.Q.Huang,M.Q.Zhao,W.Z.Qian,Y.Wang,F.Wei,Radial grow th of vertically aligned carbon nanotube arrays from ethylene on ceramic spheres,Carbon 46(2008)1152-1158.
[130]Q.Zhang,W.Z.Qian,R.Xiang,Z.Yang,G.H.Luo,Y.Wang,F.Wei,In situ grow th of carbon nanotubes on inorganic fibers with different surface properties,Mater.Chem.Phys.107(2008)317-321.
[131]Q.Zhang,M.Q.Zhao,D.M.Tang,F.Li,J.Q.Huang,B.L.Liu,W.C.Zhu,Y.H.Zhang,F.Wei,Carbon-nanotube-array double helices,Angew.Chem.Int.Ed.49(2010)3642-3645.
[132]M.Q.Zhao,Q.Zhang,W.Zhang,J.Q.Huang,Y.H.Zhang,D.S.Su,F.Wei,Embedded high density metal nanoparticles with extraordinary thermal stability derived from guest-host mediated layered double hydroxides,J.Am.Chem.Soc.132(2010)14739-14741.
[133]M.Q.Zhao,J.Q.Huang,Q.Zhang,J.Q.Nie,F.Wei,Stretchable single-walled carbon nanotube double helices derived from molybdenum-containing layered double hydroxides,Carbon 49(2011)2148-2152.
[134]M.Q.Zhao,Q.Zhang,G.L.Tian,F.Wei,Emerging double helical nanostructures,Nanoscale 6(2014)9339-9354.
[135]Q.Zhang,M.Q.Zhao,Y.Liu,A.Y.Cao,W.Z.Qian,Y.F.Lu,F.Wei,Energy-absorbing hybrid composites based on alternate carbon-nanotube and inorganic layers,Adv.Mater.21(2009)2876-2880.
[136]Q.Zhang,M.Q.Zhao,J.Q.Huang,Y.Liu,Y.Wang,W.Z.Qian,F.Wei,Vertically aligned carbon nanotube arrays grow n on a lamellar catalyst by fluidized bed catalytic chemical vapor deposition,Carbon 47(2009)2600-2610.
[137]Q.Zhang,M.Q.Zhao,J.Q.Huang,J.Q.Nie,F.Wei,Mass production of aligned carbon nanotube arrays by fluidized bed catalytic chemical vapor deposition,Carbon 48(2010)1196-1209.
[138]C.M.Chen,Q.H.Yang,Y.G.Yang,W.Lv,Y.F.Wen,P.X.Hou,M.Z.Wang,H.M.Cheng,Self-assembled free-standing graphite oxide membrane,Adv.Mater.21(2009)3007-3011.
[139]H.Bai,C.Li,G.Q.Shi,Functional composite materials based on chemically converted graphene,Adv.Mater.23(2011)1089-1115.
[140]J.-L.Shi,H.-J.Peng,L.Zhu,W.Zhu,Q.Zhang,Template grow th of porous graphene microspheres on layered double oxide catalysts and their applications in lithiumsulfur batteries,Carbon 92(2015)96-105.
[141]M.Q.Zhao,H.J.Peng,Q.Zhang,J.Q.Huang,G.L.Tian,C.Tang,L.Hu,H.R.Jiang,H.Y.Cai,H.X.Yuan,F.Wei,Controllable bulk grow th of few-layer graphene/singlew alled carbon nanotube hybrids containing Fe@C nanoparticles in a fluidized bed reactor,Carbon 67(2014)554-563.
[142]J.Li,T.Ma,L.Zhou,T.Zhang,Q.S.Zhu,H.Z.Li,Synthesis of fullerene-like WS2nanoparticles in a particulately fluidized bed:Kinetics and reaction phase diagram,Ind.Eng.Chem.Res.53(2014)592-600.
[143]J.Li,L.Zhou,Q.Zhu,H.Li,Decoupling reduction-sulfurization synthesis of inorganic fullerene-like WS2nanoparticles in a particulately fluidized bed,Chem.Eng.J.249(2014)54-62.
[144]C.N.Wu,B.H.Yan,Y.Jin,Y.Cheng,Modeling and simulation of chemically reacting flows in gas-solid catalytic and non-catalytic processes,Particuology 8(2010)525-530.
[145]Y.Cheng,J.Q.Chen,Y.L.Ding,X.Y.Xiong,Y.Jin,Inlet effect on the coal pyrolysis to acetylene in a hydrogen plasma downer reactor,Can.J.Chem.Eng.86(2008)413-420.
[146]F.X.Li,L.S.Fan,Clean coal conversion processes—progress and challenges,Energy Environ.Sci.1(2008)248-267.
[147]M.H.Han,P.T.Chang,G.S.Hu,Z.T.Chen,D.Z.Wang,F.Wei,Conversion of hydrogen chloride to chlorine by catalytic oxidation in a two-zone circulating fluidized bed reactor,Chem.Eng.Process.50(2011)593-598.
[148]Y.Cui,Q.Zhang,J.He,Y.Wang,F.Wei,Pore-structure-mediated hierarchical SAPO-34:facile synthesis,tunable nanostructure,and catalysis applications for the conversion of dimethyl ether into ole fins,Particuology 11(2013)468-474.
[149]J.Zhu,Y.Cui,Y.Wang,F.Wei,Direct synthesis of hierarchical zeolite from a natural layered material,Chem.Commun.(2009)3282-3284.
[150]Y.Wang,Z.X.Di,Y.X.Li,D.Z.Wang,F.Wei,Multiphase catalytic reactors for methanol-to-ole fins,CIESC J.65(2014)2472-2484(in Chinese).
[151]J.Li,L.Zhou,P.C.Li,Q.S.Zhu,J.J.Gao,F.N.Gu,F.B.Su,Enhanced fluidized bed methanation over a Ni/Al2O3catalyst for production of synthetic natural gas,Chem.Eng.J.219(2013)183-189.
[152]J.Li,L.Zhou,Q.S.Zhu,H.Z.Li,Enhanced methanation over aerogel NiCo/Al2O3catalyst in a magnetic fluidized bed,Ind.Eng.Chem.Res.52(2013)6647-6654.
[153]A.S.Al-Fatesh,M.A.Naeem,H.Fakeeha,A.E.Abasaeed,Role of La2O3as promoter and support in Ni/γ-Al2O3catalysts for dry reforming of methane,Chin.J.Chem.Eng.22(2014)28-37.
[154]J.J.Ding,H.Y.Liu,P.Yuan,G.Shi,X.J.Bao,Catalytic properties of a hierarchical zeolite synthesized from a natural aluminosilicate mineral without the use of a secondary mesoscale template,ChemCatChem 5(2013)2258-2269.
[155]J.Li,X.Y.Li,G.Q.Zhou,W.Wang,C.W.Wang,S.Komarneni,Y.J.Wang,Catalytic fast pyrolysis of biomass with mesoporous ZSM-5 zeolites prepared by desilication with NaOH solutions,Appl.Catal.A 470(2014)115-122.
[156]L.S.Fan,L.Zeng,W.L.Wang,S.W.Luo,Chemical looping processes for CO2capture and carbonaceous fuel conversion—prospect and opportunity,Energy Environ.Sci.5(2012)7254-7280.
[157]Y.Z.Liu,Q.J.Guo,Investigation into syngas generation from solid fuel using CaSO4-based chemical looping gasification process,Chin.J.Chem.Eng.21(2013)127-134.
[158]J.M.Valverde,Ca-based synthetic materials with enhanced CO2capture efficiency,J.Mater.Chem.A 1(2013)447-468.
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scoping biology-inspired chemical engineering☆
- Multi-functional forward osmosis draw solutes for seawater desalination☆
- Bio-inspired enantioseparation for chiral compounds☆
- Process engineering in electrochemical energy devices innovation☆
- In-situ design and construction of lithium-ion battery electrodeson metal substrates with enhanced performances:A brief review☆
- Developments in the understanding of gas-solid contact efficiency in the circulating fluidized bed riser reactor:A review☆
