Uncertainty Evaluation of Preparation of Organophosphorus Pesticide Mixed Standard Solution
2015-11-18JielianWANGZhengYANAgriculturalProductsQualitySafetyMonitoringCenterofShanxiProvinceTaiyuan030025China
Jielian WANG,Zheng YAN,Agricultural Products Quality Safety Monitoring Center of Shanxi Province,Taiyuan 030025,China
Uncertainty Evaluation of Preparation of Organophosphorus Pesticide Mixed Standard Solution
Jielian WANG*,Zheng YAN,
Agricultural Products Quality Safety Monitoring Center of Shanxi Province,Taiyuan 030025,China
The uncertainty of standard solution plays an important role in detection of pesticide residues.It may affect the accuracy of detection results.In this study,the 14 organophosphorus pesticides mixed standard solution was used as the material to analyze all the influencing factors for the preparation of mixed standard solution with uncertainty as the only judging index.The preparation uncertainty of mixed standard solution was calculated with the top-down calculation method.In the end,the expanded uncertainty was presented.The results showed that the preparation of mixed standard solution from stock solution with precise pipettes had a relatively low uncertainty.
Organophosphorus pesticide;Mixed standard solution;Preparation and dilution;Uncertainty;Evaluation
M ost of the organophosphorus pesticides have high toxicity. According to statistics[1],the cases of organophosphorus pesticide residue exceeding account for 2/3 of the total vegetables with excessive pesticide residues.So the detection of organophosphorus pesticide residues has attracted much attention.To improve the reasonability of detection results,the measurement of uncertainty is essential.In the detection of pesticide residues in vegetables,the standard solution is needed.And the uncertainty of preparation of standard solution will directly affect the accuracy of detection results[2].There have been rare reports on uncertainty evaluation of preparation of standard solution. Sun et al.[3]ever evaluated the preparation uncertainties of 6 monostandards for organophosphorus pesticides.The 6 monostandards were prepared with solid standards.However,in current pesticide residue detection,liquid standards are commonly used.The researches on uncertainty
evaluation of preparation of organophosphorus standard solution are rare. In this study,the 14 organophosphorus pesticides were used as material. Then the mixed standard solution was prepared with 2 methods respectively. The uncertainties of mixed standard solutions were prepared so as to provide a guarantee for the detection results.
Preparation of Mixed Standard Solution of Organophosphorus Pesticides
According to the requirements for standard solution preparation by "Multi-residue Detection of Organophosphorus,Organochlorine,Pyrethroid and Carbamate in Vegetables and Fruits"(NY/T761-2008)[4],the 100 μg/ml of organophosphorus standard solution,purchased from the National Standard Centre,should be diluted into 0.2 μg/ml of sampling solution.In the past,certain amount of single organophosphorus standard (100 μg/ml)should be cracked[5]and thendissolved to certain volume with repeated rinsing.During the cracking,loss of standard is forbidden.There have been currently many preparation methods.In this study,two preparation methods were selected and compared.Their expanded uncertainties were obtained.
(1)At current,the volume of organophosphorus standard solution is generally higher than 1.0 ml.Before the beginning of preparation,the crystals in standard solution must be cracked.Subsequently,1 ml of original standard solution was transferred with 1 ml graduated pipette and dissolved in acetone to 10 ml.Thus 10 μg/ml of stock solution was prepared. The stock solutions of the 14 organophosphorus pesticides were all prepared like that.Then 0.2 ml of each stock solution was mixed together and diluted to 10 ml.The sampling solution with 0.2 μg/ml of each pesticide was prepared.
(2)Certain amount(1.0 ml)of each pesticide standard solution was transferred accurately with 1 ml graduated pipette and mixed together to 25 ml.Thus the stock solution with 4 μg/ml of each pesticide was prepared. Subsequently,0.5 ml of stock solution was transferred accurately with 0.5 ml graduated pipette and diluted in acetone to 10 ml.The sampling solution with 0.2 μg/ml of each pesticide was also prepared.
(3)The prepared mixed standard solutions were detected with Agilent7890A-FDP Detector(column,DB-1701),which could completely separate the 14 kinds of pesticides.The approximate read of each standard solution was all 0.2 μg/ml.
Sources of Uncertainty
The concentration of each pesticide in the mixed standard solution was calculated according to Eq.(1). The uncertainty of each ingredient in the organophosphorus mixed standard solution mainly came from two parts: the uncertainty of each purchased standard solution itself and the uncertainty caused by two times of dilution[6].
Wherein,C refers to the final concentration of standard solution;C0refers to the concentration of purchased standard solution;V1refers to the volume of transferred standard solution;V2refers to the volume of transferred stock solution;V3refers to the final volume of transferred stock solution is diluted to;V4refers to the volume of prepared sampling solution.
Assessment of Uncertainties from Various Sources
Relative uncertainty assessment of each ingredient in the mixed standard solution caused by its own original concentration
The uncertainty of each pesticide can be obtained from the product instruction(Table 1).The relative uncertainty of each ingredient in the mixed standard solution was calculated according to Eq.(2)[7].
Wherein,u (c0)refers to the uncertainty described in the instruction;c0 refers to the concentration of standard solution;k refers to expanded factor.
Uncertainty of two times of dilution
The relative uncertainty from volumetric flasks and pipettes consists of three parts.
Calculation of uncertainty (u1rel)from volume scales In terms of relative uncertainty from volume scales[8],the tolerances of glass apparatus were first obtained through referring to verification regulations of common volumetric glassware (JJG246-2006)[3]. According to rectangular distribution,the relative uncertainties of glass apparatus were calculated according to Eq.(3)(Table 2).
Wherein,u1(v)refers to the tolerance of glass apparatus,ml;v refers to the capacity of glass apparatus,ml.

Table 1 Relative uncertainties of 14 organophosphorus pesticides in the mixed standard solution caused by their own original concentrations
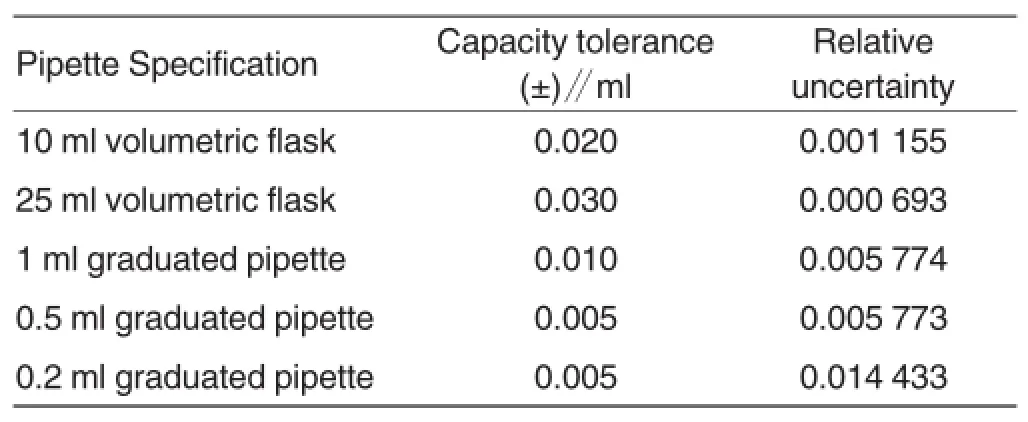
Table 2 Relative uncertainties from volume scales

Table 3 Uncertainty from difference between solution temperature and calibrated temperature
Calculation of relative uncertainty(u2rel)from temperature change
The temperature inside laboratories is required to be 20℃.Once the temperature changes,the volume of acetone will also change.Thus the volume expansion coefficient is needed.The volume expansion coefficient of acetone is 0.001 49 ml/℃.During the preparation,the acetone temperature was 23℃.So the uncertainty(rectangular distribution)of volumetric flask caused by temperature change wascalculated as follows:
0.00149(volume expansion coefficient of acetone)×3(temperature difference)×10 (capacity of glassware)=0.044 7 ml.

Table 4 Relative uncertainty of approximate read

Table 5 Combination of relative uncertainties from different sources
According to Eq.(4),the uncertainty caused by difference between solution temperature and calibrated temperature was calculated(Table 3).
Wherein,u2(v)refers to the change in volume,ml;v refers to the capacity of glass apparatus,ml.
Calculation of relative uncertainty(u3rei)of reading staff The approximate read of the same volumetric glassware by different staff may be different,resulting in the generation of different error.Under the same conditions,the same volumetric flask filled with acetone to the maximum scale was red by two different people respectively.The reads were first calibrated by temperature,and then their repeatability standard deviation was calculated.According to Eq.(5),the relative uncertainty of glass apparatus caused by approximate read was calculated(Table 4).
Wherein,u3(v)refers to the standard uncertainty of approximate read of glass apparatus,ml;v refers to the capacity of glass apparatus,ml.
Combined uncertainty of glass apparatus
During the dilution from standard solution to sampling solution,the uncertainties from various sources of various glass apparatus were calculated.Then the combined uncertainty of each glass apparatus was calculated in according to Eq.(6)(Table 5).
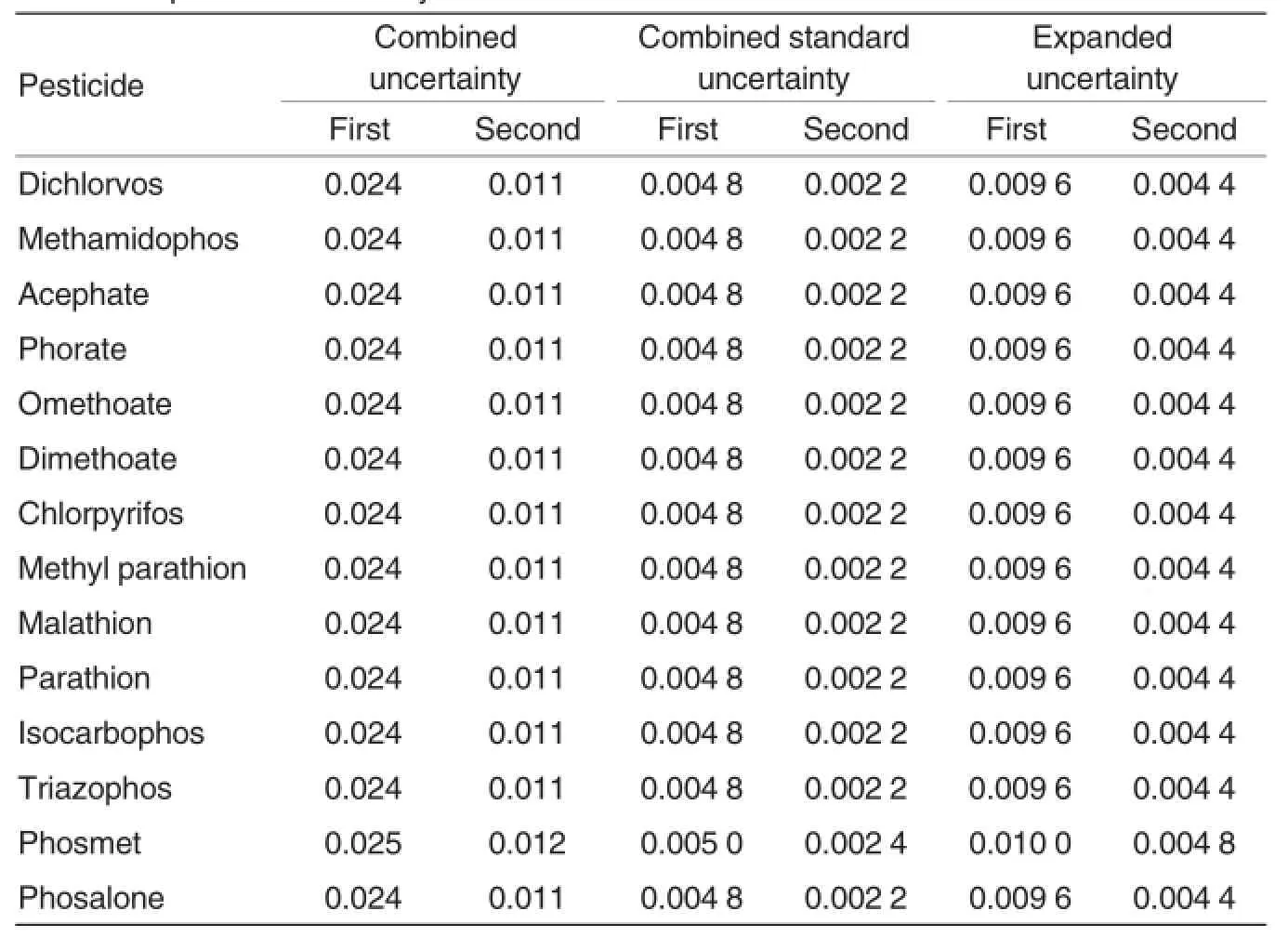
Table 6 Expanded uncertainty of each standard solution

Table 7 Final concentration of each standard solution
Expanded Uncertainty
According to analysis and calculation above,the uncertainties from different sources were calculated first.Then the combined uncertainties of the mixed standard solutions prepared by two different methods were calculated.In the first preparation method,10 ml volumetric flask was used twice;1 ml graduated pipette was used once;0.2 ml graduated pipette was used once.The uncertainty of mixed standard solution consisted of the uncertainty of each ingredient in the mixed standard solution and the uncertainties of various glass apparatus used in the preparation (Eq.(7)). The combined uncertainties of mixed standard solutions prepared by the two methods were calculated according to Eq.(8).Under conditions of normal distribution,if the coverage factor(k)wasassigned to be 2,the expanded uncertainties of the two mixed standard solutions could be calculated according to Eq.(9)(Table 6).
Final Concentrations
Based on the combination of uncertainties of preparation and dilution,the final concentrations the two mixed standard solution were obtained.As shown in Table 7,the second method was significantly better than the first one.In the first preparation method,10 ml volumetric flask was used twice;1 ml graduated pipette was used once;0.2 ml graduated pipette was used once;while in the second method,25 ml volumetric flask was used once;10 ml volumetric flask was used once;1 ml graduated pipette was used once;0.5 ml graduated pipette was used once.In addition to difference in used pipettes,the stock solutions of the two preparation methods were also different.One was monostandards,while the other was mixed standard.Therefore,the precise pipettes are recommended in the preparation of mixed standard solution with stock solution.
Discussion
The detection of pesticide residues in agricultural products has attracted much attention from researchers and detectors[8-15].However there are rare reports on uncertainty assessment of organophosphorus pesticide standards[3,9].The solid monostandards are commonly used in the past.At current,the liquid standards are often used in the detection of pesticide residues.The uncertainty assessment of mixed standard solutions consisting of various liquid organophosphorus standards is also reported rarely in China.The previous studies on uncertainty assessment of preparation of pesticide standards mostly conclude that the precise pipettes should be used in the preparation of mixed standards.But they don’t pay their attention to the procedures of the preparation.The precision of pipettes produces greater impact on detection results.However,for the detectors,it is unchangeable.Instead,the uncertainty improvement of standard solutions through comparing preparation methods is more practical for detectors.
References
[1]WANG JL(王洁莲),DONG L(董琳),CHANG H(常宏),et al.Analysis of and strategies for pesticide residue situation in vegetables in Shanxi,2010(2010年山西省蔬菜农药残留情况分析及应对策略)[J].Agricultural Technology&Equipment(农业技术与装备),2011,18:24-25.
[2]State Bureau of Technical Supervision(国家技术监督局).JJF1059-2011 E-valuation and Expression of Uncertainty in Measurement(Revised)(JJF1059-2011测量不确定度评定与表示 (修订稿))[S].Beijing:China Standard Press(北京:中国标准出版社),2011.
[3]SUN XM(孙晓梅),LIU CY(刘慈玉).Uncertainty analysis of six organophosphorus pesticides standard solution(6种有机磷农药标准溶液不确定度分析)[J].Chemical Reagent(化学试剂),2011,33(8):733-735.
[4]Environmental Quality Supervision and Testing Center(Tianjin)of Ministry of Agriculture,Institute for Environmental Protection and Research of Ministry of Agriculture(农业部环境质量监督检验测试中心(天津),农业部坏境保护科研监测所).NY/T 761-2008 Pesticide multiresidue screen methods for determination of organophosphorus pesticides,organochlorine pesticides,pyrethroid pesticides and carbamate pesticides in vegetables and fruits(NY/T 761-2008蔬菜和水果中有机磷、有机氯、拟除虫菊酯和氨基甲酸酯类农药多残留的测定)[S].Beijing:China Agriculture Press(北京:中国农业出版社),2008.
[5]ZENG Y(曾艳),LANG H(郎红),SHAO H(邵辉),et al.Uncertainty evaluation of preparation of organ chlorine pesticides mixed standard solution(有机氯农药混合标准溶液配制不确定度评定)[J].Pesticide Science and Administration(农药科学与管理),2013,3,4(8):37-41.
[6]General Administration of Quality Supervision,Inspection and Quarantine(国家质量监督检验检疫总局).JJG196-2006 Verification Regulation of Working Glass Container(JJG196-2006常用玻璃量器检定规程)[M].Beijing:China Metrology Press(北京:中国计量出版社),2007.
[7]China National Accreditation Board for Conformity Assessment(中国合格评定国家认可委员会).CNAS-GL06 Guidance on Evaluating the Uncertainty in Chemical Analysis(CNAS-GL06化学分析中不确定度的评估指南)[M].Beijing:China Metrology Press(北京:中国计量出版社),2006.
[8]PENG HJ(彭慧莲),CHENG XJ(成秀娟),XU WS(徐伟松),et al.Analysis of uncertainties in determination of four pesticide residues in vegetables by gas chromatography(蔬菜中毒死蜱等几种农药残留量测定不确定度分析)[J].Pesticide Science and Administration(农药科学与管理),2012,4:35-39.
[9]JIANG YX(蒋永祥),YE L(叶丽).Uncertainty evaluation of determination of organophosphorus pesticide multiresidues in tea(茶叶中多种有机磷农药残留量测定不确定度的评定)[J].Analytical Laboratory(分析实验室),2006,12: 54-57.
[10]WANG LJ(王立君),YANG T(杨挺),HUANGFU WG(皇甫伟国).Uncertainty evaluation for determinating pyrethroids residues in vegetables by GC(气相色谱(GC)测定蔬菜中拟除虫菊酯类农药残留量的不确定度计算与分析)[J].Chinese Agricultural Science Bulletin(中国农学通报),2009,7:219-222.
[11]HAN HX(韩红新),SUN CP(孙翠平),WU LY(吴莉宇),et al.Measurement uncertainty in determination of pesticides testing(测量不确定度在农药残留分析测试中的应用)[J].Shanghai Measurement and Testing(上海计量测试),2008,1:15-18.
[12]GONG J(龚剑),ZHAN YG(占永革). Uncertainty evaluation of dilution of standard solution(标准溶液稀释不确定度评定)[J].Experimental Technology and Management(实验室技术与管理),2011,5(28):52-54.
[13]MA LS(马丽莎),ZHENG GM(郑光明). Uncertainty evaluation for determination of chloramphenicol residue in aquatic products by gas chromatography(气相色谱法测定水产品中氯霉素的不确定度分析)[J].Chinese Fishery Quality and Standards(中国渔业质量与标准),2013,4:15-20.
[14]LONG Y(龙阳),MA XH(马新华),HOU CL(侯翠丽),et al.Application of uncertainty evaluation in determination of moisture in rapeseed seeds(不确定度评定在油菜籽水分测定中的应用)[J]. Grain and Oil Storage Technology Newsletter(粮油仓储科技通讯),2013,6:44-45.
[15]ZHANG QL(张青龄).Evaluation of uncertainty in determination of cadmium in rice(大米中镉含量的测量不确定度评定)[J].Fujian Analysis&Testing(福建分析测试),2014,1:59-62.
Responsible editor:Tingting XU
Responsible proofreader:Xiaoyan WU
有机磷农药混合标准溶液配制不确定度评价
王洁莲*,闫 征 (山西省农产品质量安全检验监测中心,山西太原030025)
标准溶液的不确定度对农药残留检测起着非常重要的作用,影响着检测结果的准确性。笔者以实际应用的14种有机磷农药混合标准溶液为例,采用配制混合标准溶液过程单元操作的不确定度计算方法(top down),分析了在配制混合标准溶液过程中的所有影响因素,得到了最终的扩展不确定度。最终结果显示使用精准的移液器将储备液配置为混合标液,得到最终的扩展不确定度相对小。
有机磷农药;混合标准溶液;配制稀释;不确定度;评价
山西省科技攻关项目(20130310011-4)。
王洁莲(1980-),女,山西临猗人,高级农艺师,研究生,研究方向:农产品中农药残留检测。*通讯作者,E-mail:agilent2006@163. com。
2014-12-16
2015-01-26
Supported by Key Science and Technology Program of Shanxi Province,China(20130310011-4).
.E-mail:agilent2006@163.com
December 16,2014Accepted:January 26,2015
猜你喜欢
杂志排行
Agricultural Science & Technology的其它文章
- Characteristics of Pollen Germination and Storage of Zygocactus truncates
- lnfluence of a New Nano-PE-film on the Greenhouse Environmental Factors
- Study on Photosynthetic and Physiological Effects of a Novel Adaxially-Rolled Character in Rice(Oryza sativa L.)
- Effects of Mutagenesis by UV lrradiation and60Co-γ lrradiation on Fermentation of Xylose to Ethanol by Pichia stipitis
- Effects of-glucosidase lnhibitors on the Function of Mulberry Leaf Extract as an Additive in Feedstuff
- Effects of Penicillium spp.and Trichoderma spp. on Pleurotus ostreatus Growth and Screening of Effective Disinfectants
