大鼠尸体细菌演替规律及其在死亡时间推断中的应用(英文)
2016-03-23郭娟娟特拉提赛依提彭钰龙郭亚东扎拉嘎白乙拉蔡继峰中南大学基础医学院法医系湖南长沙4003新疆警察学院刑侦系新疆乌鲁木齐8300
张 琳,郭娟娟,特拉提·赛依提,彭钰龙,谢 丹,郭亚东,闫 杰,扎拉嘎白乙拉,蔡继峰(.中南大学基础医学院法医系,湖南长沙4003;.新疆警察学院刑侦系,新疆乌鲁木齐8300)
大鼠尸体细菌演替规律及其在死亡时间推断中的应用(英文)
张琳1,郭娟娟1,特拉提·赛依提2,彭钰龙1,谢丹1,郭亚东1,闫杰1,扎拉嘎白乙拉1,蔡继峰1
(1.中南大学基础医学院法医系,湖南长沙410013;2.新疆警察学院刑侦系,新疆乌鲁木齐830011)
摘要:目的探讨大鼠尸体细菌演替规律并评估其在死亡时间(PMI)推断中的应用价值。方法选取成年雌性SD大鼠,处死后置于纸箱内自然腐败,采集大鼠眼周皮肤、口腔和阴道三个部位的细菌样本,使用纯培养生化方法鉴定菌种,记录菌群构成变化规律。结果大鼠三个部位细菌演替规律显示,葡萄球菌属和奈瑟菌属为死后初期的优势菌属,尤其是金黄色葡萄球菌与乳糖奈瑟菌在死后6h内相对含量较高。干酪乳杆菌规律地出现在死后第3或第4天,且在死后晚期维持较恒定水平。结论大鼠死后三个部位的正常细菌及腐败细菌呈现一定的演替规律,可用于PMI推断。
关键词:法医病理学;细菌;死亡时间;大鼠
Author: ZHANG Lin(1986—), postgraduate, major in forensic pathology; E-mail: lyre2008@126.com
Introduction
Estimation of postmortem interval(PMI)is one of the most important and difficult practical tasks in daily forensic casework, especially in putrefied corpses. Traditionally, estimation of PMI has relied on the physical changes that occur after death[1]. However, these phenomena cannot be applied to putrefied corpses, but just can provide a rough estimation of PMI. Forensic scientists have never stopped exploring new approaches to the determination of PMI, such as DNA/RNA degradation, bacterial processes and forensic entomology[2]. In practical terms, a need still exists to develop a reliable and accurate method to estimate PMI.
Various kinds of bacteria are associated with both the external and internal aspects of the human body[3]. Shortly after death, these bacteria were reported to begin digesting the body from the inside out, which was particularly evident in the areas of partial natural cavities[4]. While vertebrate scavengers are excluded, the loss of biomass is driven primarily by immature Diptera and microbes[5]. But in the pre-colonization interval(pre-CI), which spans the time period between the time of death and sarcosaphagous insects arriving[6], or in colder months when insects are not active, the metabolic activities of microbes are major components of the decomposition processes.
In the literature reviews, legal medical experts have tended to employ heart and vessel blood, spleen and cerebrospinal fluid for postmortem bacteriological cultures so that they can be served as the diagnostic procedures and indicators of nosocomial infections[7-8]. It has been proven that the evaluation of the relation between PMI and bacteriological yield in sudden and unexpected deaths in infancy takes the lead of current research[9-10]. Research studies have been reported on PMI estimation[11-15]; however,these studies failed to deal with the uncertainty in bacterial succession in natural cavity. There is a need for tentative studies of decomposition of partial natural cavities in terrestrial environments for bacterial succession in forensic investigations.
Microorganisms play a significant role in the natural decomposition of a corpse[12]. The objective of the current investigation was to seek the microbial succession involved in the decomposition of partial natural cavities on rats so that the succession regulations could be outlined on the grounds.
Materials and methods
Study site
The experiments were conducted in Changsha, Hunan province in Central South China(28.12°N, 112.58°E), in the climate of mild and humid May days with the average temperature above 21℃and humidity around 88%. The location for the experiments was chosen indoors with the temperature, humidity and aeration identical to the open air, but without direct solar radiation.
Experimental procedures
Adult female Sprague Dawley rat carcasses, which have been accepted as the models for the human body’s decomposition studies as their similar decay process and mechanism to humans and the sample preparation in circumocular, intraoral and intravaginal places are relatively manageable[3, 16], were used as the same model. Three Sprague Dawley rats, weighting 180-200 g, were killed by spinal cord transection for avoiding external wounds. Each carcass was placed in a carton box of 50cm×30 cm× 25cm, paved with thick, sterile sawdust in order to absorb the subsequent putrefactive liquid and reduce surrounding contamination, and bound up into a grenadine bag to guarantee normal air circulation and isolate exogenous insect interference.
The bacterial samples were taken at 2-hour interval for the first 24 hours as the early PMI, and then at 1-day interval for the following 10 days as the late PMI. When the carton box was opened along with the grenadine bag, 3 areas of carcass were swabbed with sterile surgical swabs. Three habitats of different normal flora on carcass were sampled: circumocular skin(oculi rimae), mouth (buccal mucosa, gingiva and lingual surface)and vagina(mucosa). The sampling lasted for 10 days. The cotton swabs were placed in 1.5 mL microcentrifuge tubes with 1 mL ultrapure water and stored at 4℃until further processing. The samples taken with the aseptic cotton swabs were sent to the laboratory to be cultured immediately in blood agar, chocolate agar, MacCONKEY agar and Salmonella Shigella agar(SS agar)as aerobic and anaerobic enrichment culture for 24-48 hours. The samples collected from the different sites of the rat remains were cultured alone. From the enrichment culture, the isolated culture was operated with attached Man Rogosa Sharpe agar and the previous conditions.
Ambient air temperature and humidity were monitored at the sampling time point during the whole 10 experimental days.
Bacterial colonization analysis
The bacterial colonies were first screened on the basis of phenotypic characteristics including colony size, shape, color, margin, and opacity, the number recorded and used to calculate relative abundance of bacteria. Each kind of independent colony was identified with automatic bacteriology identification apparatus(VITEK®2 Compact of BioMérieux Corporate)[17]and biochemical method according to Gram staining results.
Results
Temperature and humidity analysis
The time point temperature of the first 24 hours ranged from 19.3℃to 25.2℃, 18.0℃to 25.0℃during the following 10 days, while the humidity ran from 72% to 95% and 75% to 96%, respectively. The whole experiment was performed in a relatively mild and humid environment.
Bacterial succession
A total of 4 orders, 6 families and 21 species of bacteria were identified(Table 1). Overall, a significant change was observed in both the early and late PMI. And also the succession changes of the sample sites demonstrated their characteristics, respectively(Fig. 1). For the sites surrounding the eyes, Staphylococcus, Neisseria lactamica, Flavobacterium groupⅡb and Yersinia kristensenii developed in the early PMI. Staphylococcus, of major quantity and variety, predominated on the circumocular skin for nearly 5 days’exposure. Chryseobacterium meningosepticum, Lactobacillus casei and Pseudomonas stutzeri, which always frequent the carcass in the name of recurring bacterium[12], appeared on the 2-3 d, predominating in the late PMI. Regarding the oral cavity, Staphylococcus and Neisseria dominated in the early PMI. Other genera were almost not detected during the first 24 hours. Proteus mirabilis, Flavobacterium groupⅡb, Lactobacillus casei and Pseudomonas stutzeri were observed to predominate in the late PMI. The dynamics of the bacteria in vagina deserved particular mention; there were a variety of species in the early PMI. Staphylococcus aureus and Flavobacterium groupⅡb exhibited a greater proportion just for 4 and 18 hours, respectively. Lactobacillus casei developed on the 3rd day, predominating in the late PMI. However, Pseudomonas and Citrobacter freundii nearly presented themselves in vagina during the whole course.
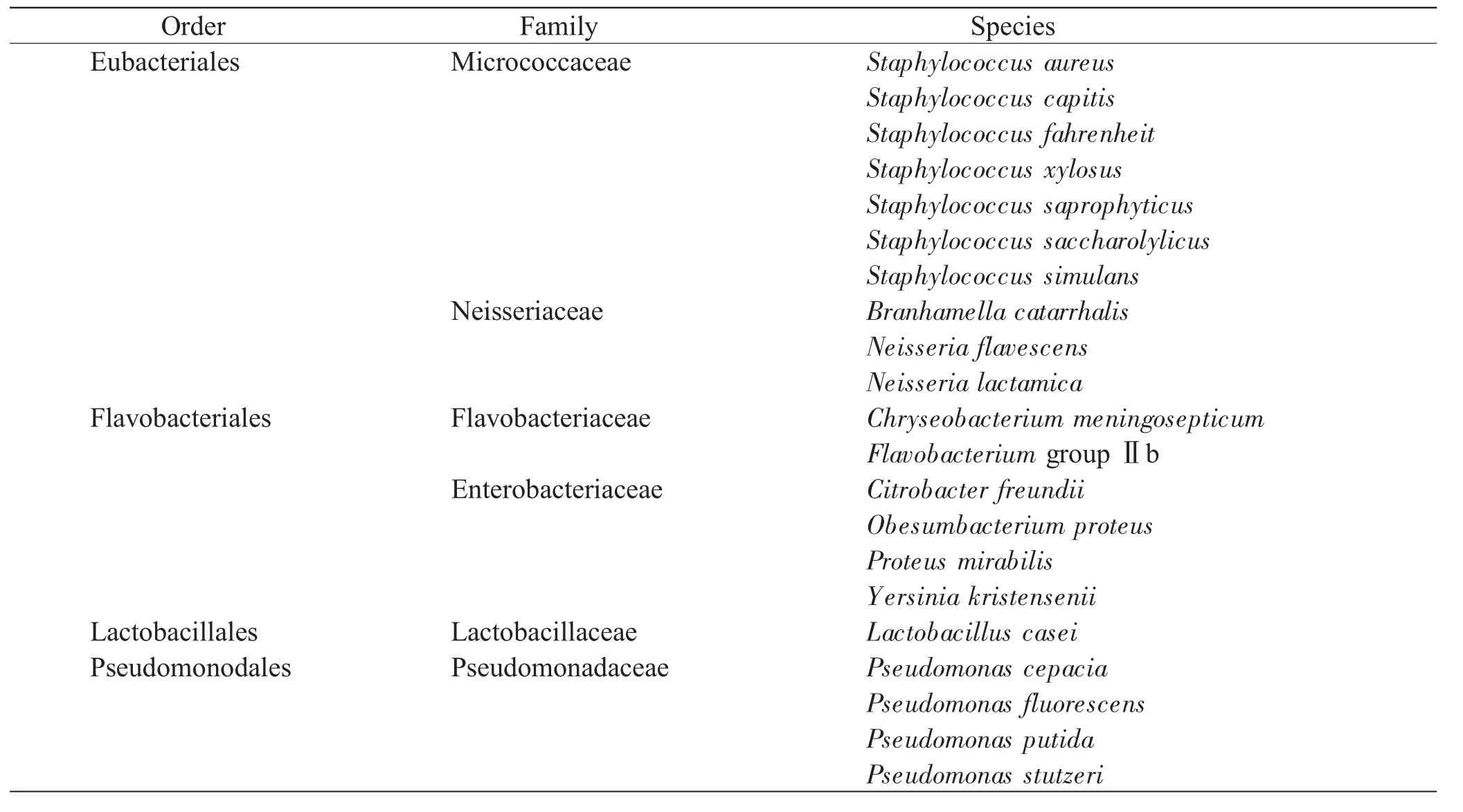
Table 1 Classification of the identified bacteria
Regardless of circumocular skin, mouth and vagina, abundant signature taxa such as Staphylococcus and Neisseria were common to these situations, especially Staphylococcus aureus and Neisseria lactamica in 6 hours following the death of the early PMI, which predominated with more stringent environmental oxygen requirements regardless of their nonspecific degrees. Meanwhile, numerous colonizing bacterial taxa were progressively observed such as Flavobacterium groupⅡb, Yersinia kristensenii and Citrobacter freundii. As time went by, Pseudomonas was found in the mouth and vagina, but absent from the circumocular skin until the beginning of the late PMI. Thus the uncertainties presented by the variety of multiple genera seemed to be unpredictable; however, there was still a clear tendency that one kind of gram-positive bacillus, identified as Lactobacillus casei, appeared regularly on the 3-4 d, with the graduate speed of accelerating extinction until a certain stable level during the later period. Furthermore, it was obscure to distinguish the colonization patterns with forensic implication of the multifarious bacterium in the trend of the whole study.
It turned out that Staphylococcus and Neisseria were inclined to develop in the early PMI, while Lactobacillus was inherently referential for the late PMI. Succession uniqueness of the vagina showed a higher stability than that of the other habitats, and the bacterial succession in the mouth was more valuable and appropriate for PMI estimation.
Discussion
The concept of normal microbial flora would be declared by a description of micropopulation, as physiological microbiota, which parasitizes in the body surface and natural cavities interacting with the outside environments, such as the oral cavity, respiratory, alimentary and urogenital tract, showed no significant effect on the livings[18]. As the insects did not contact with the corpses yet, the bacteria microbial existed on the body before death played a critical role in PMI estimation during the pre-CI stage. With the variations of micro-environment, the kind and amount of normal flora presented a gradual fluctuation in due order at an early phase, while the massive growth of putrefactive bacteria decomposed the carcass tissue protein, releasing a variety of metabolites as the extension of time. It is a practical scheme that the pre-CI deduction based on the species and quantitative changes of microbial populations is complementary to the PMI inference. The application depends on whether it is the early or late PMI. Practically, bacterial succession with forensic implication could be summarized as follows: Bacteria gradually disappear with the prolongation of time; bacteria develop from scratch and persist continuously; and bacteria grow but die away by degrees. The practical effect of germs’variability indicates that the deduction of death time is precise, which can play a unique role in case work detection.
In general, normal microbial florae distribute around some microbiotic areas. The differences of species and quantity can be reflected in various aspects, such as personal habits, occupations and environmental factors. It is necessary to identify the normal flora in these aspects, but often difficult to do so because some bacteria need special growth requirements. It was reported that organisms coexisted in complex ecologies with various symbiotic relationships instead of living in isolation in nature[19]. In the current study, we aimed to culture both aerobic and anaerobic bacteria extracted from distinct sites to create a large number of bacteria from the succession to facilitate PMI estimation; therefore, we chose circumocular skin, oral cavity and vagina.
The diversity of microbes within a given body habitat can be defined as the number and abundance distribution of distinct types of organisms, and each body habitat in almost every subject was characterized by one or a few signature taxa making up the plurality of the community[3]. The variability of different body habitats or one body habitat but long after death is high. For circumocular skin, such common microorganisms as coagulase negative Staphylococci, Enterobacteria, Pseudomonad, Diphtheroid bacillus and Mycobacterium are apt to settle down because of direct contact with air. These skin bacteria could persist on touched surfaces for prolonged periods because many are highly resistant to environmental stresses[20-21]. Given the specific temperature, humidity, nutrition and anatomic structure, oral cavity owns the most complicated microecological system of a living body. In the oral cavity, a good relationship of symbiosis, rivalry and antagonism formed by the variety of normal microflora helps preserve oral health. During the executing period and subsequent exposure to the postexecution environment, the vagina is colonized by a wide array of microbes, many of which are commensal or symbiotic. It was reported that oral communities were especially diverse in terms of community membership, while vaginal sites harbored particularly complex communities which are also intricate habitats for a diverse population of microbiota[3]. In the current study, therefore, bacterial succession in the mouth was more valuable and appropriate for PMI estimation.
Furthermore, Staphylococcus and Neisseria inclined to the early PMI, while Lactobacillus was inherently referential for the late PMI. Staphylococcusspecies have been reported to be the first microorganism to migrate from the small intestine[11]and to colonize the oral cavity[22-23]. Lactobacillus was known to be common inhabitants of the human vagina[24]. Moreover, Lactobacillus was also detected in nonvaginal samples[ 25 ]. Moreover, Pseudomonas have been labeled as the secondary invaders of sample sites, most commonly referring to the colonization of corruption[12]. And Pseudomonas have been reported to be important in the breakdown of proteins of a cadaver[26].
It has been turned to the late PMI 24 hours after death. In this stage, the microorganisms within and among body habitats exhibited relationships suggestive of driving physical factors, such as pH, oxygen, moisture as amino acids, vitamins, trace elements and electrolyte and other cofactors[27]. A shift from aerobic(Staphylococcus and Enterobacteriacae)to anaerobic(Lactobacillus, Clostridia and Bacteroides)organisms was observed and considered as the result of loss of redox potential of the tissue due to lack of oxygenated blood[26]. In fact, the majority of bacteria(96%-99%)participating in the decomposition of a dead body are anaerobic, in part coming from the surrounding soil, but mostly originating from the organism’s own intestine[28]. Moreover, putrefactive bacterium(Clostridium Perfringens, Bacteroides, Veillonella and Escherichia coli)multiplied enormously, which improved the value of pH and oxidation reduction potential (ORP)significantly, indulging the body into a neutral even alkaline environments. During this time, as the experiment sites chosen in the current study, the natural body cavities released a large number of odorant molecules. Ammonia, amine, hydrogen sulfide, benzazole and phenolic compounds had a strong appeal to sarcosaphagous insects for egg production[29]. Hence, the bacterial succession of carcasses is most likely to react on the insect succession to a certain degree, which can make the succession process more complicated.
However, the current study still had some limits. Decomposition is a mosaic system with an intimate association between biotic factors(the individuality of the cadaver, intrinsic and extrinsic bacteria and other microbes, and insects)and abiotic factors(weather, climate and humidity)and could be regarded as the function of a specific ecosystem[13]. It is critical for forensic scientists to understand the functions and influences of these abiotic factors. The current study can provide valuable bacterial succession data from adult female Sprague Dawley rat carcasses for PMI estimation. The bacterial succession in adult male Sprague Dawley rat carcasses and human dead bodies could present some differences, which needs further investigations in the future.
Conclusion
The current study presents a significant bacterial succession progress on rats without insect interference, offering a certain amount of semi quantitative data of genus species for subsequent molecular research of forensic entomology. Derived from the experiments on the animals, the data can be further integrated into our current studies which involve reinforcement of different climates, surrounding environments, insect inference, animal species and experimental techniques. A breakthrough is made in the habitual thinking about the limitations of postmortem bacteriology practicability, which paves a foundation for further studies in this discipline.
Acknowledgement
This study was supported by grants from the National Natural Science Foundation of China(81373249 and 81571855), the Science and Technology Committee of Shanghai Municipality(KF1203), the Fundamental Research Funds for the Central Universities of Central South University(2015zzts276)and the Graduate Student Education Innovation Projects of Central South University(160020009).
Reference:
[1] Li L. Forensic Medicine[M]. Beijing: People’s Medical Publishing House, 2014.
[2] Li ZQ, Zuo WD, Zhang F, et al. Latest progress in postmortem interval estimation[J]. Fa Yi Xue Za Zhi, 2012, 28(4): 287-292.
[3] Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome[J]. Nature, 2012, 486(7402): 207-214.
[4] Amendt J. Current Concepts in Forensic Entomology[M]. Berlin: Springer Netherlands, 2010.
[5] Putman RJ. Patterns of carbon dioxide evolution from decaying carrion: decomposition of small mammal carrion in temperate systems 1[J]. Oikos, 1978, 31(1): 47-57.
[6] Tomberlin JK, Mohr R, Benbow ME, et al. A roadmap for bridging basic and applied research in forensic entomology[J]. Annu Rev Entomol, 2011, 56: 401-421.
[7] Morris JA, Harrison LM, Partridge SM. Practical and theoretical aspects of postmortem bacteriology[J]. Curr Diagn Pathol, 2007, 13(1): 65-74.
[8] Tsokos M, Puschel K. Postmortem bacteriology in forensic pathology: diagnostic value and interpretation[J]. Legal Med(Tokyo), 2001, 3(1): 15-22.
[9] Weber MA, Hartley JC, Brooke I, et al. Post-mortem interval and bacteriological culture yield in sudden unexpected death in infancy(SUDI)[J]. Forensic Sci Int, 2010, 198(1-3): 121-125.
[10] Weber MA, Sebire NJ. Postmortem investigation of sudden unexpected death in infancy: current issues and autopsy protocol[J]. Diagn Histopathol, 2009, 15(11): 510-523.
[11] Melvin JR Jr, Cronholm LS, Simson LR Jr, et al. Bacterial transmigration as an indicator of time of death[J]. J Forensic Sci, 1984, 29(2): 412-417.
[12] Dickson GC, Poulter RT, Maas EW, et al. Marine bacterial succession as a potential indicator of postmortem submersion interval[J]. Forensic Sci Int, 2011, 209(1-3): 1-10.
[13] Hyde ER, Haarmann DP, Lynne AM, et al. The living dead: bacterial community structure of a cadaver at the onset and end of bloat stage of decomposition[J]. PLoS One, 2013, 8(10): e77733.
[14] Metcalf JL, Wegener Parfrey L, Gonzalez A, et al. A microbial clock provides an accurate estimate of the postmortem interval in a mouse model system[J]. Elife, 2013, 2: e01104.
[15] Pechal JL, Crippen TL, Benbow ME, et al. The potential use of bacterial community succession in forensics as described by high throughput metagenomic sequencing[J]. Int J Legal Med, 2014, 128(1): 193-205.
[16] Querido D. A preliminary investigation into postmortem changes in skinfold impedance during the early postmortem period in rats[J]. Forensic Sci Int, 1998, 96(2- 3): 107-114.
[17] Romero-Gómez MP, Gómez-Gil R, Paño-Pardo JR, et al. Identification and susceptibility testing of microorganism by direct inoculation from positive blood culture bottles by combining MALDI-TOF and Vitek-2 Compact is rapid and effective[J]. J Infect, 2012, 65(6): 513-520.
[18] Norin E. How normal is a“normal”flora in animal or man?[J]. Anaerobe, 2011, 17(6): 431-432.
[19] Saffo MB. Coming to terms with a field: words and concepts in symbiosis[J]. Symbiosis, 1993, 14(1-3): 17-31.
[20] Smith SM, Eng RH, Padberg FT Jr. Survival of nosocomial pathogenic bacteria at ambient temperature[J]. J Med, 1996, 27(5-6): 293-302.
[21] Brooke JS, Annand JW, Hammer A, et al. Investigation of bacterial pathogens on 70 frequently used environmental surfaces in a large urban U.S. university[J]. J Environ Health, 2009, 71(6): 17-22.
[22] Nakanishi H, Kido A, Ohmori T, et al. A novel method for the identification of saliva by detecting oral streptococci using PCR[J]. Forensic Sci Int, 2009, 183(1-3): 20-23.
[23] Power DA, Cordiner SJ, Kieser JA, et al. PCR-based detection of salivary bacteria as a marker of expirated blood[J]. Sci Justice, 2010, 50(2): 59-63.
[24] Forney LJ, Gajer P, Williams CJ, et al. Comparison of self-collected and physiciancollected vaginal swabs for microbiome analysis[J]. J Clin Microbiol, 2010, 48(5): 1741-1748.
[25] Benschop CC, Quaak FC, Boon ME, et al. Vaginal microbial flora analysis by next generation sequencing and microarrays; can microbes indicate vaginal origin in a forensic context?[J]. Int J Legal Med, 2012, 126(2): 303-310.
[26] Janaway RC. Microbiology and Aging[M]. Totowa: Humana Press, 2009.
[27] Faust K, Sathirapongsasuti JF, Izard J, et al. Microbial co-occurrence relationships in the human microbiome[J]. PLoS Comput Biol, 2012, 8(7): e1002606.
[28] Jawetz E, Melnick JL, Adelberg EA. Review of Medical Microbiology[M]. 17th ed. Los Altos: Appleton and Lange, 1987.
[29] Leitch O, Anderson A, Kirkbride KP, et al. Biological organisms as volatile compound detectors: A review[J]. Forensic Sci Int, 2013, 232(1-3): 92-103.
(Received date: 2014-09-15)
(Editor: HUANG Ping)
书讯
《法医病理数字化新技术理论与实践》由司法部司法鉴定科学技术研究所刘宁国与陈忆九主编,于2015年1月出版。该书以当前飞速发展的数字化技术为基础,结合作者多年从事法医病理学鉴定的经验和近年来开展的法医虚拟解剖、计算机生物力学仿真、数字化快速三维现场图、法医骨学专家系统和全息数字病理切片等领域的研究和鉴定工作,并吸纳了国内外最新科研成果和工作实践的报道编写而成。本书分为六章。每章既有数字化法医病理技术手段的最新概况,也有该技术手段所依据的基本理论和方法,同时还对相应技术在法医学各类鉴定的应用进行分别归纳、总结,涵盖了法医病理数字化技术的相关领域基础理论、应用实践和最新研究成果,可供从事法医学鉴定和科学研究的公安、检察、法院、高校和社会鉴定机构等工作人员和学者阅读参考,也可为司法审判人员、大中专院校相关专业学生以及法律工作者提供帮助。
该书精装、全彩版,定价为198元,包装邮寄费10元/本,共计208元/本。有意购书者请与本编辑部联系。
联系地址:上海市普陀区(市西)光复西路1347号《法医学杂志》编辑部邮编:200063
联系人(收款人):武胜男
联系电话:021-52361148-2319(发行),021-52360413(传真)
电子信箱:fyxzz@126.com
Bacterial Succession on Rat Carcasses and Applications for PMI Estimation
ZHANG Lin1, GUO Juan-juan1, TələT·SIYIT2, PENG Yu-long1, XIE Dan1, GUO Ya-dong1, YAN Jie1, ZHA Lagabaiyila1, CAI Ji-feng1
(1. Department of Forensic Science, School of Basic Medical Sciences, Central South University, Changsha 410013, China; 2. Department of Investigation, Xinjiang Police College, Urumchi 830011, China)
Abstract:Objective To investigate the bacterial succession on rat carcasses and to evaluate the use of bacterial succession for postmortem interval(PMI)estimation. Methods Adult female SD rat remains were placed in carton boxes. The bacterial colonization of circumocular skin, mouth and vagina was collected to be identified using culture-dependent biochemical methods. The changes in community composition were regularly documented. Results The bacterial succession in three habitats showed that Staphylococcus and Neisseria were predominated in early PMI, especially Staphylococcus aureus and Neisseria lactamica in 6 hours after death. Lactobacillus casei developed on the 3-4 days regularly, and kept stable at a certain level in late PMI. Conclusion The involvement of normal and putrefactive bacteria in three body habitats of rat remains can be used for PMI estimation.
Key words:forensic pathology; bacteria; postmortem interval; rats
Corresponding author:CAI Ji-feng, professor, major in forensic pathology and entomology; E-mail: cjf_jifeng@163.com
文章编号:1004-5619(2016)01-0001-06
中图分类号:DF795.1
文献标志码:A
doi:10.3969/j.issn.1004-5619.2016.01.001
