Identification and Culture Condition Study of Marine Actinomycete HT-8 with Nematicidal Activity against Pine Wood Nematode
2016-03-08CHENCongcongWANGChaoGUOQunqunLILiGUODaosen
CHEN Cong-cong, WANG Chao, GUO Qun-qun, LI Li, GUO Dao-sen
(College of Life Sciences, Qingdao University, Qingdao 266071)
Identification and Culture Condition Study of Marine Actinomycete HT-8 with Nematicidal Activity against Pine Wood Nematode
CHEN Cong-cong, WANG Chao, GUO Qun-qun, LI Li, GUO Dao-sen*
(CollegeofLifeSciences,QingdaoUniversity,Qingdao266071)
Pine wilt disease (PWD) is a devastating disease to pine trees, which was caused by the pine wood nematode (PWN),Bursaphelenchusxylophilus, and causes major losses in coniferous forests in many countries and regions in the world. In order to screen actinomycete strains with high nematicidal activity against PWN, marine actinomycetes were isolated from the marine environment and their nematicidal activity were tested.The marine actinomycetes were isolated from the samples collected from the subtidal zones near Qingdao coast using the dilution and streak plate method and their culture supernatant were assayedinvitrofor nematicidal activity against PWN using immersion test. The strain with high nematicidal activity was identified on the basis of morphology, cultural characteristics and 16S rDNA sequence analysis, and its optimal culture conditions for the production of nematicidal substances were investigated through the single-factor experiments.A total of 28 marine actinomycete strains were isolated from the samples. One of these strains, designated as strain HT-8 and isolated from sea sand, exhibited stronger nematicidal activity with a 88.30% corrected mortality of PWN treated with the culture supernatant for 30 h. The strain HT-8 was identified asStreptomycestermitumon the basis of morphology, cultural characteristics, 16S rDNA sequence and phylogenetic analysis. The results of the single-factor experiment demonstrated that the optimal cultivation conditions of HT-8 for the production of nematicidal substances were inoculum age was 48 h, inoculum concentration was 6%, concentration of seawater was 100%, initial pH was 7.5 and incubating at 25 ℃ for 7 days.This study provides a theoretical basis for the development of marine microorganism resources and the utilization of natural nematicidal substances.
Marine actinomycete; Nematicidal activity;Bursaphelenchusxylophilus; Identification; Culture conditions
The pine wilt disease (PWD) is a fatal disease of pine trees, which was caused by pine wood nematode (PWN),Bursaphelenchusxylophilus, and transmitted by the pine sawyer beetle. This disease has caused major economic loss and destruction of ecological environment in the word, particularly in Japan, China and South Korea[1]and western Europe, e.g. Portugal[2]. In China, pine wilt disease was first reported in Nanjing in 1982 and had been spread to 14 provinces and cities until 2011, which resulted in over 0.3 million ha of damaged forests and more than 0.5 billion dead pine trees during the last decade[3]. There are many methods to control the pine wilt disease, such as aerial application of synthetic insecticides to control the insect vector, fumigation of the dead pine trees or trunk injection with nematicides[4]. The use of chemicals in these methods is the most effective, but they also contribute to pollution problems which indirectly harm human health. In order to reducing pesticide use, the researchers have begun to look for alternative and equally effective natural products which more safer for people and the environment[5]. Approximately 71% of the earth’s surface area is covered by the ocean, and its biological diversity is far more than the land. Because of the Marine environment with high pressure, high salt and low temperature, the marine microorganism has the special metabolic pathway which is different from terrestrial microorganisms. Several marine microorganisms with nematicidal activity such asBacilliusmegaterium[6],Pseudoalteromonasnigrifaciens[7],Penicillium[8],Verticillium[9]have been reported. In this paper, we investigated the identification of one marine actinomycete HT-8 with high nematicidal activity against PWN and the influence of various cultural conditions on its nematicidal activity.
1 Materials and Methods
1.1 Preparation of nematodes
PWN were isolated by the Baermann funnel method[10]from chips of the infected Japanese black pine tree,Pinusthunbergii. The fungus,Botrytiscinerea, was cultured on PDA in Erlenmeyer flask at 28 ℃. The flasks with fully grown fungus were inoculated with PWN and incubated until the fungi were completely eated. The PWN suspension used in the experiment was prepared by washing the plates with sterile distilled water.
1.2 Isolation of marine actinomycete HT-8
The marine actinomycetes were isolated from the samples, including seawater, sea sand and marine sediments, collected in October 2014 at low tide from the subtidal beds at Qingdao, P.R. China (36.10°N,120.40°E). The seawater used in this study was filtered with three layers of medical gauze to get filtered seawater, and sterilised seawater was prepared by autoclaving the filtered seawater at 121 ℃ for 20 min. The samples were prepared as ten-fold serial dilutions using sterilised seawater. A volume of 0.1 mL of each sample dilution was spread evenly over Gause’s synthetic agar medium (containing 20 g soluble starch, 0.5 g K2HPO4, 1.0 g KNO3, 0.5 g MgSO4·7H2O, 0.5 g NaCl,0.01 g FeSO4·7H2O and 15.0 g agar dissolved in 1 000 mL filtered seawater, pH 7.2-7.4, autoclaved at 121 ℃ for 20 min) and incubated at 28 ℃ for 7 days. The candidate isolates were purified using the streak plate method and kept in Gause’s synthetic agar slants at 4 ℃ for further experiments.
1.3 Preparation of culture supernatant
The isolates were re-grown on slant medium at 28 ℃ for 7 day. The spores were washed down from slant medium with 1 mL sterile water and transferred into the seed culture medium (containing 6.0 g peptone, 4.0 g yeast extract and 4.0 g glucose dissolved in 1 000 mL filtered seawater, pH 7.5, autoclaved at 121 ℃ for 20 min). The inoculated seed culture medium (30 mL) in a 100 mL Erlenmeyer flask was incubated on a shaker at 28 ℃, 180 r/min for 2 days. Following this initial growth stage, the seed culture (6%, v/v) was transferred to a fermentation culture medium (containing 15.0 g soybean powder, 20.0 g soluble starch, 4.0 g yeast extract, 2.0 g peptone and 4.0 g CaCO3dissolved in 1 000 mL filtered seawater, autoclaved at 121 ℃ for 20 min) and grown at 28 ℃, with rotary shaking at 185 r/min for 5 days. After centrifugation at 10 000 r/min for 10 min, the culture supernatant was collected for the measurement of nematicidal activity.
1.4 Bioassay of nematicidal activity
In the bioassay, 450 μL culture supernatant was added to a 1.5 mL Eppendorf tube containing 50 μL nematode suspension (approximately 200 nematodes). Each treatment was replicated three times. Sterilised distilled water and fermentation medium were used as controls. After the tube had been incubated at 25 ℃ in the dark for 2 to 48 h, the numbers of live and dead nematodes in each treatment were counted under a stereomicroscope and mortality of nematode was calculated as a percent age of total nematodes. Nematodes were defined as dead if their bodies were straight and did not move even when transferred to fresh distilled water[11]. The observed mortality in the treatment group was corrected in relation to the observed mortality in the control group using the Schneider-Orelli formula[11]:
where CM-Corrected mortality; MTG-Mortality in treatment group; MCG-Mortality in control group.
1.5 Identification of the marine actinomycete HT-8
HT-8 was identified on the basis of morphology, cultural characteristics and 16S rDNA sequencing. Cultural characteristic parameters included colony color, size, shape, margin, elevation, opacity and surface characteristics. The genomic DNA of strain HT-8 was extracted by lysozyme method and the 16S rDNA was amplified by PCR. The sequences of primers are 5′-GCGCAAGCTTAGAGTTTGATCMTGGCTCAG-3′ and 5′-GCGCTCTAGATACGGTYACCTTGTTACGACTT-3′. The PCR amplification program: 94 ℃ for 2 min, followed by 35 cycles of 94 ℃ for 30 s, 56 ℃ for 30 s, and 72 ℃ for 2 min. The PCR product was cloned into pClone EZ cloning vector and sequenced by Sangon Biotech. The neighbor-joining method[12]was used to construct the phylogenetic tree.
1.6 Effects of cultivation conditions on nematicidal activities of marine actinomycete HT-8
The single-factor experiment was conducted for evaluating the influence of different cultivating factors on the nematicidal activity of marine actinomycete HT-8. The factors included seawater concentration (0, 200, 400, 600, 800, 1 000 mL/L), the initial pH (6.0, 6.5, 7.0, 7.5, 8.0, 8.5), the cultivation temperature (15, 20, 25, 30, 35,40 ℃), incubation time (1, 3, 5, 7, 9, 11 days), inoculum age (12, 24, 36, 48, 60, 72 h) and inoculum concentration (2%, 4%, 6%, 8%, 10%, 12%). All experiments were conducted in triplicate.
2 Results
2.1 Isolation and nematicidal activity of actinomycete strains
A total of 28 marine actinomycete strains were isolated from the samples collected from the subtidal zones near Qingdao coast and screened for their nematicidal activities against PWN. The isolate was regarded as having a clear nematicidal activity if the corrected mortality of PWN was more than 50% with-in 30 h exposure to the culture supernatant. Five strains of the isolates were classed to have nematicidal activities (Table 1). Among the active isolates, one isolate, named HT-8 which isolated from sea sand, demonstrated the highest nematicidal activity and the corrected mortality of the tested nematodes was 88.30%. Morphological changes of the nematodes treated by HT-8 culture supernatant as shown in Fig.1. Therefore, the HT-8 was selected for further investigation.

Table 1 List of the 5 isolates with a corrected mortality of PWN>50% and their nematicidal activities against Bursaphelenchus xylophilus
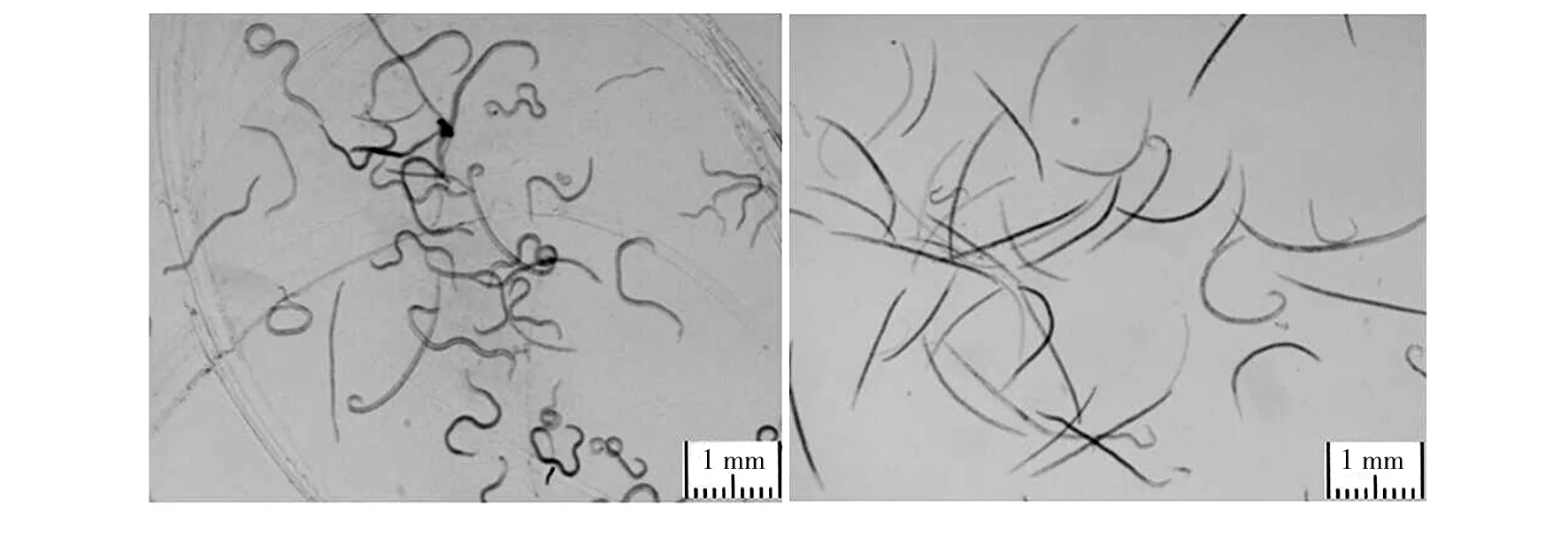
Fig.1 Morphological changes of Bursaphelenchus xylophilus treated by HT-8 culture supernatanta: Morphology of B. xylophilus in the control medium for a 30 h incubation showing the nematodes alive;b: B. xylophilus for a 30 h incubation in the culture supernatant of strain HT-8 showing the nematodes dead
2.2 Identification of the selected isolate HT-8
Pure culture of HT-8 was obtained by successive transfers of individual colony grown on the Gause’s synthetic agar medium. The colony of HT-8 was white, the surface was powdery and the substrate was pale yellow (Fig.2). The substrate mycelia were slim and did not have transverse septa. The aerial mycelium were short, thick and can produce a large number of oval spores when they matured (Fig.3).
The almost complete 16S rDNA gene sequences (1 525 bp) of HT-8 were compared with sequences of model strains in National Center for Biotechnology Information (NCBI). The sequence blast results indicated that isolate HT-8 had close homology toStreptomycestermitum. The phylogenetic tree constructed by the neighbour joining method is presented in Fig.4. Therefore, based on the morphology, cultural characteristics and 16S rDNA sequencing, the isolate HT-8 was identified asStreptomycestermitumand designated as S. termitum HT-8.
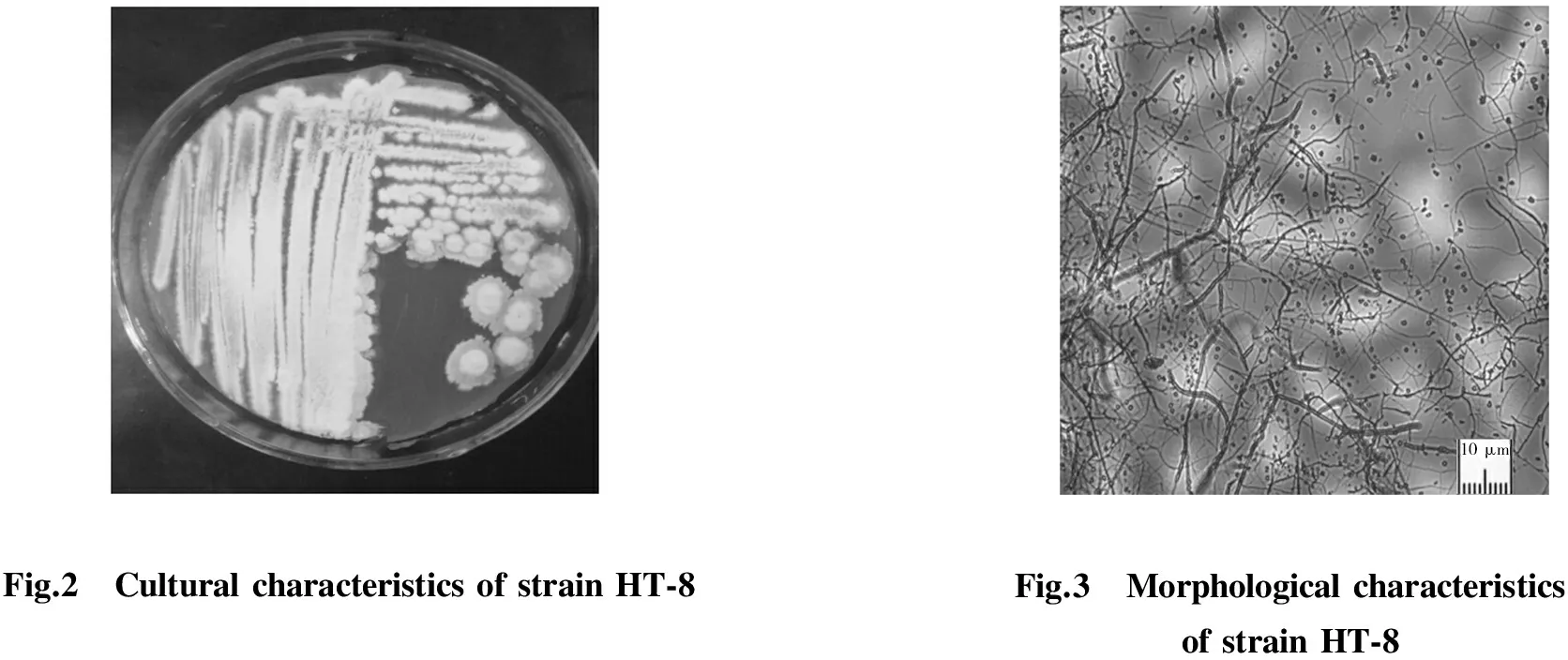
Fig.2 CulturalcharacteristicsofstrainHT⁃8Fig.3 MorphologicalcharacteristicsofstrainHT⁃8
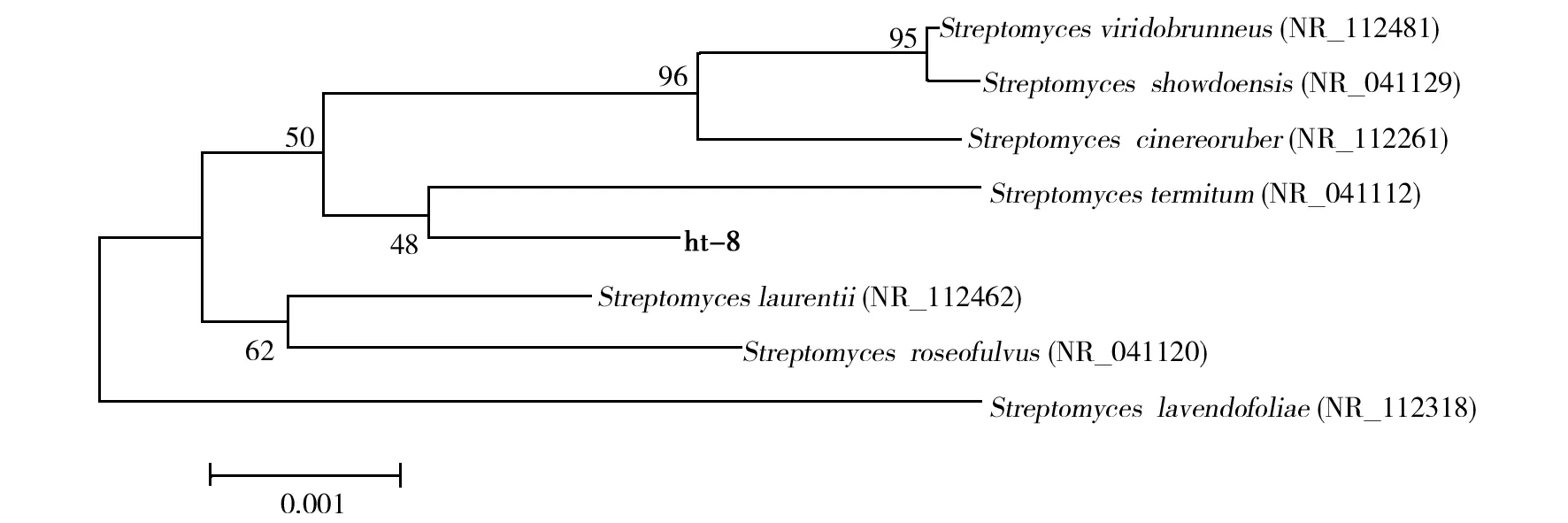
Fig.4 Neighbour-joining phylogenetic tree based on 16S rDNA sequences of Streptomyces termitum HT-8
2.3 Effects of inoculum age, inoculum concentration, concentration of seawater, initial pH, temperature and culture time on nematicidal activities
The optimal culturing conditions for the production of the active substances of strain HT-8 were investigated. The results in Fig.5 showed that the corrected mortalities of nematode were highest when the inoculum age was 48 h and inoculum concentration was 6%. It was found that the nematicidal activity of the strain HT-8 was obviously increased with the increase of seawater concentration in culture media and reached the maximum when the fermentation culture medium was prepared with 100% seawater, an initial pH of 7.5, temperature of 25 ℃ and cultivation time of 7 days were optimal culturing conditions for production of nematicidal substances (Fig.6) .
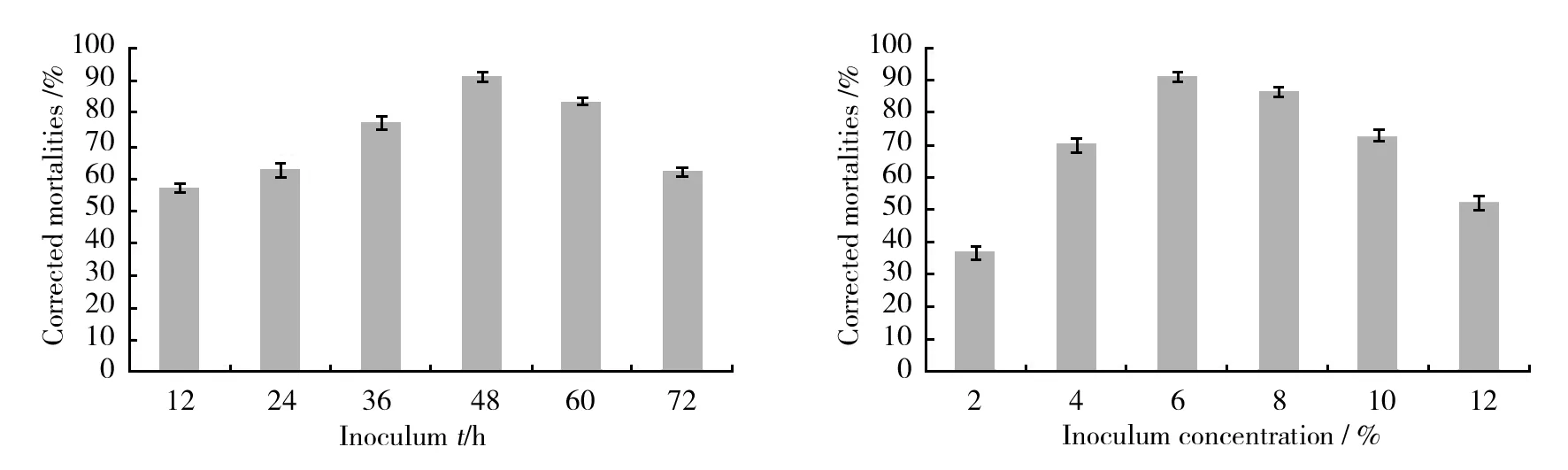
Fig.5 Effects of inoculum age and inoculum concentration on the nematicidal activities (corrected mortality,%) of Streptomyces termitum HT-8 culture supernatant against Bursaphelenchus xylophilus
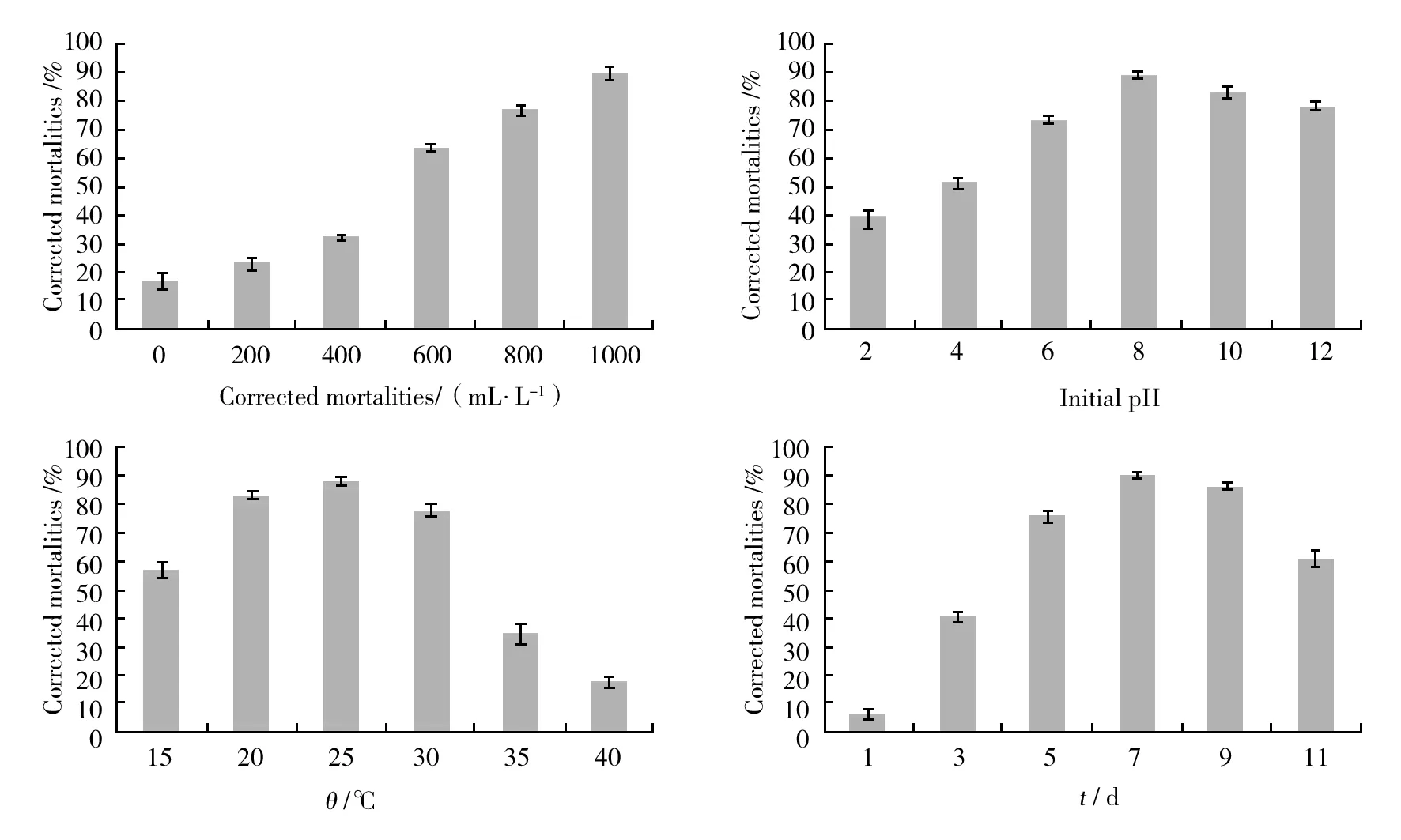
Fig.6 Effects of concentrations of seawater, initial pH, temperature and culture time on the nematicidal activities (corrected mortality,%) of Streptomyces termitum HT-8 culture supernatant against Bursaphelenchus xylophilus
3 Discussion
The pine wood nematode (PWN) is among the most important pests included in the quarantine lists of many countries around the world[13]. Chemical methods were used to control the pine wilt disease traditionally, but the wide application of chemical pesticides can cause irreversible damage to the environment. The chemical pesticide residue reduces the quality of environment, even results in the death of the natural enemies of this disease. In addition, long-term use of pesticides causes PWN with resistance. Therefore, the researchers gradually focused on natural active products from nature with nematicidal activities. Zheng et al.[14]obtained a strain ofBrevundimonasdiminutawith high nematicidal activity from 206 nematicidal endophytic bacterial strains and extracted active substance(R)-(-)-2-ethylhexan-1-ol from the fermentation broth. Zhu et al.[15]isolated a strain ofBacillusamyloliquefaciensfrom the leaves ofPinusmassonianawith high nematicidal activity onBursaphelenchusxylophilusand they found that the mortality of nematode with 24 h treatment of culture filtrate attained 100%. At present, the microorganisms with nematicidal activities which were isolated from terrestrial environment are more than from marine environment. Because of special environment of ocean, rare or novel species that have not been previously investigated for the production of natural active products might be to exist in the ocean. Hence, exploring the rare resources in marine environment is an exciting field of research[16]. In this study, a total of 28 marine actinomycete strains were isolated from the samples collected from the subtidal zones near Qingdao coast. Among them the culture supernatant of strain HT-8 showed the strongest nematicidal activity with a 88.30% corrected mortality to nematode after treated for 30 h. Results of morphology, cultural characteristics, 16S rDNA and phylogenetic tree suggested that this strain have close homology toStreptomycestermitum, so the strain HT-8 was identified asStreptomycestermitum. After the analysis of fermentation conditions, the nematicidal activity of culture supernatant was the highest when the inoculum age was 48 h, inoculum concentration was 6%, concentration of seawater was 100%, initial pH was 7.5, and incubating at 25 ℃ for 7 days. This is the first report on the nematicidal activity ofStreptomycestermitum.
This study shows that there are natural active products with nematicidal activities in the marine environment. The results of this study can provide an important reference for the development and utilization of natural active products with nematicidal activities from marine microorganisms.
Reference
[1] Zhao BG, Kazuyoshi F, Jack R, et al. Pine Wilt Disease[M].Tokyo: Springer,2008:33-34.
[2] Mota MM, Braasch H, Bravo MA, et al. First report ofBursaphelenchusxylophilusin Portugal and in Europe[J].Nematology,1999, 1:727-734.
[3] Pan Cang-sang. Development of studies on pinewood nematodes diseases[J].Journal of Xiamen University (Natural Science), 2011, 50(2):476-483.
[4] Korea Forest Service. Guideline for the control of forest diseases and insect pests[M].Korea Forest Service. Daejeon, Republic of Korea, 2013:325.
[5] Chitwood DJ. Phytochemical based strategies for nematode control[J].Annual Review of Phytopathology, 2002, 40: 221-249.
[6] Zheng Hai-ying, Yu Jie, Li Rong-gui, et al. Screening and identification of two marine bacterial strains with antinematodal activity againstBursaphelenchusxylophilus[J].Journal of Qingdao University, 2012, 27(1):1-8.
[7] Yu Jie, Li Zi, Zhou Chang-jing, et al. Identification and culture condition study of marine bacterium G-23 with nematicidal activity against pine wood nematode[J].Journal of Qingdao University, 2014, 29(2):94-100.
[8] Li Zi, Zhou Chang-jing, Chen Cong-cong, et al. Identification and culture condition study of marine fungus with nematicidal activity against pine wood nematode[J].Journal of Qingdao University, 2015, 30(2):102-107.
[9] Xu Zhong-qiang, Yu Jie, Liu Chao, et al. Screening and preliminary identification of one marine fungal strain with antinematodal activity againstBursaphelenchusxylophilus[J]. Journal of Qingdao University, 2013, 28(2):70-76.
[10]Gray NF. Ecology of nematophagous fungi: Comparison of the soil sprinkling method with the Baerman funnel technique in the isolation of endoparasites[J].Soil Biology Biochemistry,1984,16:81-83.
[11]Yu J, Du G, Li R, et al. Nematicidal activities of bacterial volatiles and components from two marine bacteria,Pseudoalteromonasmarinastrain H-42 andVibrioatlanticusstrain S-16, against the pine wood nematode,Bursaphelenchusxylophilus[J].Nematology, 2015, 17(9):1011-1025.
[12]Kumars S, Tamura K, Nei M. MEGA3:Integrated software for molecular evolutionary genetics analysis and sequence alignment[J]. Briefings in Bioinformatics,2004, 5(2):150-163.
[13]Ryss A, Kulinich O, Sutherland J. Pine wilt disease: a short review of worldwide research[J]. Forestry Studies in China, 2011, 13(2):133-138.
[14]Zheng L, Li G, Wang X, et al. Nematicidal Endophytic Bacteria Obtained from Plants[J]. Annals of Microbiology, 2008, 58(4):569-572.
[15]Zhu Li-mei, Wu Xiao-qin, Jiang Ping. A study of the nematicidal activity of bacterium XS-JS3 toBursaphelenchusxylophilus[J]. Journal of Nanjing Forestry University (Natural Sciences Edition), 2008, 32(1):79-82.
[16]Gao X, Lu Y, Xing Y, et al. A novel anticancer and antifungus phenazine derivative from a marine actinomycete BM-17[J]. Microbiological Research, 2012, 167:616-622.
欢迎订阅《微生物学杂志》
山东省自然科学基金项目(ZR2010CM009);青岛市科技计划项目(13-1-4-158-jch)
陈聪聪 女,硕士研究生。主要研究方向为微生物资源的开发与利用。E-mail:562288378@qq.com
杀松材线虫海洋放线菌HT-8的鉴定及培养条件的研究
陈聪聪, 王 超, 郭群群, 李 丽, 郭道森*
(青岛大学 生命科学学院,山东 青岛 266071)
松树萎蔫病(Pine wilt disease, PWD)是由松材线虫(Bursaphelenchusxylophilus)引起的一种毁灭性疾病,该病对世界上许多国家和地区的针叶林资源造成严重破坏。为了寻找具有高杀松材线虫活性的海洋放线菌,对分离自海洋环境中的放线菌菌株进行杀线虫活性测定。选用稀释涂布平板法对采自青岛近海海域的样品进行放线菌分离,采用浸渍法测定菌株的杀松材线虫活性,并对所筛选出的高杀线虫活性菌株的形态学、培养特征和16S rDNA序列进行测定,运用单因素试验测定菌株产生杀线虫活性物质的最适培养条件。结果显示,从采集的样品中共分离到28株放线菌,经杀线虫活性筛选得到1株具有高杀线虫活性的放线菌HT-8。该菌株分离自海沙,其培养上清液处理松材线虫30 h时,线虫的校正死亡率高达88.30%。根据形态学、培养特征和16S rDNA序列测定及其系统发育分析结果,将菌株HT-8鉴定为Streptomycestermitum。单因素试验结果表明,该菌株产生杀线虫活性物质的最适培养条件为接种体菌龄48 h,接种量6%,培养基海水浓度100%,初始pH 7.5,培养温度25 ℃,培养时间7 d。该研究为海洋微生物资源的开发及其杀松材线虫天然活性物质的利用提供理论依据。
海洋放线菌;杀线活性;松材线虫;鉴定;培养条件
Q93-331;Q939.11+8
A
1005-7021(2016)06-0055-07
* 通讯作者。男,教授,博士,硕士生导师。研究方向为松树萎蔫病病理研究。E-mail:15806598505@163.com
10.3969/j.issn.1005-7021.2016.06.009
Funds Project: Shandong Provincial Natural Science Foundation (ZR2010CM009); Basic Research Projects of Qingdao (13-1-4-158-jch)
Author profile: CHEN Cong-cong female, graduate.major research directions: Development and utilization of microbial resources.
E-mail:562288378@qq.com
*Corresponding author:male, Ph.D, Master tutor.principal research: Pathology of pine wilt disease, E-mail:15806598505@163.com
Draft accepted date:2016-01-28;Revision returned date:2016-03-25
