Antitumor effect of recombinant human endostatin combined with cisplatin on rats with transplanted Lewis lung cancer
2015-11-30ZhanWuYuYingHuaJuChengLiangYangHanBingYuQuanLuoYeGangMaYongYuLiu
Zhan-Wu Yu, Ying-Hua Ju, Cheng-Liang Yang, Han-Bing Yu, Quan Luo, Ye-Gang Ma,Yong-Yu Liu*
1Department of Thoracic Surgery, Liaoning Cancer Hospital and Institute, Dalian Medical University Affiliated Tumour Hospital, Shenyang City,Liaoning Province 110042, China
2Department of Biochemistry and Molecular Biology, China Medical University, Shenyang City, Liaoning Province 110122, China
1. Introduction
Lung cancer is a common malignancy in respiratory system,ranging the top of incidence of all the malignant cancers[1-3].According to the pathological characteristics, the lung cancer can be divided into small cell lung cancer and non-small-cell lung cancer;according to statistics, non-small-cell lung cancer in lung cancer patients accounts for about 75%-85%[4]. The conventional therapy for lung cancer is basically surgery, radiotherapy and chemotherapy,with toxic side effects, liability to relapse and metastasize, and low survival rate of 5 years for patients[5]. Therefore, how to improve the efficiency of non-small-cell lung cancer treatment and reduce the toxic side effects have been urgent problems in antitumor research field. It has been widely and clinically recognized that tumor grows depending on angiogenesis and that the tumor invasion and metastasis are closely connected to angiogenesis of tumor tissues; hence, anti-angiogenesis has become the research highlight of oncotherapy[6]. Angiostatin is a new angiogenesis inhibitor and can play a specific inhibitory role in vascular endothelial cells with heparin[5-7]. Researches confirm that vascular endostatin at normal level has the particular inhibitory effect in tumor growth. In the present research, the antitumor effect of Endostarinjection in tumor combined with intraperitoneal injection of cisplatin on subcutaneous transplanted Lewis lung cancer in rats was observed by taking C57 rats as models of subcutaneous transplanted Lewis lung cancer ready for the medication. The antitumor mechanism and effect were observed, aiming to provid a experimental basis for the clinical treatment.
2. Materials and methods
2.1. Experimental animals
A total of 30 clean C57/6J rats were purchased from Shanghai Lab,Animal Research Center, with age of 5-7 weeks, weight of (21±3)g,humidity for raising of (60±5)%, and temperature at (25±2)℃, and food and water were available ad libitum. The whole experimental process was conducted strictly sticking to Regulations for the Administration of Affairs Concerning Experimental Animals.
2.2. Equipments and reagents
Endostar injection was purchased from Shandong Simcere-Medgenn Bio-pharmaceutical Co.,Ltd., with batch number 20130404 and standard of 15 mg/piece. Cisplatin injection was provided by Mayne Pharma Pty. Ltd., with batch number U131881AA and standard of 50 mg/piece. The monoclonal antibodies of rabbit-antirat endothelial cell CD34 antigen and rabbit-anti-rat VEGF antigen were provided by Beijing Zhongshan Golden Bridge-Biotechnology Co., Ltd. Phosphate buffered saline (buffer, Strept Avidin-Biotin Complex kit, 3,3'-diaminobenzidine color-substrate solution and related reagents were purchased from Shanghai Senxiong Biotechnology Co., Ltd. Olympus BH-2 microscope was from Japan.
2.3. Model preparation and group treatments
Well-grown tumors in tumor-bearing rats were taken for the preparation of monoplast suspension 2×106/mL by homogenization.A total of 0.2 mL of the prepared suspension was taken to inoculate in the subcutaneous tissues of axilla beneath rats' right upper limb, after which the rats were divided randomly into Groups A,B, and C, with 10 in each. Medication was performed when the tumor volume reached 400 mm3. Group A was the control group without any medication. Group B was given Endostar 5 mg.kg-1.d injection directly in tumor for continuous 10 d while Group C was given Endostar injection 5 mg.kg-1.d combined with intraperitoneal injection of cisplatin 5 mg.kg-1.d for continuous 10 d.
2.4. Observation indicators
All the rats were executed the day after the 10-d medication. The volume and mass of tumor were then measured and the antitumor rate was calculated. The blood from rats' eyeballs was taken for the determination of vascular endothelial growth factor (VEGF)level in plasma by double antibody sandwich ELISA assay. After the measurement and weighing of tumor, conventional tissue biopsies were prepared. After hematoxylin-eosin staining was conducted,the necrosis and metastasis of tumor were observed under light microscope. VEGF and microvessel density (MVD)of tumor tissue were determined by immunohistochemical method.
2.5. Result determination
According to method of Rahman et al, the VEGF expression was scored and radio of positive cells were scored based on dyeing range and intensity. Dyeing intensity was ranged 0-3 levels, namely,level 0: negative; level 1: weakly positive; level 2: positive; level 3:strongly positive. Dyeing techniques were ranged from 0-4 levels,namely, level 0: negative; level 1: positive cells 1%-25%; level 2:positive cells 26%-50%; level 3: positive cells 51%-75%; level 4:positive 76%-100%. The densest dyeing area of blood vessels of tumor in biopsies at high magnification was for the MVD count and 5 random counts were taken for the average value of microvessel numbers.
2.6. Statistical processing
The data were processed by SPSS 13.0 and measurement data were expressed by mean±sd. One-way ANOVA was performed by Pairwise comparison and Q test was conducted. If P < 0.05,statistical significance was considered to exist.
3. Results
3.1. Comparison of tumor volume, mass and antitumor rates in groups
Both tumor volume and mass in Groups B and C were significantly lower than Group A (P<0.05).Tumor volume and mass in Group C were significantly lower than Group B (P<0.05). The antitumor rate in Group C was significantly higher than Group B (P<0.05). Specific results were shown in Table 1.
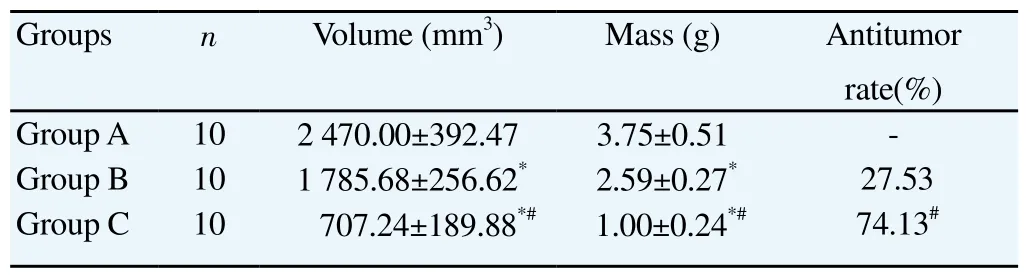
Table 1Comparison of tumor volume, mass and antitumor rates in groups.
3.2. Comparison of tumor VEGF, MVD and plasma VEGF in groups
The tumor VEGF score, MVD and plasma VEGF level in Group C were significantly lower than Groups A and B (P < 0.05). The tumor VEGF score, MVD and plasma VEGF level in Group B were significantly lower than Groups A (P < 0.05). Specific results were shown in Table 2.
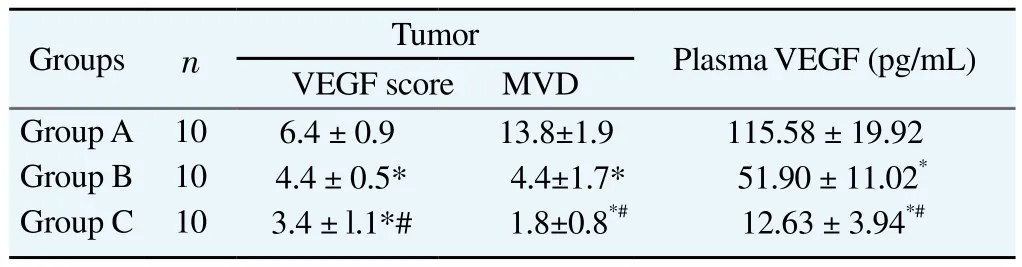
Table 2Comparison of tumor VEGF, MVD and plasma VEGF in groups.
3.3. Histological observation
In Group A, there was clear demarcation between tumor and the surrounding tissues. The tumor was with hilic growth, complete but easy-to-peel capsule. The cut of tumor was grey white. The tumor cells grew vigorously and the tumor was surrounded by abundant blood capillary. In Group B, several caseous necrosis and large area of focal necrosis were seen in the central part of tumor. The number of tumor cells and blood capillary surrounding tumor was obviously smaller than Group A. In Group C, the active tumor cells were obviously fewer than Groups A and B. Atrophy and liquefaction necrosis were easily seen in parts of tumor, and the blood capillary surrounding tumor was the fewest in Group C. Specific results were shown in Figure 1.
4. Discussion
Lung cancer is the common malignant disease in respiratory system, with increasing incidence in recent years, and has topped the morbidity and mortality of malignancy. At present, the surgery,radiotherapy and chemotherapy are still the major therapeutic regimens, but radiotherapy and chemotherapy are of heavy toxic side effects which are detrimental to the survival rate of patients[10-13]. The growth and metastasis of malignant tumor are dependent on the vasifaction of new blood vessels which can ensure the nutrient supply of tumor cells; hence, anti-angiogenesis has been the new focus in therapy for malignant tumor[14]. Anti-angiogenesis targeted drugs that are not prone to show drug resistance and of low toxicity have become the research focus in tumor therapeutic area. The present research observed the effect of anti-angiogenesis drug, endostatin, combined with cisplatin, on growth and metastasis of tumor in rats with transplanted Lewis lung cancer and aimed to provide the experimental basis for clinical treatment.
Experiments have confirmed that angiostatin carries significant inhibitory effect on growth of malignant tumors of all kinds and can reduce the proliferation and metastasis of tumors[15-18]. Other experiments also confirm that endostatin at normal level can significantly inhibit tumor growth but when it is lower than normal level, the tumor would grow 2-3 times faster. Many researchers believe that the process is the result of multiple target points and multiple steps[19]. Endostar is the new recombinant human endostatin in recent years, whose functional mechanism is to inhibit the new tumor angiogenesis by inhibiting the metastasis of endothelial cells in blood vessels, so that the nutrient supply of tumor can be blocked and therefore the proliferation and metastasis of tumor can be inhibited[20]. In-vitro experiment results showed that Endostar had inhibitory effect in the metastasis of human microvascular endothelial cells and formation of tubes, and meanwhile could significantly inhibit angiogenesis of chick chorioallantoic membrane which implied that Endostar carries in-vitro anti-angiogenesis effect; in addition, Endostar plays an inhibitory effect in human lung adenocarcinoma cell SPC-A4 and gastric carcinoma cell SGC7901[21,22]. cis-Dichlorodiamineplatinum(Ⅱ)or DDP is the first line chemotherapy for treatment in kinds of solid tumors, with broad anticancer spectrum, strong function, no cross-resistance and other advantages, and it can be used with many antineoplastic drugs,producing a synergistic effect which makes it the most common drug in current combined chemotherapy; VP-16 plus EP regimen is the first line therapy for treatment in small cell lung cancer or nonsmall-cell lung cancer, with significant antitumor effect[23,24]. The present research treated the rats with subcutaneous transplanted Lewis lung cancer, having Endostar injection in tumor combined with intraperitoneal injection of cisplatin. After the treatment,in Group C, the volume and quality of tumor were significantly lower than Groups A and B (P < 0.05)and the antitumor rate was significantly higher than Group B (P < 0.05), which showed that Endostar injection in tumor can significantly inhibit the growth and proliferation of tumor, and that injection combined with intraperitoneal injection of cisplatin can play a synergetic inhibitory effect with better efficacy than single medication. The present research showed that tumor VEGF, MVD and plasma VEGF levels in Group C were significantly lower than Groups A and B (P <0.05)and tumor VEGF, MVD and plasma VEGF levels in Group B were significantly lower than Group A (P < 0.05), suggesting that Endostar injection combined with cisplatin can effectively reduce the tumor VEGF expression and plasma VEGF level of rats with subcutaneous transplanted Lewis lung cancer so that the tumor angiogenesis can be reduced and antitumor goal can be achieved.Histopathological observation showed that the positive tumor cells in tumor tissue of rats in Group C were obviously fewer than Groups A and B, with atrophy and liquefaction necrosis in parts of tumor and the fewest blood capillary around tumor; that also confirmed that Endostar can effectively inhibit the tumor angiogenesis so that the nutrient supply of tumor cells can be blocked and tumor cell apoptosis can be accelerated. Furthermore, cisplatin can directly kill tumor cells and cause atrophy and liquefaction necrosis in tumor tissue. The combination of two medications makes a better antitumor effect.
The research confirmed that Endostar injection in tumor combined with intraperitoneal injection of cisplatin can significantly inhibit the VEGF and MVD of rats with subcutaneous transplanted Lewis lung cancer so that the nutrient supply of tumor cells can be blocked and tumor cells can be killed directly, so the proliferation and metastasis of tumor cells can be inhibited.
Conflict of interest statement
We declare that we have no conflict of interest.
[1]Bai ZQ, Wang YW, Kang YB, Liu YL. Application of human recombinant human endostatin combined with chemotherapy in the treatment of NSCLC. J Pract Oncol 2013; 28(2): 218-221.
[2]Zheng YH, Wang L, Jiang ZY, Qin SK. Research on continuous or intermittent administration of recombinant human endostatin by intraperitoneal injection in mice H22 ascite tumor model. Chin Clin Oncol 2012; 17(3): 202-206.
[3]Jia YT, Liu M, Huang WG, Wang ZB, He YT, Wu JH, et al. Recombinant human endostatin Endostar inhibits tumor growth and metastasis in amouse xenograft model of colon cancer. Pathol Oncol Res 2012; 18(2): 315-323.
[4]Wei HM, Qin SK, Yin XJ, Chen YL, Hua HQ, Wang L, et al. Research on intercalated combination of recombinant human endostatin and cisplatin in H22 tumor ascite model. Chin Clin Oncol 2014; 19(2): 122-127.
[5]Zhao WY, Chen DY, Chen JH, Ji ZN. Effects of intracavitary administration of Endostar combined with cisplatin in malignant pleural effusion and ascites. Cell Biochem Biophys 2014; 70(1): 623-628.
[6]Yue L, Zhai FY, Xue HO, Wang Y, Gao Y, Wang F, et al. The clinical research of Endostar topical combined with chemotherapy on treating malignant hydrothorax and ascites. J Jiangxi Univ Trad Chin Med 2013;25(4): 10-13.
[7]Zhang DW, Li HL, Yao Q, Yang WL, Wang HL, Zhai DX, et al. The synergistic effect of recombinant human endostatin (YH-16)combined with oxaliplatin on human colorectal carcinoma. J Int Med Res 2010;38(1): 111-126.
[8]Wei HM, Qin SK, Yin XJ, Chen YL. Therapeutic features of Endostar,a modified endostatin, on ascites tumor in mice. J First Milit Med Univ 2010; 30(7): 1509-1531.
[9]Rahman MA, Dhar DK, Yamaguchi E, Maruyama S, Sato T, Hayashi H, et al. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res 2001; 7(5): 1325-1332.
[10]Manegold C, Vansteenkiste J, Cardenal F, Schuette W, Woll PJ, Ulsperger E, et al. Random-ized phase Ⅱ study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer. Invest New Drugs 2013; 31(1):175-182.
[11]Li N, Jin ZL, Liu ZJ, Wang J, Li K. Efficacy of Endostar combined with chemotherapy in multi-cycle treatment of patients with advanced nonsmall cell lung cancer. Chin J Oncol 2011; 33(12): 937-942.
[12]Du CX, Chen SS, Liu XY, Zhang HG. Rh-endostatin combined with docetaxel, platinates and fluoropyrimidines as first-line chemotherapy for advanced gastric cancer. Chin Clin Oncol 2014; 19(10): 925-928.
[13]Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V,et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer:results from a randomised phase Ⅲ trial (AVAiL). Ann Oncol 2010;21(9): 1804-1809.
[14]An SJ, Huang YS, Chen ZH, Su J, Yang Y, Chen JG, et al. Posttreatment plasmaVEGF levels may be associated with the overall survival of patients with advanced non-small cell lung cancer treated with bevacizumab plus chemotherapy. Med Oncol 2012; 29(2): 627-632.
[15]Yang ZP, Du YG, Xie YH, Li JR, Wang XM, Song YW. Inhibition effects of combination of radiotherapy and Endostar on rats with nasopharyngeal carcinoma xenografts and its mechanism. Chin J Cancer Prev Treat 2012;19(4): 259-262.
[16]Xu Y, Li Q, Li XY, Yang QY, Xu WW, Liu GL. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J Exp Clin Cancer Res 2012;31(1): 16-19.
[17]Wu SF, Xie YY, Chen PF. Clinical study of recombinant human endostatin combined with intravenous chemotherapy and intraperitoneal hyperthermic perfusion chemotherapy for advanced ovarian cancer. Chin J Postgrad Med 2013; 36(3): 10-13.
[18]Zhang CC, Li K, Wei XY, Chen C, Yuan J, Wang J. Comparison of the effect of rh-endostatin on intratumoral and myocardial micrangium in mice. Chin J Oncol 2011; 33(6): 415-420.
[19]Yu SH, Wu YZ. Effect of recombinant human endostatin injection combination with chemotherapy treatment for elderly patients with advanced non-small cell lung cancer. China Mod Med 2014; 21(21): 94-96.
[20]Wang LH, Li K. The anti-angiogenic and anti-metastatic effect of Endostatin on Lewis lung carcinoma xenograft in mice. Chin J Clin Oncol 2008; 35(10): 587-598.
[21]Meng W, Cai WJ, Shao GF, Zhang Y. Clinical efficacy of docetaxel combined with recombinant human endo-statin in the treatment of nonsmall cell lung cancer. China Mod Med 2015; 22(6): 132-137.
[22]Wang LF, Gu ZF, Wang BC, Cui W, Bi JW. Antitumor effect of local injection of recombinant human endostatin in tumor on rats with Lewis lung cancer. J Pract Oncol 2014; 29(6): 556-559.
[23]Wei HM, Qin SK, Yin XJ, ChenYL, Hua HQ, Wang L, et al. Inhibition andmechanism on combination of recombinant human endostatin and cisplatin in mouse model bearing S180 tumor ascite. Chin J Cancer Prev Treat 2015; 22(6): 442-446.
[24]Zhao JZhao J, Chen X, Zhang A, Xu F, Hu M, et al. Apilot study of combination intraperitoneal recombinant human endostatin and chemotherapy for refractory malignant ascites secondary to ovarian cancer. Med Oncol 2014; 31(4): 930-940.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Antidiabetic and antioxidant activities of Nypa fruticans Wurmb. vinegar sample from Malaysia
- Anti-inflammatory and analgesic activities with gastroprotective effect of semi-purified fractions and isolation of pure compounds from Mediterranean gorgonian Eunicella singularis
- Natural products: Perspectives in the pharmacological treatment of gastrointestinal anisakiasis
- Upregulated hepatic expression of mitochondrial PEPCK triggers initial gluconeogenic reactions in the HCV-3 patients
- Analysis of human B cell response to recombinant Leishmania LPG3
- Rifabutin reduces systemic exposure of an antimalarial drug 97/78 upon co- administration in rats: an in-vivo & in-vitro analysis
