Imaging protein crystal grow th behaviour in batch cooling crystallisation☆
2016-05-29JingLiuCaiMaXueWang
Jing J.Liu ,Cai Y.Ma ,Xue Z.Wang ,*
1 School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510640,China
2 Institute of Particle Science and Engineering,School of Chemical and Process Engineering,University of Leeds,Leeds LS2 9JT,UK
1.Introduction
Experimental studies of protein crystallisation have focused on obtaining high quality large crystals in order to perform X-ray diffraction analysis with the aim of obtaining the 3-dimensional structure of protein molecules.The experiments[1-11]often used micro-volume protein solutions and were carried out by changing concentration via evaporation.Typical equipment involves high-throughput micro-crystallisers,in which alargenumber of experiments are conducted and repeated,sometimes by applying external fields such as electric,magnetic,electromagnetic,and micro-gravity[12].How ever,it is obviously difficult to achieve large volume production of protein crystals by the method of changing concentration using evaporation.It is also slow and difficult to exercise automatic control.As a contrast,it is a much easier and more efficient technique to alter temperature during a crystallisation process.
In contrast to the large number of publications on cooling crystallisation of small molecules,there are only very limited reports on protein cooling crystallisation in the literature,and few can be considered as comprehensive studies.As a result,as some researchers have stressed,the effect of temperature or cooling on protein crystallisation[13-15]needs more attention.Murai and co-w orkers[16]also stressed the importance of temperature and cooling in protein crystallisation and developed a laboratory device for such investigation,but the equipment was not able to maintain efficiently the optimal temperature and cooling pro file in experiments.Adachi and co-w orkers[17]reported a high throughput experimental system that could be used to study the effect of temperature on protein crystallisation,know n as TACON(temperature at once)system.The system is composed of 45-w ell(9×5)plates or 84-w ell(12×7)plates,the temperature of each well in the platecan be controlled to be the same or totally different.How ever,the original TACON system was found unable to be directly used in protein cooling crystallisation experiments.Murai and co-w orkers[16]therefore made some improvements to the TACON,and then applied it to controlled cooling crystallisation.Although the HEW lysozyme cooling crystallisation experiment was demonstrated in the new TACON system,much of the discussion in the paper was directed at describing the new instrument;little details were given about the effect of temperature on the cooling protein crystallisation.It is not clear how sensitive TACONcan bein controlling the temperature.In this study,a hot-stage reactor which manages to execute the temperature pro file accurately within a wide range of cooling rates was applied.Repeatable crystallisation condition was achieved and the influence of cooling rate fully investigated.
The purpose of the current study is to carry out an experimental investigation on the temporal and spatial grow th behaviour of protein crystals under the influence of cooling in a crystalliser.Crystallisation via evaporation often takes a long time,requires small volume,and is difficult to exercise closed-loop control,therefore is not suitable for large scale production of protein crystals.In 2004,Basu and co-w orkers[18]pointed out that there are many potential advantages of delivering biopharmaceuticals in the form of crystals,and predicted that although there was only one biopharmaceutical that was marketed in the form of crystals at the time which was insulin,more in future would be manufactured in crystalline form.Today there have been more than 150 crystalline form biopharmaceuticals on the market.The efficiency of large scale protein crystallisation can be significantly improved by employing cooling strategies,and research on this subject has been carried out in our previous work using morphological population balance models which demonstrated that operational condition(e.g.cooling rate and seed loading)can be optimised and desired shape and size distributions for a population of crystals can be closed-loop controlled[19-23].The focus of this paper is experimental study on cooling crystallisation of proteins,investigating the temporal and spatial grow th behaviour of protein crystals using on-line real-time imaging instrument.
2.Experimental Stud y of Hen-Egg-White Lysozym e Cooling Crystallisation
2.1.Materials,equipment and instruments
2.1.1.Materials
The materials used in this study include dimethyldichlorosilane(DMDC)(purchased from the Sigma-Aldrich company),1,1,1-trichloroethane(methyl chloroform)w ritten as C2H3Cl3or CH3CCl3,Hen-Egg-White Lysozyme,L62971(purchased from the Sigma-Ald rich company),sodium hydroxide,sodium chloride,sodium azide,hydrochloric acid or acetic acid,and deionised water(>15 MQ·cm-1).
2.1.2.Equipment and instrument
The main experimental rig of HEW lysozyme crystallisation comprises a LTS350 hot-stage reactor and a video imaging system.The LTS350 hot-stage reactor was supplied by the Linkam[24]company,which is combined with the video imaging system to carry out on-line monitoring of the HEW lysozyme crystallisation process.The LTS350 hot-stage reactor consists of a hot-stage reactor,LNP94 Liquid Nitrogen Cooling Pump and CI94 temperature controller,which are show n in Fig.1.The LTS350 is an easy to use versatile heating or freezing stage,which includes a large area temperature controlled element with a platinum resistor sensor embedded close to the surface for accurate temperature measurements.The details of the hot-stage reactor are listed in Fig.2.LNP94 Liquid Nitrogen Cooling Pump and CI94 temperature controller are the main components for accurate temperature control of the hot-stage reactor,and the precise control of liquid nitrogen flow enables specific stages to control cooling rates as fast as 130 °C·min-1or as slowas 0.1 °C·min-1.
The video imaging system is made up of a camera interface,computer and Polytec xenon strobe box with a liquid light guide,which is designed to capture digital video using specialised video software(Video Savant)running on a custom built high specification PC,as show n in Fig.3.The experiments were carried out on the stage of an optical microscope real-time or time-lapsed and non-invasive.Professor Wang and his group[25-32]have carried out much research on small molecular crystallisation processes using the imaging video system.
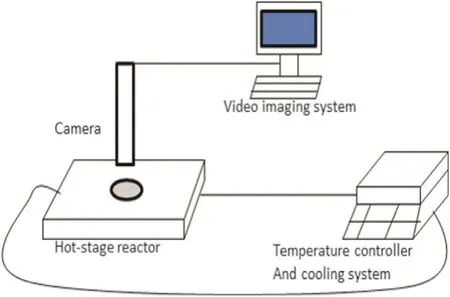
Fig.1.Set-up of HEWL cooling crystallisation experiment.
Other facilities include a vacuum oven,glass or ceramic containers,a p H meter(Mettler Toledo 320 digital meter),a glass stirring rod,a plastic head dropper,Buchner funnel,centrifugal pump,pipette,sitting-drop crystallizer tray(6 × 4 w ell),and membrane of 0.22 μm,and thermostat(water).
2.2.Experiment
As is know n,lysozyme is easily crystallised as the form of dimer or polymer(oligomer)in the condition of low er ionic strength solution and in values of p H higher than 5.How ever,lysozyme exists as a type of monomer in the form of co-existing aggregates,so the value of p H was set as 4.5 in this study.In addition,lysozyme crystals have polymorphism in their solution,of which lysozyme crystals with tetragonal structure are easily crystallised and more stable.Therefore,lysozyme crystals in tetragonal structure are applied and all data derived from the literature is utilized in the base of tetragonal lysozyme crystals.Silanization[33]process is necessary for all glassware,including the sitting-drop crystallizer.Details about silanization are not given here.
Preparation of HEWL solution with a concentration of 20-50 mg·ml-1is detailed below,w here sodium chloride is chosen as precipitate with a concentration of 2.5 w t%and sodium acetate is used as buffer solution with a concentration of 0.1 mol·L-1and p H of 4.5.
Preparation experiments were carried out at a room temperature of 20°C and involve the follow ing steps:
(1)Preparation of the buffer solution:sodium chloride was dissolved into deionized water to make a sodium chloride solution with a concentration of 0.1 mol·L-1;the value of p H is adjusted by titrating acetate or sodium hydroxide.
(2)Dissolving sodium chloride into solution 1 and making sodium chloride-sodium acetate solution with a concentration of 50 mg·ml-1;if the value of p H needs to be re-calibrated,sodium chloride must be added into solution 1 before calibration.
(3)Triassic nitride solution was made with a concentration of 0.05 wt%and added into the buffer solution to inhibit the interference of bacteria.
(4)Dissolving lysozyme solution into solution 1(without sodium chloride)and titrating it to be a 40-100 mg·ml-1solution.
(5)Mixing solutions 2 and 4 with the same volume to produce HEWL solution with a concentration of 20-50 mg·ml-1sodium chloride solution with a concentration of 2.5%and sodium acetate solution with a concentration of 0.1 mol·L-1and a pH of 4.5.
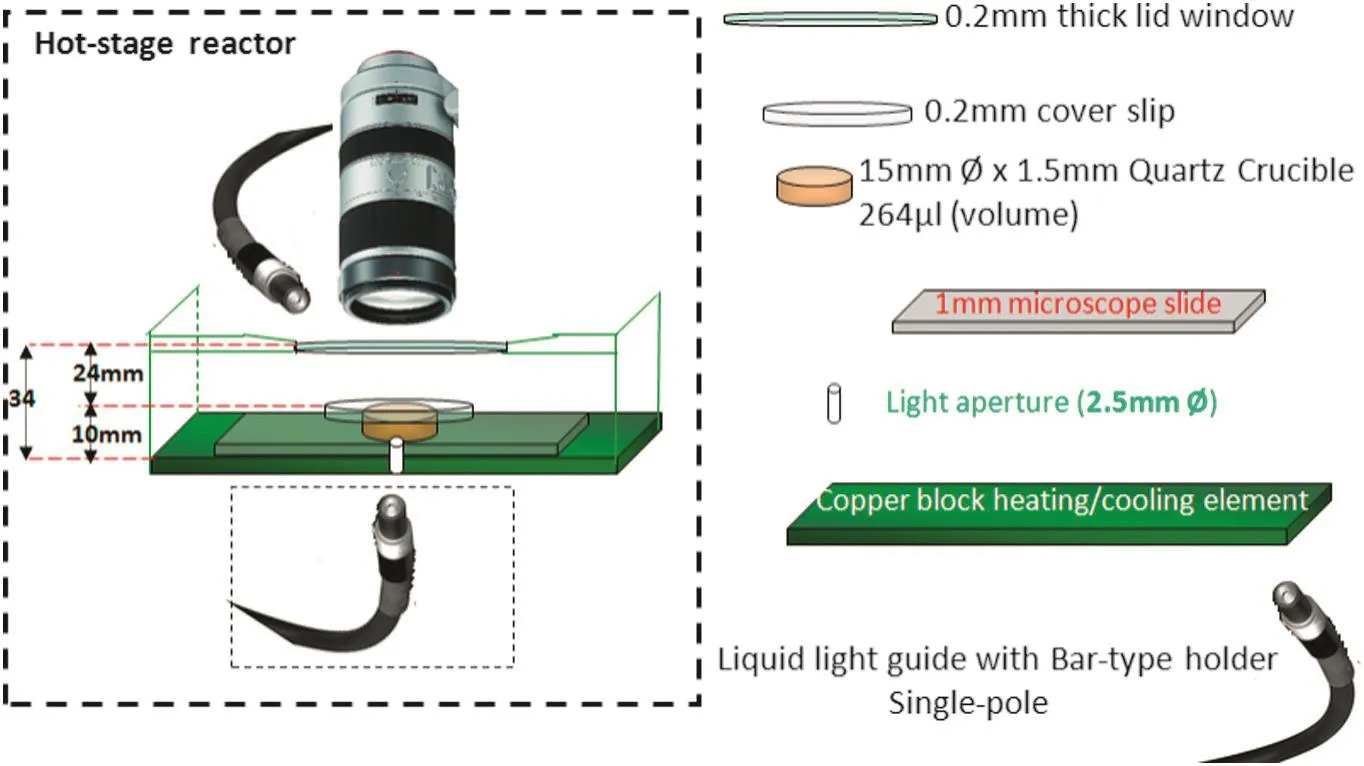
Fig.2.Details of the LTS350 hot-stage reactor integrated with the imaging system.

Fig.3.HEWL crystals were generated 18°C overnight and observed by microscope.
It is noted that all solutions from 1 to 5 must be centrifuged for 15 min(1300 r·min-1)after preparation and then all solutions were placed into membranes of 0.22 μm to avoid impurities.
2.3.Effect of lysozyme solution on protein crystallisation
It is important to have some prior know ledge of lysozyme crystallisation behaviour before the cooling crystallisation experiments using the hot-stage.As is already know n,protein crystallisation is more complex than small molecules as a result of the complicated effects of solution,precipitate,concentration,temperature and p H.Therefore,it is necessary to explore the behaviour of protein crystallisation itself under fixed initial conditions,including solution,precipitate,concentration,temperature and p H.After that the cooling protein crystallisation experiment was carried out in order to study the behaviour of cooling protein crystallisation within different cooling rates.In this experiment,room temperature was kept at 18°C with p H at 4.5 and the purpose of the experiment was to discuss the effect of initial solution concentration and precipitate concentration on lysozyme crystallisation under a consistent temperature.
2.3.1.Thermodynamic study of HEW lysozyme
The driving force for protein crystallisation is the difference in chemical potential between the solution and solid phase;therefore,solubility is always needed in order to determine the supersaturation at any given operational condition.There is a considerable amount of research concerning the thermodynamic study of HEW lysozyme,such as experiments on measuring HEW lysozyme solubility[6,15,34-36],efforts to measure solubility using different techniques[2,11,35,37].Protein solubility can be described as a function of four variables,including p H,precipitant concentrations,solution concentrations and temperature[38],which are the basis for the selection of crystallisation conditions,including initial solution concentration,temperature and crystallisation methods.It needs to point out here that if the p H and temperature are given,precipitant concentrations and solution concentrations are the main factors for crystallisation in limited volume protein crystallisation experiments.In traditional protein crystallisation experiments,protein solutions are left to settle overnight to produce protein crystals,of which the w hole process is time consuming.It is important to find out initial solution conditions of protein crystallisation within cooling operation for further study.Therefore,sitting-drop micro-plate protein crystallisation is carried out before cooling protein crystallisation in a hot-stage reactor in order to explore the effects of the initial solution conditions on protein crystallisation.HEW lysozyme solution for crystallisation is prepared based on the solubility equation in the paper[38]discussed previously.
2.3.2.Linbro micro-plate sitting-drop crystallisation
In traditional protein crystallisation experiments which were usually in biological area under the principle of vapour diffusion,temperature is set as constant.The size of the Linbro 24-well plate is 150 mm×106 mm×28 mm with a lid,which has 24 w ells having a diameter of φ 16.5 mm.24 samples can be used at one time and the temperature can be kept at±1 °C.A drop of HEW lysozyme solution mixed with precipitate solution of different concentrations is placed in vapour equilibration with a liquid reservoir of precipitate and put into each cell of micro-well plate.Typically,the drop contains a lower precipitate concentration than thereservoir in the well.To achieve equilibrium,water vapour leaves the drop and eventually reaches the reservoir.As water leaves the drop,the protein undergoesan increase in relatives upersaturation.Equilibration is reached when the reagent concentration in the drop is approximately the same as that in the reservoir and finished until protein crystals leave the drop mixture.The sitting drop crystallisation method is easy to monitor using microscopic techniques.
In terms of the solubility phase of HEW lysozyme,36 sets of HEWlysozyme crystallisation experiments were run at the same time in the Linbro micro-well plate with different mixtures of initial concentration and precipitate concentration.HEW lysozyme initial concentration is ranged as 10,20,30,40,50,and 60 mg·ml-1and precipitate concentration was prepared as5%,10%,15%,20%,25%,and 30%.The Linbro micro well sitting drop plate was stored overnight at 18°C in the culture room and covered with alid.After 24 h,it was clearly observed under a microscope that tetragonal HEW lysozyme crystals were generated in some wells as shown in Fig.3.However,crystallisation conditions were different in each w ell as given in Table 1,w here ‘Clr’means clear in the cell,the number in each cell denoting the number of crystals,‘pre’means precipitation took place in the well and no crystals were available.
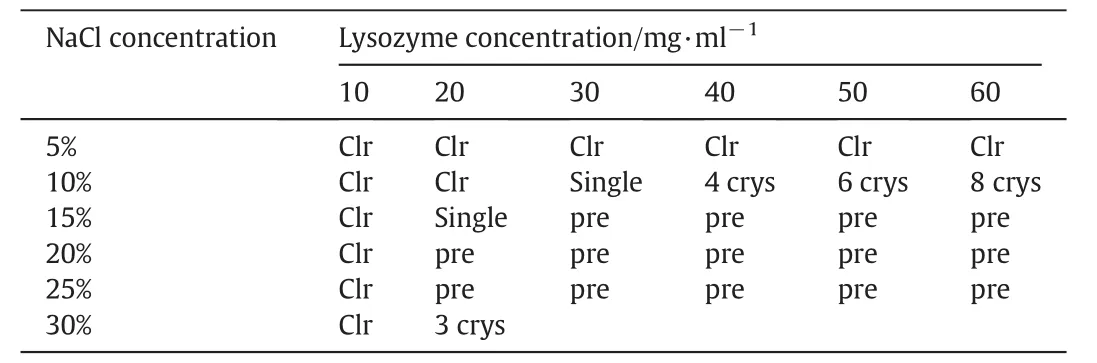
Table 1 HEW lysozyme crystallisation status in each well of Linbro micro-well plate after leaving overnight
As is known,protein crystallisation is very complicated because it is easily affected by initial solution concentration,precipitate species,precipitate concentration,impurity,temperature and p H in the solution.The purpose of this experiment is to make sure of suitable initial solution conditions for the mixture of initial solution concentration and precipitate concentration,given precipitate species(sodium chloride),temperature(18°C)and a p H of 4.5.
It is shown in Table 1 that no crystals appeared in the cell with the initial mixture of HEW lysozyme concentration from 10,20,30,40,and 50 to 60 mg·ml-1and a precipitate concentration of 5 wt%.Several crystals were observed in the w ell with mixtures of initial concentrations from 30,40,50 to 60 mg·ml-1and precipitate concentration of 10 w t%.How ever,there were no crystals in thew ell with initial concentrations of 10 to 20 mg·ml-1and precipitate concentration of 10 w t%.There were no crystals appearing in the w ell with an initial HEW lysozyme concentration of 10 mg·ml-1,no matter w hat the precipitate concentration was from 5%,10%,15%,20%,25%,and 30%.Precipitate only appeared in w ell 16 with an initial HEW lysozyme concentration over 30 mg·ml-1and precipitate concentrations over 15 w t%.
It is necessary to state the principles of precipitate effect on protein crystallisation.Typically,salt precipitate is often applied in protein crystallisation instead of organic solvent precipitate,where salt precipitate is mainly used to destroy the hydration layer within protein and decrease the binding capacity between protein and water,as a contrast,increasing the binding forces between proteins and facilitating bond formation in protein crystals,as HEW lysozyme has higher solubility in low er precipitate concentrations.It was easy to see that the solution was still clear in the column with HEW lysozyme concentration at 20 mg·ml-1,even if the initial solution concentration was over 60 mg·ml-1.The same conclusion was observed by Han[39]and his group w here an optimal initial HEW lysozyme solution concentration from 10 to 30 mg·ml-1for crystal growth was obtained.During the concentration range,it was easy to grow large protein crystals with less crystal nuclei.Howard et al.[34]carried out similar research to obtain better initial conditions for grow ing protein crystals of a larger size,with an initial protein concentration of 2.5-3 times protein solubility often being applied.
2.4.Cooling HEW lysozyme crystallisation experiment
For the cooling HEW lysozyme crystallisation experiment,initial solution conditions were chosen based on the testing experiment where the solution in the cell of sitting-drop is still clear at 18°C with the solution concentration mixture,so that the cooling HEW lysozyme crystallisation experiment can be carried out under chosen initial conditions.It can be concluded from Table 1 that the initial conditions should be as those in the well with a mixture of initial concentration below 60 mg·ml-1and precipitate concentration no more than 5 w t%.Therefore the HEW lysozyme solution was prepared with a concentration of 20 and 40 mg·ml-1and precipitate concentration 2.5 w t%and 5 w t%.
Firstly,calibration of the objective lens was necessary using a ‘1 mm’standard ruler before the experiment until there was clear image on the screen as shown in Fig.4.It is important to keep the w hole system stable,including the camera and the liquid light guide during the w hole experiment.Secondly,initial HEW lysozyme solutions were transferred into the LTS hot stage reactor sample cell,which had a lid,by pipette to prevent evaporation and carrying out the experiments using the imaging video system.Thirdly,the LNP94 cooling systems and CI94 temperature controller were switched on,and a Linkam RS232 Video Interface was used to get a video camera signal and the Linksys temperature control software was used to link temperature pro files with the Linkam Real Time Video Measurement software.As shown in Fig.5,the abscissa of the cooling pro file represented the recording time in the unit of‘second’and ordinate means crystallisation temperature in the unit of°C,the slope of the temperature curve means cooling rate in the unit of°C·s-1.The w hole process is recorded and monitored by Video Savant software where the intervals of capturing images and storage space for images are controlled.So then the temperature control information could be connected and record a relationship with digitally captured images by Linksys temperature control software and Linkam RS232.

Fig.4.Video imaging system and lens calibration for HEWL crystallisation experiments.
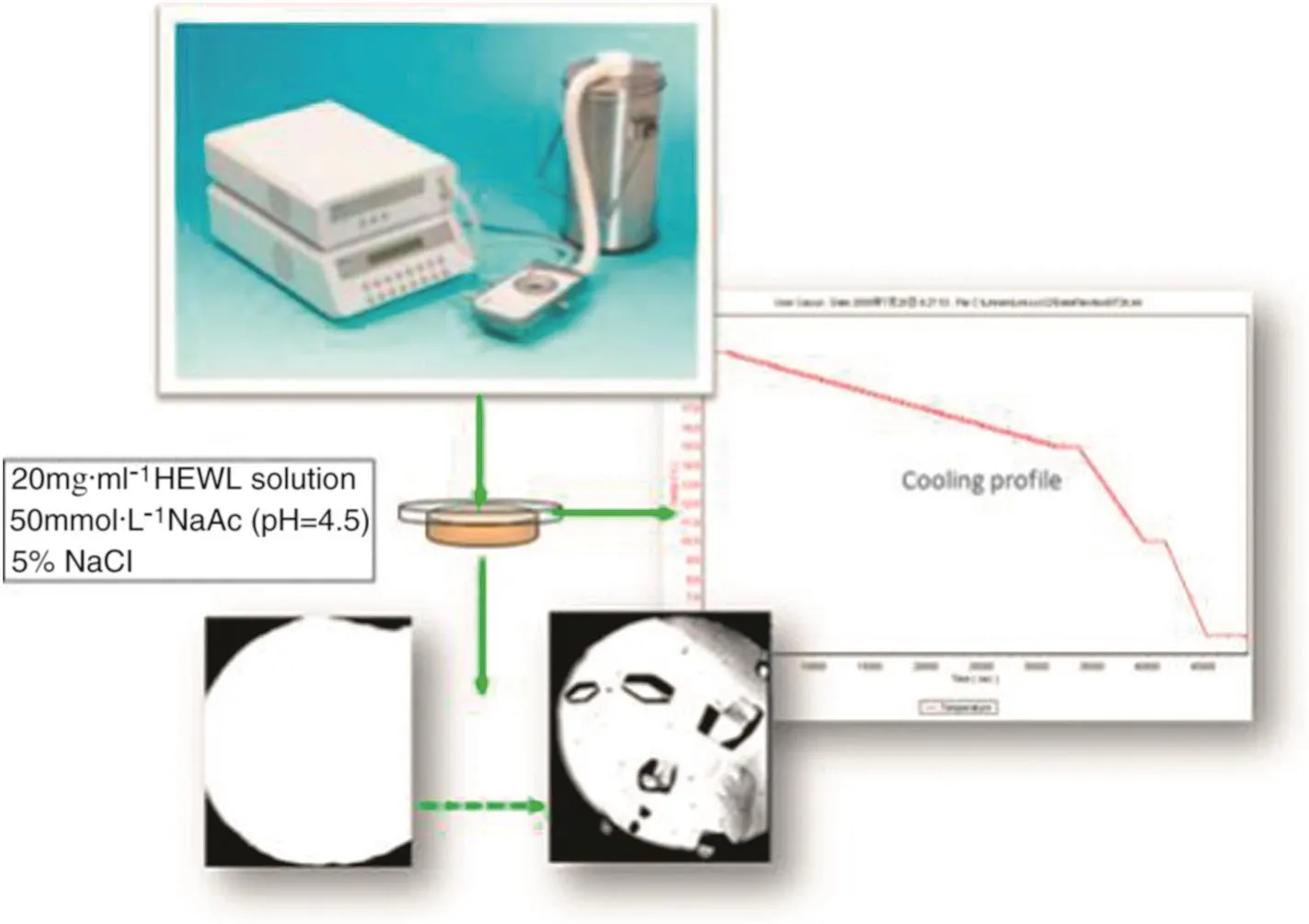
Fig.5.Monitoring the HEWL cooling crystallisation.
3.Results and Discussion
3.1.On-line imaging of HEW lysozyme cooling crystallisation
Four sets of experiments were carried out using the LTS350 and imaging video system.Initial HEW lysozyme solution concentration was 20 and 40 mg·ml-1,precipitate concentrations were 2.5 w t%and 5 w t%,solution p H was 4.5 and the initial temperature was set at 18°C.Crystals are generated in all four sets of experiments by adjusting cooling rates and the whole process can be logged by recording images as in Fig.5,parts of images are selected from 460 piece images set to be recorded during cooling crystallisation.The initial HEW lysozyme concentration is 20 mg·ml-1,NaCl precipitate concentration is 5 w t%,p H is 4.5,the cooling rate was 0.04 °C·min-1and the crystallisation time was 6.25 h.45 images in Fig.6 covered the cooling crystallisation process from clear solutions at the beginning to the end with crystals,w here the time scale was recorded at the beginning,in the images of 169,330 and 420.A ‘1 mm’standard ruler image is recorded in the final images so that the crystal size can be measured with the help of a ruler.
It was observed in Fig.6 that it is time consuming to transport HEW lysozyme crystals from the clear solutions and some crystals are clearly seen with regular tetragonal crystal shapes.How ever,some protein crystals are not clear and irregularities in the images are not only because the crystals are smaller in size,but also because the camera resolution was not high enough to get the shape of crystals.The crystals finally become clear and regular.It is also concluded from the images of the experiments that it is an effective operation for protein crystallisation by adjusting temperature and the effects of cooling rateson HEW lysozymecrystallisation will bediscussed in the following section.
3.2.Effect of cooling rates on HEW lysozyme crystallisation
Although there is literature covering the effects of cooling rates on micro-molecular crystallisation[40-44],the purpose of HEW lysozyme cooling crystallisation is to explore the relationship of cooling rates and protein crystallisation qualitatively.The initial HEW lysozyme concentration was 40 mg·ml-1.The concentration of NaCl precipitate was 2.5 w t%,p H was 4.5 and crystallisation time was 14 h.The behaviour of HEW lysozyme crystals was recorded by the imaging video system at different cooling rates.
The experiments were carried out from 18 °C to 5 °C at different cooling rates;different HEW lysozyme behaviour and crystallisation phenomena were observed as shown in Fig.7.Images at the beginning of crystal birth and the end were selected with the temperature information.It is shown in Fig.7(a)that some precipitate and small irregular crystals birthed at the beginning with higher cooling rates,no more crystals were generated with continued decreasing temperatureor constant temperature.As a contrast,plenty of precipitates are produced with decreasing temperature and precipitates almost filled the w hole w ell.Obviously,it is not a good choice for protein cooling crystallisation with high supersaturation and high cooling rates at the beginning.
Fig.7(b)show s that at a cooling rate lower than that used in the experiment of Fig.7(a)nucleation of some HEW lysozyme crystals in the solution occurred but turning to a faster cooling rate still generated precipitates.Therefore,cooling rates were not chosen at random as if regular crystal nuclei generated,it is not certain that crystal grow th was based on regular nuclei with continued decreasing cooling rates.Decreased cooling rates at the beginning compared to experiment Fig.7(b),kept the cooling rates constant after birth of crystal nuclei and made the nuclei grow,increasing cooling rates properly after crystals achieved a regular shape from the nuclei.It was observed that further grow th was happening based on the stable crystals,which continued until the growth behaviour ended,as shown in Fig.7(c).HEW lysozyme crystals of larger and more regular shape were obtained in the final solution.All the experiments concerned with the effect of cooling rates on HEW lysozyme crystallisation behaviour and on-line measurement are supplementary and useful for protein crystallisation.The phenomena observed are consistent with the know ledge that fast cooling can generate precipitates in crystallisation,and often slow cooling produces structured crystals.This is because crystal formation has to consider growth kinetics as well as thermodynamics.Fast cooling generates large supersaturation,i.e.large driving force,given little time for the molecules to arrange in a structured way therefore precipitates can be formed.While the observed phenomena are in agreement with our know ledge for small molecules,there is a major difference between protein crystallisation and small molecule crystallisation as to w hat is a slow cooling rate.The cooling rate to obtain crystals of Fig.7(c)is 0.04 °C·min-1,much slow er than the cooling rates used in small molecule crystallisation.This is because it takes much longer for proteins to reach solid-liquid phase equilibrium.For some proteins it can take months to reach equilibrium.Considering both the nucleation kinetics as w ell as thermodynamics,it is understandable that different polymorphs can be formed as a result for different initial concentrations,cooling rates as w ell as other conditions such as p H.Even for the same polymorph,the surface chemistry of each face can vary and therefore have different grow th kinetics(grow th rate m·s-1as a function of operational conditions such as supersaturation),resulting in varied morphology as a result of altering cooling rates.

Fig.6.Selected images obtained during HEWL cooling crystallisation.
4.Summary and Conclusions
Cooling strategy can shorten the crystallisation time w ithout yield reduction.In addition,in comparison with other methods of protein crystallisation such as evaporation,temperature controlling is easier and well established.HEW lysozyme cooling crystallisation was investigated in a LTS350 hot-stage reactor and on a Linbro sitting-drop micro-w ell plate.The purpose of the Linbro micro 36-well parallel experiments was to understand the effects of initial HEW lysozyme concentration and precipitate concentration on HEW lysozyme crystallisation at a room temperature of 18°C(summarized in Table 1),which also gave a clue to the choice of the initial solution conditions for further cooling crystallisation studiesusing thehot-stage.Clear solution conditions were used in cooling crystallisation experiments without consideration of the effects of HEW lysozyme solution itself on the cooling rates.

Fig.7.HEWL crystallisation under different cooling strategies.(a)Precipitates were produced if a high initial cooling rate was applied.(b)Crystals were generated at appropriate initial cooling rate,but precipitates were still generated if high cooling rates were applied later.(c)Suitable initial and later slow er cooling rates produce good crystals.
An efficient HEW lysozyme cooling crystallisation was carried out using a LTS350 hot-stage reactor,HEW lysozyme crystal shape changes were tracked real-time and the effect of cooling rates on lysozyme crystallisation was examined.It is concluded that higher cooling rates at the beginning were not a sensible choice as they led to precipitates(Fig.7(a)).It was also observed that a low er cooling rate generated nuclei but by turning to a faster cooling rate later can still lead to generation of precipitates(as show n in Fig.7(b)).How ever,it can be seen from Fig.7(c)that crystal nuclei are generated by proper cooling rates at the beginning and keep constant cooling rates for a w hile,further grow th continued by improving cooling rate slowly.Keeping constant cooling rates and improving cooling rates slow ly in turn,made it possible for lysozyme crystals to achieve a regular shape and large size finally.Therefore such on-line imaging systems described in this work made it possible to record and observe crystal's interesting growth behaviour subjected to varied cooling strategies.Such insight know ledge can be useful information for developing both qualitative and quantitative understanding(if combined with image analysis and other measurements such as supersaturation)of protein crystal formation and grow th,as w ell as for designing,optimising and controlling cooling crystallisation processes.
[1]J.K.Baird,S.C.Hill,J.C.Clunie,Kinetics of protein crystal nucleation and grow th in the batch method,J.Cryst.Growth 196(2-4)(1999)220-225.
[2]E.Cacioppo,S.Munson,M.L.Pusey,Protein solubilities determined by a rapid technique and modification of that technique to a micro-method,J.Cryst.Growth 110(1-2)(1991)66-71.
[3]A.C.Dumetz,A.M.Chockla,E.W.Kaler,A.M.Lenhoff,Effects of p Hon protein-protein interactions and implications for protein phase behavior,Biochim.Biophys.Acta Proteins Proteomics 1784(4)(2008)600-610.
[4]E.Forsythe,M.Lee Pusey,The effects of temperature and NaCl concentration on tetragonal lysozyme face growth rates,J.Cryst.Growth 139(1-2)(1994)89-94.
[5]E.F.Forsythe,M.L.Pusey,Studies on tetragonal lysozyme crystal growth rates,Acta Crystallogr.D50(1994)614-619.
[6]E.L.Forsythe,R.A.Judge,M.L.Pusey,Tetragonal chicken egg white lysozyme solubility in sodium chloride solutions,J.Chem.Eng.Data 44(3)(1999)637-640.
[7]J.Dong,J.Z.Pan,X.T.Niu,Y.C.Zhou,R.C.Bi,The influence of microgravity on crystal structure of protein,Chin.Sci.Bull.45(1)(2000)55-59.
[8]X.Y.Liu,G.L.Dai,S.J.Wang,Study of the effect of impurities on protein crystal,Chin.J.Biotechnol.27(12)(2007)101-108.
[9]X.H.Dai,R.C.Bi,Effect of precipitation agent types on the molecular packing of protein crystals,J.Biol.Chem.18(4)(2002)394-398.
[10]G.L.Dai,Y.Xie,Q.Kang,W.R.Hu,Study of intrinsic reason of effect of solution heat history on protein crystal growth,J.Chem.65(13)(2007)1202-1206.
[11]K.M.Gernert,R.Smith,D.C.Carter,A simple apparatus for controlling nucleation and size in protein crystal-grow th,Anal.Biochem.168(1)(1988)141-147.
[12]L.J.Delucas,C.D.Smith,H.W.Smith,S.Vijaykumar,S.E.Senadhi,S.E.Ealick,D.C.Carter,R.S.Snyder,P.C.Weber,F.R.Salemme,D.H.Ohlendorf,H.M.Einspahr,L.L.Clancy,M.A.Navia,B.M.McKeever,T.L.Nagabhushan,G.Nelson,A.McPherson,S.Koszelak,G.Taylor,D.Stammers,K.Pow ell,G.Darby,C.E.Bugg,Protein crystalgrow th in microgravity,Science 246(4930)(1989)651-654.
[13]W.Heinrichs,M.Heinrichs,H.Schonert,Grow th of protein single-crystals in a periodically changing solubility gradient—Description of the method and 1st results,J.Cryst.Growth 122(1-4)(1992)186-193.
[14]R.C.Demattei,R.S.Feigelson,Controlling nucleation in protein solutions,J.Cryst.Growth 122(1-4)(1992)21-30.
[15]F.Rosenberger,S.B.How ard,J.W.Sowers,T.A.Nyce,Temperature dependence of protein solubility—Determination and application to crystallisation in X-ray capillaries,J.Cryst.Growth 129(1-2)(1993)1-12.
[16]R.Murai,S.Nakata,M.Kashii,H.Adachl,A.Niino,K.Takano,H.Matsumura,S.Murakami,T.Inoue,Y.Mori,T.Sasaki,Cooling-rate screening system for determining protein crystal growth conditions,J.Cryst.Growth 292(2)(2006)433-436.
[17]H.Adachi,A.Niino,K.Takano,H.Matsumura,S.Murakami,T.Inoue,Y.Mori,T.Sasaki,Temperature-screening system for determining protein crystallisation conditions,Jpn.J.Appl.Phys.44(6)(2005)4080-4083.
[18]S.K.Basu,C.P.Govardhan,C.W.Jung,Protein crystals for the delivery of biopharmaceuticals,Expert.Opin.Biol.Ther.4(3)(2004)301-317.
[19]J.J.Liu,Y.D.Hu,X.Z.Wang,Optimization and control of crystal shape and size in protein crystallisation p rocess,in:I.A.Karim i,R.Srinivasan(Ed s.),11th international symposium on process systems engineering,Pts a and B,vol.31 2012,p p.1160-1164.
[20]J.J.Liu,Y.D.Hu,X.Z.Wang,Optimization and control of crystal shape and size in protein crystallisation process,Comput.Chem.Eng.57(2013)133-140.
[21]J.J.Liu,C.Y.Ma,Y.D.Hu,X.Z.Wang,Morphological population balance models for the dynamic evolution of particle shape and size distribution in protein crystallisation,in:R.M.de Brito Alves,C.A.O.do Nascimento,E.C.Biscaia(Eds.),10th international symposium on process systems engineering,vol.27 2009,pp.1911-1916.
[22]J.J.Liu,C.Y.Ma,Y.D.Hu,X.Z.Wang,Effect of seed loading and cooling rate on crystal size and shape distributions in protein crystallisation—A study using morphological population balance simulation,Comput.Chem.Eng.34(12)(2010)1945-1952.
[23]J.J.Liu,C.Y.Ma,Y.D.Hu,X.Z.Wang,Modelling protein crystallisation using morphological population balance models,Chem.Eng.Res.Des.88(4A)(2010)437-446.
[24]Linkam,www.linkam.co.uk.
[25]J.Calderon De Anda,X.Z.Wang,X.Lai,K.J.Roberts,Classifying organic crystals via inprocess image analysis and the use of monitoring charts to follow polymorphic and morphological changes,J.Process Control 15(7)(2005)785-797.
[26]J.Calderon De Anda,X.Z.Wang,X.Lai,K.J.Roberts,K.H.Jennings,M.J.Wilkinson,D.Watson,D.Roberts,Real-time product morphology monitoring in crystallisation using imaging technique,AIChE J.51(5)(2005)1406-1414.
[27]J.Calderon De Anda,X.Z.Wang,K.J.Roberts,Multi-scale segmentation image analysis for the in-process monitoring of particle shape with batch crystallisers,Chem.Eng.Sci.60(4)(2005)1053-1065.
[28]C.Y.Ma,X.Z.Wang,K.J.Roberts,Multi-dimensional population balance modeling of the grow th of rod-like L-glutamic acid crystals using growth rates estimated from in-process imaging,Adv.Powder Technol.18(6)(2007)707-723.
[29]J.Wan,C.Y.Ma,X.Z.Wang,A method for analyzing on-line video images of crystallisation at high-solid concentrations,Particuology 6(1)(2008)9-15.
[30]X.Z.Wang,J.Calderon De Anda,K.J.Roberts,R.F.Li,G.B.Thompson,G.White,Advances in on-line monitoring and control of the morphological and polymorphic forms of organic crystals grow n from solution,KONA Powder Part.J.(23)(2005)69-85.
[31]X.Z.Wang,J.C.De Anda,K.J.Roberts,Real-time measurement of the growth rates of individual crystal facets using imaging and image analysis—A feasibility study on needle-shaped crystals of L-glutamic acid,Chem.Eng.Res.Des.85(A7)(2007)921-927.
[32]X.Z.Wang,K.J.Roberts,C.Y.Ma,Crystal growth measurement using 2D and 3D imaging and the perspectives for shape control,Chem.Eng.Sci.63(5)(2008)1173-1184.
[33]https://en.wikipedia.org/w iki/Silanization,accessed,September 2015.
[34]S.B.Howard,P.J.Tw igg,J.K.Baird,E.J.Meehan,The solubility of hen egg-w hite lysozyme,J.Cryst.Growth 90(1-3)(1988)94-104.
[35]E.Cacioppo,M.L.Pusey,The solubility of the tetragonal form of hen egg white lysozyme from p H 4.0 to 5.4,J.Cryst.Growth 114(3)(1991)286-292.
[36]G.Sazaki,K.Kurihara,T.Nakada,S.Miyashita,H.Komatsu,A novel approach to the solubility measurement of protein crystals by tw o-beam interferometry,J.Cryst.Growth 169(2)(1996)355-360.
[37]A.G.Jones,Optimal operation of a batch cooling crystallizer,Chem.Eng.Sci.29(5)(1974)1075-1087.
[38]J.J.Liu,Modelling,optimization and experiment study of protein crystallisation,Ph D thesis,Ocean University of China,June 2010.
[39]Y.Han,H.X.Cang,R.C.Bi,Determination of NaCl solution system lysozyme crystallisation phase diagram,J.Biol.Chem.14(3)(1998)573-576.
[40]H.Hojjati,S.Rohani,Cooling and seeding effect on supersaturation and final crystal size distribution(CSD)of ammonium sulphate in a batch crystallizer,Chem.Eng.Process.44(9)(2005)949-957.
[41]H.A.Mohameed,B.Abu-Jdayil,M.Al Khateeb,Effect of cooling rate on unseeded batch crystallisation of KCl,Chem.Eng.Process.41(4)(2002)297-302.
[42]N.Kubota,N.Doki,M.Yokota,A.Sato,Seeding policy in batch cooling crystallisation,Powder Technol.121(1)(2001)31-38.
[43]N.Doki,N.Kubota,A.Sato,M.Yokota,Effect of cooling mode on product crystal size in seeded batch crystallisation of potassium alum,Chem.Eng.J.81(1-3)(2001)313-316.
[44]R.A.Judge,R.S.Jacobs,T.Frazier,E.H.Snell,M.L.Pusey,The effect of temperature and solution p H on the nucleation of tetragonal lysozyme crystals,Biophys.J.77(3)(1999)1585-1593.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scoping biology-inspired chemical engineering☆
- Review on the nanoparticle fluidization science and technology☆
- Multi-functional forward osmosis draw solutes for seawater desalination☆
- Bio-inspired enantioseparation for chiral compounds☆
- Process engineering in electrochemical energy devices innovation☆
- In-situ design and construction of lithium-ion battery electrodeson metal substrates with enhanced performances:A brief review☆
