Natural products: Perspectives in the pharmacological treatment of gastrointestinal anisakiasis
2015-11-30ValeroRomeroMCmezMateosHierroNavarroMC
Valero A, Romero MC, Gómez-Mateos M*, Hierro I, Navarro MC
1Department of Parasitology. Faculty of Pharmacy. University of Granada , Spain
2Department of Pharmacology. Faculty of Pharmacy. University of Granada, Spain
1. Introduction
Digestive anisakiasis is caused by the local effect of the parasite on the walls of the gastrointestinal tract. After a series of symptoms which vary in pattern, it normally resolves itself spontaneously,although there are cases in which real complications arise which require abdominal surgery. Because this illness is closely related to dietary habits, its appearance and diagnosis in humans is more common in countries where raw or undercooked fish is regularly consumed. Several fish and cephalopod species carry Anisakis L3 larvae and many of these species are of great commercial value.Humans act as accidental hosts since the larvae do not evolve inside them. Although this illness can be caused by other anisakids,the main etiological agent of this parasitosis is considered to be Anisakis simplex s.s.[1,2]. In Italy, however, it has been attributed to Anisakis pegreffii[3-5]. It is not yet known whether these two species are equally pathogenic, but trials performed on laboratory animals indicate that Anisakis simplex penetrates the mucous membrane of the digestive tract to a greater proportion[6]. Japan figures at the top of the list for this parasitosis, followed by Spain, due to the high level of fish consumption by inhabitant per year and the wide variety of high-risk preparation methods, although nowadays cases are reported all over the world. Various treatments have been proposed for this illness in cases where symptoms do not require urgent intervention, but in the last two decades clinicians have mainly opted for anthelmintics such as mebendazole, thiabendazole and especially albendazole in an effort to avoid surgery[7-11]. In fact, albendazole is listed as a treatment for anisakiasis on the Centers for Disease Control and Prevention webpage - Anisakiasis (USA)[12], although it points out that it is not FDA approved to treat this illness, as there is no clear evidence of its effectiveness. Some authors[8,13]argue that the results are similar to those obtained with a regime of fasting,fluid therapy and treatment of symptoms. Despite the fact that more than 50 years have passed since the first case of anisakiasis was reported in humans[14], there is still no effective drug on the market to treat this digestive parasitosis. Moreover, the anthelmintics which act against other nematodes of the gastrointestinal tract are not capable of killing Anisakis L3 larvae. In view of this fact, it is worth noting that many natural products containing pharmacologically active ingredients are increasingly used in the treatment of numerous pathologies and that these may offer a good alternative in the fight against organisms such as Anisakis.
2. Materials and methods
A literature search on investigations into the activity of natural products against the L3 larvae of Anisakis simplex was collected from scientific journals, books, theses and reports via a library and electronic search (using Pubmed, Scopus, Medline, Web of Science and ScienceDirect). The search terms included: natural products, medicinal plants, essential oils, terpenic derivatives,Anisakis, antinematodal activity. All articles were reviewed with especial attention; in addition, the reference lists of each article were reviewed to identify additional relevant articles.
In this review, covering the literature published from 1970 through to the end of October 2014, various studies were found describing both the in vitro and in vivo activity against Anisakis larvae of a large variety of natural products of varying chemical nature.
3. Results
Comparative data for in vitro studies of trialled plant extracts,essential oils and components are shown in Tables 1, 2 and 3; in vivo trials are represented in Figure 1.
3.1. In vitro studies
It has been demonstrated that Anisakis L3 larvae lose mobility completely 24 h after coming into contact with saline extracts from the plants Zingiber officinale (Z. officinale)(5% and 2.5%), Perilla frutescens (P. frutescens)(5%), Wasabia japonica (W. japonica)(5%)and Allium sativum (A. sativum)(5%), whilst others are ineffective at the stated concentrations [Allium fistulosum (A. fistulosum),Petroselinum sativum (P. sativum), Raphanus sativus (R. sativus),Brassica oleracea (B. oleracea), Spinacia oleracea (S. oleracea),Laminaria angustata (L. angustata), Capsicum annuum (C. annuum)and Thea sinensis (T. sinensis)][15]. Other authors[16]subsequently corroborated the data obtained by Kasuya et al[15]for A. sativum.Studies conducted on the fruit of Punica granatum (P. granataum)(juice and skin extract)- a plant containing various bioactive compounds and mainly found in warm, subtropical areas[17-20]-showed that it was not effective against Anisakis L3, as was also the case for extract of Tagetes lucida (T. lucida)(data not published).Trials were also conducted on enzymes found in certain plants whose anti-inflammatory properties are widely recognised: for papain at a concentration of 2.5 mg/mL the biocidal effect was 90.9%[21], whilst,the larvicidal activity of bromelain was much lower (31.7%)(data not published).
The in vitro effectiveness against Anisakis of various essential oils has also been demonstrated. Of these, tea tree oil [Melaleuca alternifolia(M. alternifolia)]gave the most significant results, killing all the larvae in 48 h at concentrations of 7 and 10 µg/mL[22]. The essential oils of several aromatic plants were found to have similarly lethal effects in 4-48 h (125 µg/mL), some of which are widely used in condiments and infusions, such as cumin [Cuminum cyminum(C. cyminum)], lemongrass [Cymbopogon citratus (C. citratus)],palmarosa [Cymbopogon martinii (C. martinii)], Java citronella[Cymbopogon winterianus (C. winterianus)], litsea [Litsea cubeba(L. cubeba)], geranium [Pelargonium graveolens (P. graveolens)],oregano [Origanum vulgare (O. vulgare)]and chamomile [Matricaria chamomilla (M. chamomilla)]. Essential oil of peppermint [Mentha piperita (M. piperita)]was shown to be lethal at concentrations of 250 µg/mL; and a mixture of this essential oil with chamomile essential oil displayed activity at concentrations of 187.5 µg/mL.Essential oil of Thymus vulgaris (T. vulgaris)was found to act on L3 larvae when added to the muscle tissue of the parasitized fish at a concentration of 10% in sunflower oil. In most cases, alterations were observed in the morphology of the larva, mainly involving the cuticle and the digestive tract, especially the ventricular area[23-28].Essential oil of Piper nigrum (P. nigrum)killed 83.3% of parasites at 125 µg/mL while Myristica fragans (M. fragans)showed no activity(data not published).
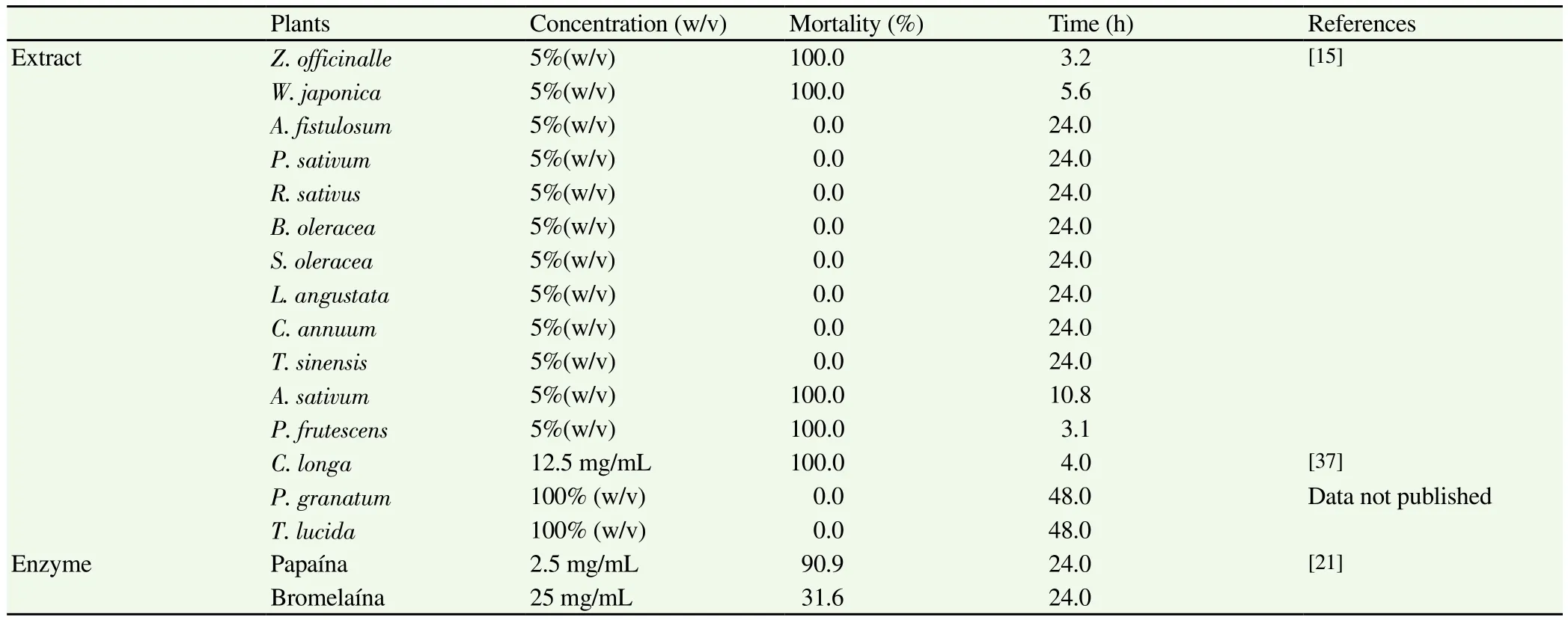
Table 1Extracts and enzymes in vitro assayed against Anisakis type I.
With regard to the components of essential oils, the data obtained by Kasuya et al[15,29]and Goto et al[30]showed that 100% of the larvae died in presence of various different concentrations of certain compounds found in Z. officinale and P. frutescens, such as [6]-shogaol(62.5 µg/mL), [6]-gingerol (250 µg/mL), perillaldehyde (125 µg/mL)and perillyl alcohol (250 µg/mL). Moreover, Lin et al[31]studied four phenolic components of Z. officinale: [10]-shogaol, [6]-shogaol,[10]-gingerol and [6]-gingerol, all of which had a larvicidal effect,particularly [10]-gingerol and [10]-shogaol, which killed 80 to 100%of the larvae (200 µM)in a period of 48 to 72 h.
Suzuki et al[32]studied the effect of other compounds of natural origin. Of these, (+)-ar-turmerone, one of the main components of essential oil from the rhizomes of Curcuma longa (C. longa),was the most effective (25 µg/mL)at the 24-hour point. Other compounds present in various different essential oils (paeonol,(-)menthol, menthyl ester, trans-anethole, allyl isothiocyanate,eugenol, isoeugenol, methyl eugenol, safrole, cinnamic aldehyde,geraniol and linalool)proved less lethal than ar-turmerone,requiring concentrations between 125 µg/mL and 500 µg/mL to kill the larvae, in a period of 24 h. Santonin, dehydrosantonin and(-)-8-phenylmenthyl ester showed no activity, even at the highest concentrations used.
After this, in vitro trials were conducted on several terpenes,showing significant results forα-pinene, ocimene, geraniol,carvacrol, thymol, citronellol, citral, cuminic aldehyde, nerolidol,farnesol andα-bisabolol, all of which produced a mortality rate of 100% at 125 µg/mL at 4-24 h; in all cases, the histological studies showed an alteration of the cuticle of the parasite and/or the gut wall[24,25,27,33-36]. Carvone (12.5 µg/mL), an oxygenated monoterpene found in various essential oils [Carum carvi (C. carvi),Anethum graveolens (A. graveolens)and Mentha spp.], displayed no significant activity in 48 h (data not published). No biocidal activity was observed forβ-pinene, myrcene and p-cimene against Anisakis simplex L3 larvae.
3.2. In vivo studies
The aim of these studies was to demonstrate the effectiveness of the trialled products in terms of preventing the larvae from attaching and causing lesions to the digestive tract, as well as their larvicidal capacity. Dosages were calculated taking into account the LD50of the trialled products, which ranged from 9.50 to 46.9 mg in a carrier of 0.5 mL of olive oil.
The C. longa showed high larvicidal activity, being all the larvae found dead in the gastric cavity of the rats[37]. The essential oils of M.piperita and M. chamomilla were studied in vivo by Romero et al[25-27],and it was observed that in rats treated with chamomile essential oil in conjunction with the larvae, only 2.2% had lesions in the gastric mucous membrane, as opposed to 93.3% in the control group. In rats treated with peppermint essential oil, no lesions appeared in the gastrointestinal tract, nor were there any larvae attached to the digestive tract, from which we can deduce that this essential oil significantly (P<0.001)reduces the pathogenic capacity of the larvae when compared to the control. In the case ofα-bisabolol, one of the main components of chamomile essential oil, lesions were only found in 5.5% of the animals in the treated group. Menthol, menthyl acetate and menthone, the main components of peppermint essential oil, provided complete protection against this parasitosis.
Several compounds present in different essential oils were also trialled in vivo, revealing the significant larvicidal activity of various oxygenated monoterpenes such as thymol, geraniol, citronellol,citral and perillaldehyde; no lesions were found on the animals and the mortality rate of the larvae was as high as 92.8% in some cases, unlike the results obtained forα-pinene, myrcene, ocimene,carvacrol, cineol, cuminic aldehyde and geranyl acetate. According to the histological study of L3s, the alteration in the intestine andcuticle may be the cause of death[34-36, 38].
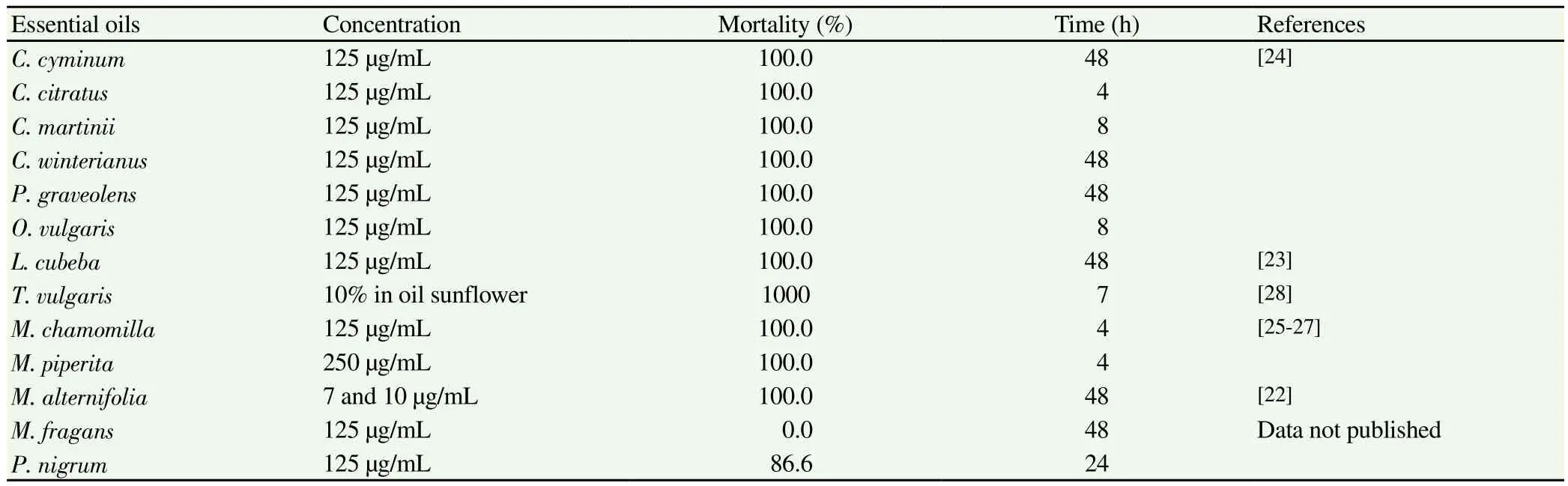
Table 2Essential oils in vitro assayed against Anisakis type I.
With regard to nerolidol and farnesol, sesquiterpenoid derivatives tested in in vivo trials, it was found that 20% of the rats from the treated group had lesions, and the larvicidal activity of elemol in vivo was even lower, as 40% of the rats given this sesquiterpenoid had lesions, in comparison to 86.6% in the control group[36].
Moreover, it is known that essential oils and/or their components can cause inflammation of the mucous membrane of the gastrointestinal tract; this was not the case, however, for these essential oils or their main components, as when the enzyme Myeloperoxidase was measured, no differences with respect to the control group were detected in any of the cases.
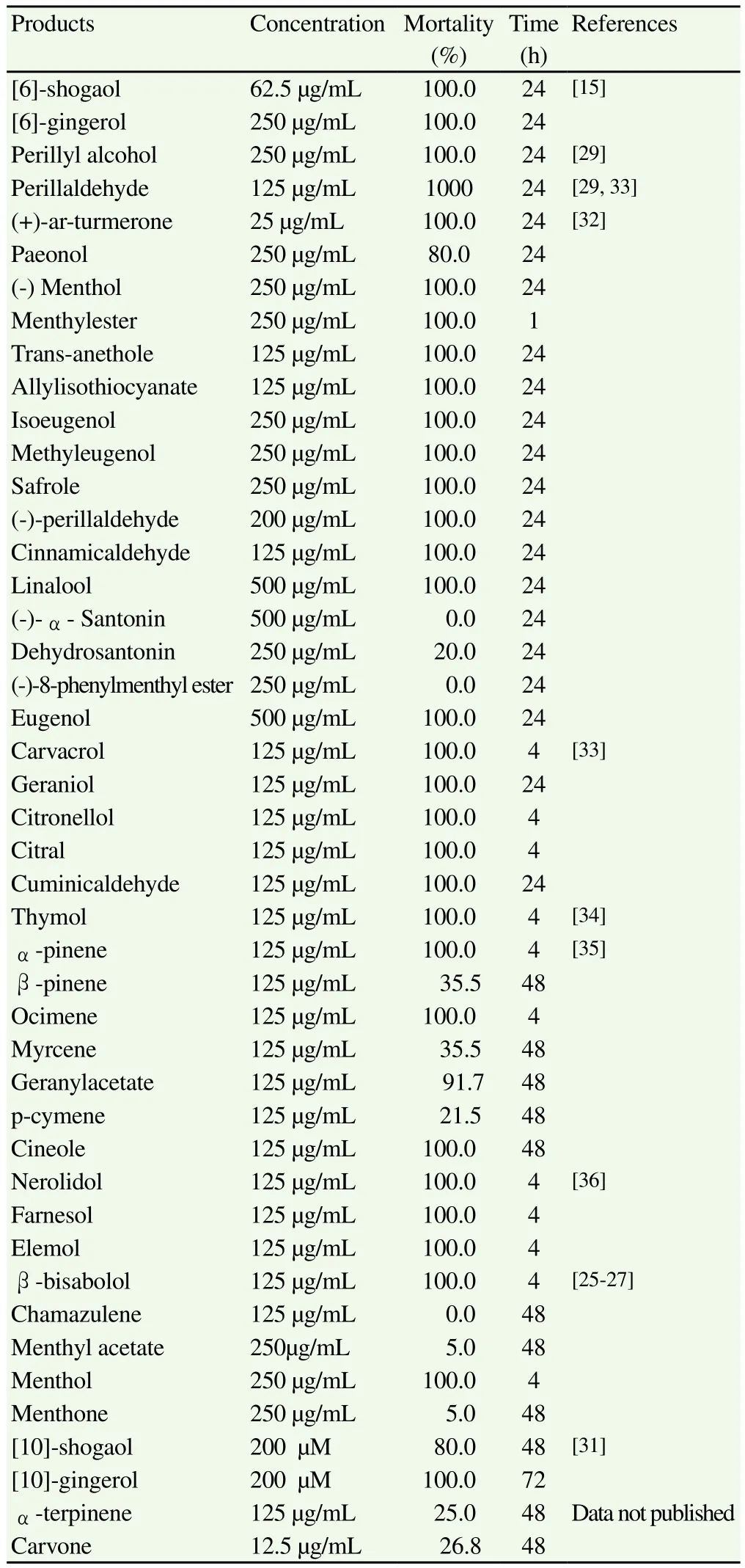
Table 3Natural products in vitro assayed against Anisakis type I.
4. Discussion
Considering the increasing worldwide public health risk posed by anisakiasis, it is imperative to make an effective drug available that kills the parasite as swiftly as possible and shortens the pathological process brought about by the mechanical action of the larvae attaching to the walls, subsequently eliminating the larva and thus reducing exposure times to excreted and secreted substances; these products, which have allergenic and proinflammatory properties,can cause hypersensitive reactions in humans ranging from mild to severe in character. The ideal drugs to treat anisakiasis are those which are do not cause alterations in the tissues of the digestive tract. This poses serious difficulties, as these nematodes do not appear to respond effectively to the anthelmintics available in the therapeutic arsenal. Thus there is a clear need to find new active agents against Anisakis L3 larvae. Due to the great chemical diversity of the compounds that it produces, the plant kingdom is one of the most ancient sources of medicine and offers a considerable range of raw material in which to search for new products for therapeutic uses. In particular, whether it is used as a source of pure compounds or extractive products, it offers a wealth of opportunities for the development of new drugs[39]. It is estimated that less than 10% of the secondary metabolites in plants have been identified, substances which frequently act as defence mechanisms for the plants against microorganisms, insects and other threats. Furthermore, around 14%-28% of higher plant species are used for therapeutic purposes, thus constituting a significant source of medicinal preparations (extracts,infusions, essential oils, etc.), which have been used in traditional medicine since ancient times.
In view of this, the effectiveness of certain components of P. frutescens, a plant used to season raw fish and as an ingredient in many dishes, may explain the reason why the prevalence of anisakiasis is lower in certain areas of China where it is used for this purpose[29]. This observation has been used as a springboard by various research groups to investigate the possible effects of different natural products on this parasitosis.
Considering the high mortality rate of Anisakis larvae in the presence of many essential oils and/or their components, it is conceivable that this may be due to the alterations that occur in the cuticle and digestive tract, especially the oesophagus, as is demonstrated in histological studies. With this in mind, some studies focus on determining the action mechanism of certain natural products. Thus many essential oils display significant biological activity both in vitro and in vivo on various different pathogenic agents[40]. One important factor to take into account here is the characteristic hydrophobia of these essential oils. According to Sikkema et al[41], this explains their toxic capacity against microorganisms whose cell walls are formed by polysaccharides, within which glucopeptides are found in abundance. The cytoplasmic membrane, a phospholipid bilayer,has low permeability for polar and charged molecules, although nonpolar compounds, such as cyclic hydrocarbons and, therefore,terpenes, can easily penetrate the bilayer. The interaction of the essential oils with the cellular and mitochondrial membranes of the bacteria brings about significant changes in the structures of these essential oils accumulates in the hydrophobic part of the membranes,which modifies their impermeability, sometimes causing them to swell[42]. Changes to the integrity of the membrane also alter its functioning, as various parameters are affected by the interaction of the lipid compounds with the membrane, altering the behaviour of the proteins it contains.
Another factor to take into consideration is the lack of agreement in some cases between in vitro and in vivo biocidal activity. The fact that menthol, menthyl acetate and menthone are active in vivo and not in vitro may be because in the presence of gastric pH(2.5)the menthyl acetate is hydrolyzed, thus producing menthol,whilst in the case of menthone, there may be a reduction of the ketone group, leading to the formation of this same monoterpenic alcohol. Given the concentrations of each of these compounds in the peppermint essential oil [menthyl acetate (68.1%), menthone(23.1%)and menthol (<1%)], we can infer that this essential oil, due to the hydrolyzing and reducing conditions of the gastric pH, would have a menthol content of more than 90%. A similar case is to be found in chamomile essential oil, which has a much lower larvicidal activity (23.9%)than that of peppermint, but much higher than that of α-bisabolol (4.4%). This set of data corroborates the thesis that the different compounds present in each essential oil act in synergy,which explains why, both for mint essential oil and chamomile essential oil, the larvicidal activity is clearly higher than that of each of its separate components[43].
Having evaluated the data from in vitro trials on essential oils, we can conclude that the essential oil obtained from M. alternifolia (tea tree oil)is the most active against Anisakis L3 larvae. With regard to the plant extracts trialled against this nematode, we can confirm that the extract obtained from the root of Z. officinale (ginger)displays the highest larvicidal activity. In terms of compounds, ar-turmerone,a component isolated from the root of C. longa (turmeric), displayed the highest activity in vitro, killing all the larvae in 24 h at a concentration of 25 µg/mL.
Of the products tested in in vivo trials, perillaldehyde takes first place given that, firstly, no lesions were found in the gastric mucous membrane of the laboratory animals and secondly, the percentage of dead larvae found in the gastric cavity was 91%. Also found to be effective were essential oil of peppermint, its different components(menthol, menthone and menthyl acetate)and other terpenic derivatives such as geraniol, citral, citronellol and thymol, as lesions were not found in any of these cases, although the percentage of dead larvae was lower in all cases than that of perillaldehyde.
In view of the fact that, in the trials carried out on laboratory animals, the different plant products tested have been promising,serving to avoid infection in the animal and thus the appearance of lesions, along with their lack of toxicity at the dosages used, we can conclude that these compounds represent a good starting point in the search for an effective drug to treat an internationally widespread parasitosis such as human anisakiasis.
Conflict of interest statement
We declare that we have no conflict of interest.
[1]Umehara A, Kuwakami Y, Araki J, Uchida A. Molecular identification of the etiological agent of the human anisakiasis in Japan. Parasitol Int 2007; 56(3): 211-215.
[2]Arizono N, Yamada M, Tegoshi T, Yoshikawa M. Anisakis simplex sensu stricto and Anisakis pegreffii: Biological characteristics and pathogenetic potential in human anisakiasis. Foodborne Pathog Dis 2012; 9(6): 517-521.
[3]Fumarola L, Monno R, Ierardi E, Rizzo G, Giannelli G, Lalle M, et al.Anisakis pegreffii etiological agent of gastric infections in two italian women. Foodborne Pathog Dis 2009; 6(9):1157-1159.
[4]Mattiucci S, Paoletti M, Borrini F, Palumbo M, Palmieri RM, Gomes V, et al. First molecular identification of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae)in a paraffin-embedded granuloma taken from a case of human intestinal anisakiasis in Italy. BMC Infect Dis 2011; 31(11): 82.
[5]Mattiucci S, Fazi P, De Rosa A, Paoletti M, Megna AS, Glielmo A, et al.Anisakiasis and gastroallergic reactions associated with Anisakis pegreffii,Italy. Emerg Infect Dis 2013; 19(3): 496-499.
[6]Romero MC, Valero A, Navarro-Moll MC, Martín-Sánchez J.Experimental comparison of pathogenic potential of two sibling species Anisakis simplex s.s. and Anisakis pegreffii in Wistar rats. Trop Med Int Health 2013; 18(8): 979-984.
[7]Bao Pérez F, Álvarez Rubio M, Martí Cabané J. Anisakis simplex sobre ulcus en paciente con Billroth II. Rev Esp Enferm Dig 2005; 97(7): 532-533.
[8]Ponferrada A, Matilla A, Borrego GM, Beceiro I, Núñez O, Lamónaca M, et al. Hemoperitoneo espontáneo secundario a yeyunitis por Anisakis.Rev Esp Enferm Dig 2005; 97(4): 292-293.
[9]Kim SG, Jo YJ, Park YS, Kim SH, Song MH, Lee HH, et al. Four cases of gastric submucosal mass suspected as anisakiasis. Korean J Parasitol 2006; 44(1):81-86.
[10]Filauro M, Rollandi GA, Cassola G, Quilici P, Angelini G, Belli F, et al. Gastrointetinal bleeding due to suspected anisakiasis: Challenging differential diagnosis for a rare disease. Updates Surg 2011; 63(3): 213-217.
[11]Pontone S, Leonetti G, Guaitoli E, Mocini R, Manfredelli S, Catania A, et al. Should the host reaction tu anisakiasis influence the treatment?Different clinical presentations in two cases. Rev Esp Enferm Dig 2012;104(11):607-610.
[12]Centers for Disease Control and Prevention. Parasites - Anisakiasis,resources for health professionals. Atlanta: CDC; 2012 [Online].Available from: http://www.cdc.gov/parasites/anisakiasis/health_professionals/index.html [Accessed on 21 Nov. 2012]
[13]Castán B, Borda F, Iñarrairaegui M, Pastor G, Vila J, Zozaya JM.Anisakiasis digestiva: clínica y diagnóstico según la localización. Rev Esp Enferm Dig 2002; 94(8): 463-472.
[14]Van Thiel PH, Kuipers FC, Roskam TH. A nematode parasitic to herring,causing acute abdominal syndromes in man. Trop Geograph Med 1960;12(2): 97-113.
[15]Kasuya S, Goto C, Ohtomo H. Studies on prophylaxis against anisakiasis-A screening of killing effects of extracts from foods on the larvae. Jpn Assoc Infect Dis 1988; 62(12 ):1152-1156.
[16]Navarro MC, Hierro I, Pérez-Galindo MP, Valero A. Actividad de un licuado de Allium sativum (cultivar morado)frente a la larva L3 de Anisakis simplex s.l. Rev Fitoter 2005; 5(2):149-152.
[17]Ajaikumar KB, Asheef M, Babu BH, Padikkala J. The inhibition of gastric mucosal injury by Punica grantum L. (pomegranate)methanolic extract. J Ethnopharmacol 2005; 96(1-2): 171-176.
[18]Adhami VM, Mukhtar H. Anti-oxidants from green tea and pomegranate for chemoprevention of prostate cancer. Mol Biotechnol 2007; 37(1): 52-57.
[19]Pai MB, Prashant GM, Murlikrishna KS, Shivakumar KM, Chandu GN. Antifungal efficacy of Punica granatum, Acacia nilotica, Cuminum cyminum and Foeniculum vulgare on Candida albicans: an in vitro study.Indian J Dnt Res 2010; 21(3): 334-336.
[20]Abdollahzadeh Sh, Mashouf R, Mortazavi H, Moghaddam M,Roozbahani N, Vahedi M. Antibacterial and antifungal activities of Punica granatum peel extracts against oral pathogens. J Dent 2011;8(1):1-6.
[21]Gómez-Mateos M, Romero MC, Polo-Vico R, Navarro MC, Valero A.Actividad larvicida de la papaína frente a L3 de Anisakis tipo I. IX Cong Cienc Vet Biomed 2010 April, Madrid, España; 2010.
[22]Gómez-Rincón C, Langa E, Murillo P, Valero MS, Berzosa C, López, V.Activity of tea tree (Melaleuca alternifolia)essential oil against L3 larvae of Anisakis simplex. Biomed Res Int 2014. doi: 10.1155/2014/549510.
[23]González P, Hierro I, Pérez P, González P, Cabo MM, Valero A, et al.Actividad de aceites esenciales de los géneros Litsea y Cymbopogon frente a las larvas de Anisakis simplex s.l. In: Proceedings of the I Cong Nac Cienc Tecnol Alim 2001 May, Granada: 95, España; 2001.
[24]Valero A, Hierro I, Gozález P, Montilla P, Navarro MC. Activity of various essential oils and their main components against L3 Anisakis simplex s.l. In: Govil JN, Singh VK, Arunachalam P. (eds.)Recent progress in medicinal plants. drug development from molecules. Houston: Estudium Press; 2006, p. 247-265.
[25]Romero MC, Valero A, Martín-Sánchez J, Navarro-Moll MC. Activity of Matricaria chamomilla essential oil anisakiasis. Phytomedicine 2012;19(6): 520-523.
[26]Romero MC, Navarro-Moll MC, Martín-Sánchez J, Valero A. Actividad de albendazol y los aceites esenciales de menta (Mentha piperita)y manzanilla (Matricaria chamomilla)frente Anisakis tipo I. Ars Pham 2013; 55(1):45-49.
[27]Romero MC, Navarro MC, Martín-Sánchez J, Valero A. Peppermint(Mentha piperita)and albendazole against Anisakiasis in an animal model. Trop Med Int Health 2014; 19(12):1430-1436.
[28]Giarratana F, Muscolino D, Beninati C, Giuffrida A, Panebianco A.Activity of Thymus vulgaris essential oil against Anisakis larvae. Exp Parasitol 2014; 142: 7-10.
[29]Kasuya S, Goto C, Koga K, Othomo H, Kagei N, Honda G. Lethal efficacy of leaf extract from Perilla frutescens (traditional Chinese medicine)of perillaldehyde on Anisakis larvae in vitro. Jpn J Parasitol 1990; 39(2): 220-225.
[30]Goto C, Kasuya S, Koga K, Othomo H, Kagei N. Lethal efficacy of extract from Zingiber officinale (traditional chinese medicine)or [6]-shogaol and [6]-gingerol in Anisakis larvae in vitro. Parasitol Res 1990;76(8): 653-656.
[31]Lin RJ, Chen CY, Lee JD, Lu CM, Chung LY, Yen CM. Larvicidal constituents of Zingiber officinale (ginger)against Anisakis simplex.Planta Med 2010; 76(16): 1852-1858.
[32]Suzuki J, Murata I, Enokida R, Yasuda I. Effects of chinese medicine for helminth (VII)Minimum lethal concentration on 3rd stage larvae of Anisakis simplex with the natural compounds, isolated from crude drugs and several kinds of derivatives. Ann Rep Tokyo Metr Res Lab PH 1994;45: 35-44.
[33]Hierro I, Valero A, Pérez P, González P, Cabo MM, Montilla MP, et al.Action of different monoterpenic compounds against Anisakis simplex s.l.L3 larvae. Phytomedicine 2004; 11(1): 77-82.
[34]Hierro I, Valero A, González de Selgas JM, Navarro MC. Actividad larvicida del timol frente a Anisakis simplex s.l. Rev Fitoter 2004; 4(2):175-176.
[35]Navarro MC, Noguera MA, Romero MC, Montilla MP, González de Selgas JM, Valero A. Anisakis simplex s.l.: Larvicidal activity of various momoterpenic derivatives of natural origin against L3 Larvae in vitro and in vivo. Exp Parasitol 2008; 120(4): 295-299.
[36]Navarro-Moll MC, Romero MC, Montilla MP, Valero A. In vitro and in vivo activity of three sesquiterpenes against L3 larvae of Anisakis simplex type I. Exp Parasit 2011; 127(2): 405-408.
[37]Navarro-Moll MC, Abatoy AH, Hierro I, Ruiz-Negrillo A, Romero MC,Montilla P, et al. In vitro and in vivo activity against Anisakis simplex s.l.L3 of an extract proceeding from the subterranean parts of Curcuma longa. Acta Parasitol Port 2005; 12(1-2): 526.
[38]Hierro I, Valero A, Navarro MC. In vivo larvicidal activity of monoterpenic derivatives from aromatic plants against L3 larvae Anisakis simplex s.l. Phytomedicine 2006; 13(7): 527-531.
[39]Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: How to develop a stronger in vitro `proof -of – concept´.J Ethnopharmacol 2006; 106(3): 290-302.
[40]Katiki LM, Chagas AC, Bizzo HR, Ferreira JF, Amarante AF.Anthelmintic activity of Cymbopogon martinii, Cymbopogon schoenanthus and Mentha piperita essential oils evaluated in four different in vitro tests.Vet Parasitol 2011; 183(1-2): 103-108.
[41]Sikkema J, de Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 1995; 59(2): 201-222.
[42]Burt S. Essential oils: their antibacterial properties and potential applications in foods-a-review. Int J Food Microbiol 2004; 94(3): 223-253.
[43]Bruneton J. Farmacognosia, fitoquímica, plantas medicinales. 2nd ed.Zaragoza : Acribia; 2001.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Antidiabetic and antioxidant activities of Nypa fruticans Wurmb. vinegar sample from Malaysia
- Anti-inflammatory and analgesic activities with gastroprotective effect of semi-purified fractions and isolation of pure compounds from Mediterranean gorgonian Eunicella singularis
- Upregulated hepatic expression of mitochondrial PEPCK triggers initial gluconeogenic reactions in the HCV-3 patients
- Analysis of human B cell response to recombinant Leishmania LPG3
- Rifabutin reduces systemic exposure of an antimalarial drug 97/78 upon co- administration in rats: an in-vivo & in-vitro analysis
- Protection effect of trigonelline on liver of rats with non-alcoholic fatty liver diseases
