Immunomodulatory effect of garlic oil extract on Schistosoma mansoni infected mice
2015-10-31ReemKamelNashwaElShinnawy
Reem O. A. Kamel, Nashwa A. El-Shinnawy
Department of Zoology, Women College for Arts, Science and Education, Ain Shams University, Asmaa Fahmey St., Heliopolis, Cairo 11757, Egypt
Immunomodulatory effect of garlic oil extract on Schistosoma mansoni infected mice
Reem O. A. Kamel, Nashwa A. El-Shinnawy*
Department of Zoology, Women College for Arts, Science and Education, Ain Shams University, Asmaa Fahmey St., Heliopolis, Cairo 11757, Egypt
ARTICLE INFO
Article history:
in revised form 20 October 2015
Accepted 3 November 2015
Available online 20 December 2015
Schistosoma mansoni Garlic oil extract
Worm load
TNF-α
ICAM-1
Objective: To assess the effect potency, and the immunomodulatory response of garlic oil extract in enhancing the host's immune system against the disorders caused by Schistosoma mansoni (S. mansoni) in mice at different stages of worm maturation. Methods: A total of 70 male CD-1 Swiss albino mice were divided into 7 groups. Group Ⅰ: healthy control. Group Ⅱ:garlic oil group orally administrating 100 mg garlic oil extract /kg b.wt. 3 d a week for 6 weeks. Group Ⅲ: infected with S. mansoni cercariae and left untreated for 42 d. Group Ⅳ: treated with garlic oil extract from day 1 to day 7 post infection (PI). Group Ⅴ: treated with garlic oil extract from day 14 till day 21 PI. Group Ⅵ: administrating garlic oil extract from day 35 until day 42 PI. Group Ⅶ received oil extract from the first day of infection for 42 d. Results:Garlic oil extract showed changes in the parasite tegument with a significant decrease in worm burden, hepatic and intestinal ova count with a decline in granuloma number and diameter. These alterations were accompanied with a reduction in serum TNF-α, ICAM-1, IgG and IgM after 7 and 42 d post S. mansoni cercarial infection. Conclusions: Results obtained confirmed the effect of garlic oil extract on the larval and mature stage of the parasite and in enhancing the host's immune system against the disorders caused by S. mansoni in mice.
Document heading doi: 10.1016/j.apjtm.2015.11.016
1. Introduction
Parasitic helminths of genus Schistosoma are the causative agents of schistosomiasis, an infectious disease affecting humans and animals. Schistosomiasis has attracted increased focus and funding for control[1]. Schistosomiasis tops all the endemic parasitic diseases world-wide particularly in Egypt[2].
Studies on the relationship of the disease and immune response in schistosomiasis during the acute phase of the infection demonstrated that the pathology caused by the blood fluke Schistosoma mansoni(S. mansoni) is induced by the host's granulomatous response to eggs deposited in the liver and the intestines[3] which is maximal bythe 8th week of infection. The toxic egg material destroys the host tissue cells and the antigenic material stimulates the development of large inflammatory reactions (granuloma) around the egg[2].
Cytokines have a major role in the development of pathology and resistance to infection. There are different outcomes that are determined by a balance between different immune responsesmodulated by certain cytokines-which are directed both against larval and adult stages of the parasite, as well as parasite eggs trapped in the tissues[4].
Eggs trapped in the pre-sinusoidal portal venules secrete soluble egg antigens which are taken up by macro-phages. Subsequently,macrophages stimulates T helper cells to secrete tumor necrosis factor-α (TNF-α), which in turn drive a cell-mediated response and attract more immune cells around the ova. As the granuloma becomes more organized, the T helper cells, produce different interleukins completing granuloma maturation towards the late stage of granuloma formation[4]
Besides, Intracellular adhesion molecule-1 (ICAM-1) which ispresent on endothelial cells, antigen presenting cells and fibroblasts plays an important role in inflammatory and immunological responses. It has been focused on the interplay between ICAM-1 expression and S. mansoni induced granulomogenesis[5].
TNF-α triggers the release of ICAM-1 which plays key role in early granuloma formation and aggravation of hepatic fibrosis[6]. In addition, ICAM-1 expression is induced by products of deposited S. mansoni eggs[7]. Also, immunoglobulins IgG and IgM have been shown to have a pivotal role in the humoral response to schistosomal infection. High levels of IgG have been associated with periportal fibrosis and portal hypertension in patients with advanced schistosomiasis mansoni[8].
Due to the lack of a vaccine, patient therapy is heavily reliant on chemotherapy with praziquantel[9], but concerns over drug resistance and possible reoccurrence of infection encouraged the search for new drug from natural resources[10]. Nowadays, there is an increased demand for using plants in therapy "back to nature" instead of using synthetic drugs which may have adverse effects that may be more dangerous than the disease itself[11].
Additionally, a comprehensive knowledge of tegumental components would be helpful in the development of new drugs. Thus the importance of studying the tegument of schistosomes arises because it acts as an interface between the parasite and its environment in the host. This interface is used to evade the immune responses of the host[12].
Ancient Egyptians realized the benefits of garlic as a folk remedy for a variety of ailments. Garlic has been shown to have plentiful medicinal effects[13]. Lately, the anthelmintic effect of garlic has been a matter of interest for researchers[14]. Moreover, garlic (Allium sativum) has been described by having some immunomodulatory activity against parasities. The therapeutic effect of garlic extract may be maintained by increasing phagocytosis along with killing the parasite by macrophages in vivo[15].
It is[16] also reported that treatment with garlic greatly improved the antioxidant status in S. mansoni infection with a noticeable reduction in worm burden .and egg load by acting upon improving the immunological host system. One of the main immunonological responses of garlic administration is the decrease of lipopolysaccharide induced proinflammatory cytokines, such as TNF-α[17].
Considering the promising activities of garlic oil extract as anti parasite, this work was carried out to assess the effect potency, and the immunomodulatory response of garlic oil extract in enhancing the host's immune system against the disorders caused by S. mansoni in mice at different stages of worm maturation.
2. Materials and methods
2.1. Experimental animals
Male CD-1 Swiss albino mice [weight, (20 ± 2) g], were bred and maintained under conventional conditions at the experimental animal research unit of the Schistosome Biological Supply Program at Theodor Bilharz Research Institute (Giza, Egypt). They were fed a standard commercial pellet diet. They were given carrot, lettuce and milk as source of vitamins and water and were monitored daily for health status. The animal experiments were carried out according to the internationally valid guidelines in an institution responsible for animal ethics (Theodor Bilharz Research Institute).
2.2. Infection of animals
S. mansoni cercariae were provided by the Malacology Laboratory of Schistosome Biological Supply Program, Theodor Bilharz Research Institute, where laboratory-bred Biomphalaria alexandria were maintained. Infection was subcutaneously injected using freshly shed (60 ±10) cercariae to each mouse[18].
2.3. Drugs
Each capsule contains 10 mg/ kg of concentrate pure garlic oil which equivalent to 1 000 mg of fresh garlic bulb. Manufactured by The Vitamin Shoppe Co. U.S.A. Garlic oil extract was given to mice in a dose of 100 mg/kg b.wt. by the method described before[19]using an oesophageal tube 3 d a week for 6 weeks (42 d) according to the following experimental design.
2.4. Experimental design
Animals were divided into seven groups, each group of 10 mice. Group Ⅰ: healthy control. GroupⅡ: garlic oil group orally administrating 100 mg garlic oil extract /kg b.wt. 3 d a week for 6 weeks. Group Ⅲ: infected with S. mansoni cercariae and left untreated until the end of the experiment (42 d). Group Ⅳ: treated with garlic oil extract from day 1 to day 7 post infection (PI). GroupⅤ: orally treated with garlic oil extract from day 14 till day 21 PI. Group Ⅵ: administrating garlic oil extract from day 35 until day 42 PI. While the last group (Ⅶ) received oil extract from the first day of infection till the end of the experiment (42 days). All mice were necropsied 42 days after cercarial exposure and worms were recovered from the portal system and mesenteric veins by perfusion technique as explained earlier[20]. The worms were classified according to sex and counted. Adult male worms were prepared for scanning electron microscopic examination. The number of eggs/ g tissues (liver and intestine) were assessed following digestion with 4% KOH as expressed before[21]. The percentage of egg developmental stages (Oogram pattern ) was examined earlier[22].
2.5. Histopathology
Sampling slices from the liver tissue were taken from mice and fixed in 10% formalin. Paraffin sections (4 μm thickness) were stained with Ehrlich's haematoxylin and eosin (H&E)[23]. Theassociated histopathological changes were observed. Granuloma number and diameter were measured using an ocular micrometer[24].
2.6. Scanning electron microscope
Adult worms of S. mansoni were washed several times with normal saline and then fixed in 2.5% gluteraldehyde and dehydrated by serial dilution of ethanol using automatic tissue processor (Leica EM TP). Then the samples was dried using CO2critical point drier (Tousimis Audosamdri-815). Specimens coated by gold sputter coater (SPI- Module) and examined with scanning electron microscope (JEOL- JSM-5500 LV) by using high vacuum mode at the Regional Center of Mycology and Biotechnology, El Azhar University, Cairo, Egypt.
2.7. Serum sample preparation for immunological studies
Blood was collected from mice in different groups by heart acupuncture. The sera were separated, centrifuged at 2 000 rpm for 15 min and stored in aliquots at -20 ℃ for serum immunological studies.
2.9. Determination of serum immunoglobulins (total IgG and IgM) levels using ELISA test
Determination of anti-SEA immunoglobulin IgG and IgM were measured using indirect ELIZA, as based previously[26].
96-well microtiter plates (Costar, Corporate Headquarters,Cambridge, MA, USA) were coated with 100 μL/well of 30 μg/mL of SEA. Sera were added individually as 100 μL/well at dilution 1:250. All samples and standards with known concentrations were tested in duplicate and the optical density (OD) was read with micro plate ELlSA-reader laboratory set to 490 nm. A curve was drawn from the standards then from this curve. The concentrations of total IgG and IgM were calcuated in ng/mL.
2.10. Statistical analysis
Data were analyzed by comparing values of different garlic oil extract treated groups with the values of individual controls. The significant differences among values were analyzed using analysis of variance (one-way ANOVA) using SPSS 17.0.coupled with posthoc least significance difference (LSD). The data were considered significantly different if P<0.05.
2.8. Determination of serum TNFα and ICAM-1
Briefly, the concentrations of mouse TNF-α and ICAM-1 in serum samples were determined by a sandwich ELISA Kit(Thermo Scientific). The assays were performed as suggested by the manufacturer as mentioned before[25].
All serum samples were measured in duplicate. Sample dilutions were 1:100, and 1:50 for s ICAM-1 and s TNF-α respectively. The absorbance of samples was measured on an ELISA plate reader set at 450 nm. The mouse s ICAM-1 and s TNF-α were calculated in pg/mL using the standard curve drawn for the different standard concentrations.
3. Results
3.1. Parasitological results
Table 1 showed the worm burden and Table 2 indicated the percentage of egg developmental stages and ova count/g tissues(hepatic and intestine) in infected and treated mice with garlic oil extract at different time intervals respectively. Oral administration of garlic oil showed a significant reduction in worm burden (32.29% and 39.83%) which coincide with a decrease in the percentageof ova count (26.86%&39.38%) and also increase in the number of dead eggs (22.00±0.63&30.00±1.85) in groups treated with garlic from 1 to 7 d PI and from 1 to 42 d PI, when compared with their corresponding infected untreated group. Reduction in these parameters between the animals treated with garlic oil extract in groups (Ⅴ&Ⅵ) and the corresponding infected control group was insignificant.

Table 1 Effect of garlic oil extract on worm burden in S.mansoni infected mice.

Table 2 Effect of garlic oil extract on the oogram pattern and ova count/g tissues (hepatic and intestine) in S.mansoni infected mice.
3.2. Histopathological studies
Histopathological studies verified the parasitological results. Figure 1A showed a liver of control mice which contain a normal structure of central vein, surrounded by hepatocytes. The number of granuloma was significantly decreased by 25.14% and 35.90% in groups (Ⅳ& Ⅶ), respectively, while, its diameters decreased by 22.58% and 30.32%, respectively related to infected untreated mice. Whereas, no significant change in both granuloma number and diameter in groups (Ⅴ&Ⅵ) compared to infected untreated group(Table 3). Treatment with garlic oil extract at different intervals recorded a modulation in the hepatic granuloma with a trapped or disintegrating central Schistosoma ova (Figures 1 B, C, D, E &F)compared to infected untreated group (Figure 1A).
3.3. Electron microscopy investigations
In infected untreated group the dorsal surface of the tegument of S. mansoni male worm was moderately rough covered with numerous tubercles, bearing directed spines. Between the tubercles the tegumental surface was composed of tegumental ridges (Figure 2A). The male worm showed changes in the tubercles, namely swelling and most of the tubercles are without spines however, some of them bearing spines with a few blebs around the tubercles in mice treated with garlic oil extract from 1to 7 d PI (Figure 2B). Administration of garlic oil extract from1-42 d PI led to a pronounced change in the aspect of the tubercles which often appeared collapsed, wrinkled,reduced in size and showed spine loss (Figure 2C). On the other hand, groups (Ⅴ&Ⅵ) did not show any sign of a change in the tegument compared to infected untreated group.

Table 3 Effect of garlic oil extract on mean granuloma number and diameter in S.mansoni infected mice.
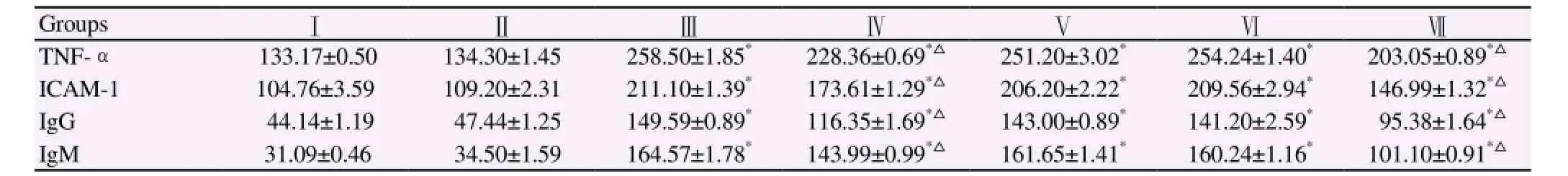
Table 4 Serum TNFα , ICAM-1 in pg/mL and serum IgG and IgM in ng/mL in different experimental groups.
3.4. Immunological results
Table 4 revealed insignificant changes in serum TNF-α, ICAM-1 and serum IgG and IgM levels in normal healthy mice or garlic oil extract administrated mice. On the other hand, mice infection with S. mansoni showed a significant (P<0.001) increase in serum TNF-α,ICAM-1, IgG and IgM recording mean values of 258.50 pg/mL,211.20 pg/mL, 149.59 ng/mL, 164.57 ng/mL, respectively as compared to normal control group. Whereas significant (P<0.001)reduction in the mean values of serum TNF-α, ICAM-1 were detected to be 228.36 pg/mL and 173.61 pg/mL, respectively after 7 d of garlic oil extract treatment and 203.05 pg/mL and 146.99 pg/mL after 42 d of garlic oil extract treatment when compared to Schistosoma infected mice. Again, significant amelioration was noticed in serum IgG, IgM denoting the highest improvement after 42 d post garlic treatments with mean values of 95.38 ng/mL and 101.10 ng/mL respectively. In contrast, infected mice treated with garlic oil extract from day 14 to day 28 or from day 35 to 42 PI didn't show a significant change in all the above mentioned parameters.
4. Discussion
The search for bioactive plants which can be used as nonconventional anthelmintics has received considerable attention in recent times because of the increasing, worldwide development of resistance to chemical anthelmintics in worm populations[27]. In the last decades, plant extracts were widely used for the treatment of S. mansoni infection[28]. This work was carried out to assess the effect and potency of garlic oil extract in treating S. mansoni. In the current study a significant decrease in worm load (26.86% and 39.28%) in groups infected and treated with garlic oil extract in a dose of 100 mg/kg b.wt. from 1 to 7 d PI and 1-42 d PI respectively, compared to infected untreated animals. The antiparasitic mode of action of garlic is made by enhancing the immunity of the host to attack the parasite[2]. This assumption is thought to be due to the antioxidative properties of aqueous garlic extract in Schistosoma infected mice.
In this study, there was a significant increase in the number of dead eggs accompanied with a significant reduction in hepatic and intestinal egg load in mice treated with garlic oil extract either for 7 or 42 d post infection compared to infected untreated group. The effect of garlic may not be on ovum itself, since no effective antischistosomal drugs acts on the eggs themselves. Eggs continue their development in the tissue until maturation, then the mature ova remain alive in the tissue till their death and eliminated in the stool. Accordingly, the significant reduction of ova might be due to the effect of garlic on the reproductive organs of the worms[27].
Moreover, aqueous garlic extract impaired the development and maturity of Schistosoma eggs, as the treatment resulted in the appearance of high numbers of dead eggs in the oogram assessment[2]. This is possibly due to a positive linear relationship between the egg output and the worm burden, where the reduction of the number of worms is correlated with the reduction in the ova count[19]. However, several other factors may explain such reduction in schistosomal egg count. These factors are a probable diminished fecundity of the worm pairs and an increased rate of egg excretion due to the egg death[17].
The clinical stages of the disease schistosomiasis are a direct result of an immunopathological reaction to the deposition of eggs which induces granuloma in the liver of the host with severe liver damage. Granuloma formation is controlled by several, cytokines and cell adhesion factors[29].
Increasing levels of serum TNF-α in Schistosoma infected mice observed in the current study (258.50 pg/mL compared to 133.17 pg/ mL of normal control) has been related to schistosome oviposition and circumoval granuloma formation[30]. TNF-α is a potential marker of disease progression and liver damage[6, 31]
In addition, the current study reveals an increase in the mean value of ICAM-1in the infected group to record (211.10 pg/mL). The T helper cytokines and TNF-α, both trigger the release of soluble ICAM-1. The soluble form of ICAM-1 is involved in lymphocyte and eosinophil recruitment and inflammatory, immune-mediated mechanisms causing the raised levels of s ICAM-1 in schistosoma infected mice[32].
Moreover, the significant elevation in immunoglobulins in schistosomaisis was attributed to the decrease in reactive oxygen species scavenging capacity by antioxidants defenses from infected hepatic cells increasing constant oxidative stress and oxidation of lipids, protein and other macromolecules such as DNA[17].
The results of the current study shows that the therapy with garlic oil extract for 7 d and 42 d PI significantly decreased TNF-α and ICAM-1 to record 228.36 pg/mL and 203.05 pg/mL for TNF-α and 173.61 pg/mL and 146.99 pg/mL respectively compared to infectedmice. This study confirmed the decline in TNF-α because garlic derived compounds inhibits the transcription of TNF-α involved in proinflammatory responses. This in turn reduces the damage in the hepatic tissue of hosts infected with S. mansoni[33]. In addition,allicin, a component of garlic extract modulates the expression of ICAM-1[32]
These antischistosomal effects of garlic may be attributed to its immunomodulator fraction, which affects the course of infection and shifts the cytokine pattern from T helper-lymphocytes mediated immune response, responsible for granuloma formation to T helper lymphocytes mediated immune responses, responsible for immune resistance[34].
Also, activated macrophages are essential for host survival during S. mansoni infection; and in their absence, the egg induced inflammatory response is lethal[35].
Garlic oil extract can directly or indirectly stimulate macrophages nitric oxide production in an additive manner. This in turn generates an antifibrogenic substance inside the schistosome granuloma decreasing both granuloma number and size which considered a major pathway in the induction of protective immunity against this disease[36].
Histopathological examination of treated mice with garlic oil extract for 7 and 42 d PI denoted a significant decrease in both granuloma number and diameter accompanied with a partial histological recovery.
In accordance with this, the infiltration of circulating fibroblasts into granulomas may be important for attracting lymphocytes as well as forming collagen, the fibrinolytic effect of garlic may possibly explain the reduction in the diameter and cellularity of the granuloma[14].
Many functions and features of the tegument of S. mansoni have raised the importance of studying it. Since it acts as an interface between the parasite and its environment in the host[12]. The ultrastructural evaluation was performed on male specimens for two reasons: females are not in frequent contact with the host microenvironment. Also, studies in the literature have shown that soft tissue alterations are more pronounced in males than in females[37]. Therefore, the present study examined the surface topography of only male worms. The male worm showed changes in the tubercles, namely swelling and most of the tubercles are without spines however, some of them bearing spines with a few blebs around the tubercles in mice treated with garlic from 1 to 7 d PI. Administration of garlic oil from 1-42 d PI led to a pronounced change in the aspect of the tubercles which often appeared collapsed,wrinkled, reduced in size and number and showed spine loss. This confirms that the treatment with garlic or its extract allicin resulted in various ultrastructural alternations in the tegument of the surviving worms[38]. The most prominent damage observed in this study was in the tegument of garlic treated worms in the form of oedema and blebbing. From the above discussion it seems that the alterations on the tegument will lead to the disappearance of the immunological disguise of the worm. Thus, it could be easily attacked by the host's immune system[12]. Moreover, such tegumental alterations induced by garlic could probably be exerting a profound effect on the worm's metabolism and consequently resulting in their death[38].
Hence, the day 7 post cercarial infection was our concern. We herein explored the immune response during the critical lung larval stage of schistosomiasis infection. At this phase, after 7 d our study indicated a significant ameliorative effect of garlic post infection in parasitological, immunological and histopathological results showing that the action of garlic was on the critical larval stage. Schistosomules remain for a period of 3 weeks (wk) of development in the liver transforming into adult worms. At this stage our study indicated no significant changes in the parasitological,histopathological or immunological parameters occurred under garlic oil treatment either from day 14 to day 21 or from day 35 to day 42 post infection. Whereas, when sexual maturity is reached within 25-30 d at the final habitat in the host, mating and pairing occurs at the 5th wk post infection[39]. Then, the mature worms migrate to gut or bladder to lay eggs around 400-500 in S. mansoni. At this stage of worm maturation, the present study exhibited a significant reduction in all above mentioned experimental investigations that is after 42 d of garlic oil administration post cercarial infection. Thus we conclude that the most prominent action of garlic oil extract administration was on the critical larval stage of the parasite (7 d post infection) and after 42 d PI which is suggested to be due to its action on the parasite viability, mobility and fecundity associated with an enhanced immune response of the host towards the parasites.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors would like to thank Theodor Bilharz Research Institute(Giza, Egypt) for their help and support in this study.
[1] Fenwick A, Webster JP. Schistosomiasis: Challenges for control treatment and drug resistance. Curr Opi infect Dis 2006; 19(6) :577-582.
[2] EL-Shenawy NS, Soliman MFM, Reyad SI. The effect of antioxidant properties of aqueous garlic extract and Nigella sativa as antischistosomiasis agents in mice. Rev Inst Med trop (Sau Paulo) 2008;50(1): 29-36.
[3] De Jesus AR, Silva A, Santana LB, Magalaes A, De Jesus AA, Almeida RP, et al. Clinical and immunologic evaluation of 31 patients with acute Schistosomiasis mansoni. J Infect Dis 2002; 185(1): 98-105.
[4] Olveda DU, Olveda RM, Mc-Manus DP, Cai P, Chau TNP, Lam A, et al. The chronic enteropathogenic disease schistosomiasis. Int J Inf Dis 2014; 28: 193-203.
[5] Zaremba J, Losy J. Adhesion molecules of immunoglobulin gene superfamily in stroke. Folia Morphol (Warsz) 2002; 61(1): 1-6.
[6] Hassanein H, Hanallah S, El-Ahwany E, Doughty B, El-Ghorab N,Badir B, et al. Immunolocalization of intercellular adhesion molecule-1 and leukocyte functional associated antigen-1 in schistosomal soluble egg antigen-induced granulomatous hyporesponsiveness. APMIS 2001;109(5): 376-382.
[7] Mohamed AH, Osman GY, Salem TA, Elmalawany AM. The hepatoprotective activity of blue green algae in Schistosoma mansoni infected mice. Exp Parasitol 2014; 145: 7-13.
[8] Tawfeek GM, Alafifi AM, Azmy MF. Immunological indicators of morbidity in human schistosomiasis mansoni: role of vascular endothelial growth factor and anti-soluble egg antigen IgG4 in disease progression. J Egypt Soc Parasitol 2003; 33(2): 597-614.
[9] Abdulla MH, Lim KC, Sajid M, McKerrow JH, Caffrey CR. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLoS Med 2007; 4(1): e14.
[10] Abebe F. Novel antischistosomal drugs from medicinal plants. In:Walson RR, Preedly VR, editors. Botanical medicine in clinical practice. Cambridge. CABI; 2008, p. 175-183.
[11] Mady NI, Allam AF, Salem AI. Evaluation of the addition of Nigella sativa oil triclabendazole therapy in the treatment of human fascioliasis. J Egypt Pharmacol Exp Ther 2001; 20: 807-827.
[12] Xiao S, Shen B, Utzinger J, Chollet J, Tanner M. Ultrastructural alterations in adult Schistosoma mansoni caused by artemether. Mem Inst Oswaldo Cruz 2002; 97(5): 717-724.
[13] Riad NHA, Taha HA, Mahmoud YI. Effects of garlic on Schistosoma mansoni harbored in albino mice : Molecular characterization of the host and parasite. Gene 2013; 518(2): 287-291.
[14] Alghabban AJM. Garlic treatment reduces granuloma and P53 expression in experimental schistosomiasis. Int J Life Sci 2014; 3(1): 5-10.
[15] Arreola R, Quintero-Fabián S, López-Roa RI, Flores-Gutiérrez O,Reyes-Grajeda JP, Carrera-Quintanar S, et al. Immunomodulation and anti-inflammatory effects of garlic compounds. J Immunol Res 2015; ID 401630. DOI: 10.1155/2015/401630.
[16] Metwally NS. Potency of Allium sativum and Allium cepa oils against Schistosoma mansoni infection in mice. Egypt J Hosp Med 2006; 23: 319-332.
[17] Mantawy MM, Ali HF, Rizk MZ. Therapeutic effects of Allium sativum and Allium cepa in Schistosoma mansoni experimental infection. Rev Inst Med Trop (Sao Paulo) 2011; 53(3): 155-163.
[18] Peters PA, Warren K S. A rapid method of infecting mice and other laboratory animals with Schistosoma mansoni: subcutaneous injection. J Parasitol 1969; 55: 558.
[19] Riad NH, Fares NH, Mostafa OM, Mahmoud YI. The effect of garlic on some parasitological parameters and on hepatic tissue reactions in experimental schistosomiasis mansoni. J Appl Sci Res 2007; 3: 949-960.
[20] Smithers SE, Terry R J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and recovery of worms. Parasitol 1965; 55: 695-700.
[21] Cheever AW, Anderson LA. Rate of destruction of Schistosoma mansoni eggs in the tissues of mice. Am J Trop Med Hyg 1971; 20(1): 62-68
[22] Pellegrino J, Oliveira CA, Faria J, Cunha A. New approach to the screening of drugs in experimental schistosomiasis mansoni in mice. Am J Trop Med Hyg 1962; 11: 201-215.
[23] Bancroft JD, Stevens A. Theory and practice of histological technique. 3rd ed. Churchill Livingstone. Edinburgh, London; 1990.
[24] Mahmoud AA, Warren KS. Anti inflammatory effects of tartar emetic and niridazole suppression of schistosoma egg granuloma. J Immunol 1974; 112: 222-228.
[25] Peristin C, editor. A practical guide in immunology. NewYork: Academic Press; 1987, p71.
[26] Engvall E, Perlman P. Enzyme linked immunosorbent assay (ELISA)quantitative assay of immunoglobulin G. Immuno Chem 1971; 8: 871-874.
[27] El-Kott AF, Mohammed RT, Ismail NR. Efficacy of garlic and mirazid in treatment of the liver granuloma in mice infected with Schistosoma mansoni. Res J Parasitol 2011; 6(5): 151-159.
[28] Molgaard P, Nielsen SB, Rasmussen DE, Drummond RB, Makaza N, Andreassen J. Antheminthic screening of Zimbabwean plants traditionally used against schistosomiasis. J Ethnopharmacol 2001;74(3): 257-264.
[29] Stadecker MJ, Asahi H, Finger E, Hernandez HJ, Rutitzky LI, Sun J. The immunobiology of Th1 polarization in high-pathology schistosomiasis. Immunol Rev 2004; 201: 168-179.
[30] Abdalla A, Sheesha AA, Shokeir M, El-Agrody O, El-Regal ME, Abdel-Khalik MK, et al. Serum intercellular adhesion molecule-I in children with chronic liver disease: relationship to disease activity. Dig Dis Sci 2002; 47(6): 1206-1208.
[31] Haseeb MA, Shirazian DJ, Preis J. Elevated serum levels of TNF-alpha, sTNF-RI and sTNF-RII in murine schistosomiasis correlate with schistosome oviposition and circumoval granuloma formation. Cytokine 2001; 15(5): 266-269.
[32] Rassoul F, Salvetter J, Reissig D, Schneider W, Thiery J, Richter V. The influence of garlic (Allium sativum) extract on interleukin 1 alphainduced expression of endothelial intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Phytomedicine 2006; 13(4): 230-235.
[33] Ho CY, Weng CJ, Hang JJ, Cheng YT, Huang SM, Yen GC. Diallyl sulfide as a potential dietary agent to reduce TNFalpha- and histamineinduced proinflammatory responses in A7r5 cells. Mol Nutr Food Res 2014; 58(5):1069-1078.
[34] Ghazanfari T, Hassan ZM, Khamesipour A. Enhancement of peritoneal macrophages phagocytic activity against Leshmania major by garlic(Allium sativum) treatment. J Ethnopharmacol 2006; 103(3): 333-337.
[35] Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A,Radwanska M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and down modulates T helper 1 responses and immunopathology. Immunity 2004; 20: 623-635.
[36] Keiss HP, Dirsch VM, Hartung T, Haffner T, Trueman L, Auger J,et al. Garlic (Allium sativum L.) modulates cytokine expression in lipopolysaccharide-activated human blood thereby inhibiting NF-kappaB activity. J Nutr 2003; 133(7): 2171-2175.
[37] Mostafa OMS, Soliman MI. Ultrastructural alternations of adult male Schistosoma mansoni harbored in albino mice treated with Sidr honey and / or Nigella sativa oil. J King Saud Univ 2010; 22(3): 111-121.
[38] Lima CMBL, Freitas FI D, Lima de Morais LCS,Cavalcanti1 MGS, DaSilva LF, Padilha RJR, et al. Ultrastructural study on the morphological changes to male worms of Schistosoma mansoni after in vitro exposure to allicin. Rev Soc Bras Med Trop 2011; 44(3): 327-330.
[39] Coon DR. Schistosomiasis: Overview of history, biology, clinic pathology and laboratory diagnosis. Clin Microbiol News Lett 2005;27(21): 163-169.
15 September 2015
Nashwa A. El-Shinnawy, Assistant Professor, Department of Zoology, Women College for Arts, Science and Education, Ain Shams University,Asmaa Fahmy St., Heliopolis, Cairo 11757, Egypt.
Tel: ±2/ 01065393939
Fax: (202)6847824
E-mail: elshinnawy_nashwa@hotmail.com
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Larvicidal activity, inhibition effect on development, histopathological alteration and morphological aberration induced by seaweed extracts in Aedes aegypti (Diptera: Culicidae)
- Human ocular dirofilariasis due to Dirofilaria repens in Sri Lanka
- Childhood brucellosis: Review of 317 cases
- Effect of cyclophosphamide on fungal infection in SLE mice detected by fluorescent quantitative PCR
- Therapeutic effect of okra extract on gestational diabetes mellitus rats induced by streptozotocin
- Effect of low intensity pulsed ultrasound on expression of TIMP-2 in serum and expression of mmp-13 in articular cartilage of rabbits with knee osteoarthritis
