Therapeutic effect of okra extract on gestational diabetes mellitus rats induced by streptozotocin
2015-10-31ZhaoHuaTianFengTaiMiaoXiaZhangQiaoHongWangNaLeiLiChenGuo
Zhao-Hua Tian, Feng-Tai Miao, Xia Zhang, Qiao-Hong Wang, Na Lei, Li-Chen Guo
1Department of Gynaecology and Obstetrics, People's Hospital of Zhengzhou, Zhengzhou, China
2Department of Gynaecology and Obstetrics, Second Affiliated Hospital of Zhengzhou University, Zhengzhou, China
3Department of Gynaecology and Obstetrics, Central Hospital of Shanghai Jiading District , Shanghai, China
Therapeutic effect of okra extract on gestational diabetes mellitus rats induced by streptozotocin
Zhao-Hua Tian1, Feng-Tai Miao1, Xia Zhang2, Qiao-Hong Wang1, Na Lei1, Li-Chen Guo3*
1Department of Gynaecology and Obstetrics, People's Hospital of Zhengzhou, Zhengzhou, China
2Department of Gynaecology and Obstetrics, Second Affiliated Hospital of Zhengzhou University, Zhengzhou, China
3Department of Gynaecology and Obstetrics, Central Hospital of Shanghai Jiading District , Shanghai, China
ARTICLE INFO
Article history:
in revised form 20 October 2015
Accepted 3 November 2015
Okra extract
Gestational diabetes mellitus
Pregnant rats
Antioxidant enzyme
Insulin resistance
Objective: To explore the effect of okra extract on gestational diabetes mellitus (GDM) rats and its probable molecular mechanism. Methods: A total of 30 female SD rats were caged with male rats for pregnancy, 27 pregnant rats were obtained and weighed. The pregnant rats were equally randomized into the control group, GDM group and intervention group. Once the pregnancy was verified, GDM group and intervention group were given 45 mg/ kg streptozotocin by peritoneal injection for inducing GDM, control group was given equal volume of citrate buffer. Once the model was established successfully, intervention group was administered orally the solution containing 200 mg/kg/d okra extract, the other groups were given the diet and water only. On the 19th day of pregnancy, the blood samples and fetal rats of all groups were collected, fetal rats weight and placental weight was recorded and the serum glucose, lipids, serum insulin and C-peptide of pregnant rats before the delivery were determined. Results: The pregnant rats weight before the delivery, fetal rats weight and placental weight of GDM group were lower than control group and intervention group(P<0.05). After the treatment of okra extract, serum glucose and lipids levels of intervention group were both improved significantly (P<0.05), especially, the FBG, HDL, FINS, serum m insulin and hepatic glycogen levels were equivalent to control group (P>0.05). Antioxidant enzymes levels of GDM group in liver and pancreas tissues were lower than the other groups,and after treatment of okra extract, antioxidant enzymes levels in liver and pancreas tissues were equivalent to control group (P>0.05). Conclusions: Okra extract, rich in antioxidant substances, could avoid the excessive consuming of antioxidant enzymes, then, suppresses the oxidative stress and insulin resistance, thereby improving blood glucose level of GDM rats.
Document heading doi:10.1016/j.apjtm.2015.11.002
1. Introduction
Gestational diabetes mellitus (GDM) seriously impairs the health of maternity and infant, and it is also closely related to adverse pregnancy outcome. Mother in the period of high-incidence season of diabetes also pose a significant risk on the growth and development of the fetus[1,2]. The incidence of GDM is increasing year by year and accompanying with advancing maternal age, racial/ethnic disparities, and obesity[3]. It is thought that insulin resistance(IR) is the common pathogenesy of GDM and type 2 diabetes mellitus (T2DM). The increase of IR is more common in the late stage of pregnancy of GDM women [4,5].
Abelmoschus esculentus (L.) Moench. (synonym-Okra) is a flowering plant in the mallow family[6]. This plant has a wide range of medicinal values and has been used to treat many diseases. Some references reported that okra owns the activity of anti-diabetes[7], reducing blood lipid[8] and neuroprotection[9],and it also has the activity of antioxidation in vitro[10]. Zhou et al examined the preventive effect of the total flavone glacosides of Flos Abelmoschus manihot on urinary albumin and glomerulusapoptosis in experimental diabetic nephropathy rats, and they found that the major active constituent of total flavone glacosides of Flos Abelmoschus manihot could significantly mitigate the podocyte apoptosis induced by the advanced glycation end-products and decrease urinary albumin excretion in early-stage diabetic nephropathy[11]. Fan and colleagues[12] found that treatment with extract of okra, isoquercitrin and quercetin 3-O-gentiobioside reduced blood glucose, serum triglyceride, total cholesterol and serum insulin levels and improved glucose tolerance in obese mice, results indicated that okra may serve as a dietary therapy for hyperglycemia and hypertriglyceridemia. Sabitha et al treated the streptozotocin-induced diabetic rats with Abelmoschus esculentus peel and seed powder (AEPP and AESP), they found the significant reduction in blood glucose level and increase in body weight;meanwhile, the blood lipid level also returned to normal level[13].
(4)目前,针对掺砾心墙料的冻融特性的研究较少,冻融循环后引起心墙料的强度与变形、孔隙率的变化、渗透特性的研究需要进一步探讨。
At present, whether okra can be applied to treat GDM has not been reported. In the study, diabetes mellitus (DM) was induced by intraperitoneal injection of streptozotocin in pregnant rats for establishing GDM model. Then, okra extract were given to the GDM rats and then the levels of serum glucose and blood lipids and the content of antioxidant enzymes in liver and pancreas tissues were detected to investigate the therapeutic effect of okra extract on GDM rats and its possible molecular mechanism.
2. Materials and methods
2.1. Establishment of GDM model
A total of 30 female SD rats and 15 male SD rats weighing 180-200 g were selected. Rats were adaptively feeding with standard diet, and the rats body weight and serum glucose level were determined (If the serum glucose level was greater than 7.0 mmol/L, the rats were excluded). Vaginal smears were collected daily for estrous cycle determination, and then the estrous rats were paired with male rats without diabetes mellitus according to the rate 2:1 for conception. On the following morning, the appearance of mucus plug or sperm observed by microscopy meant being pregnant for 0 d. Then the pregnant rats were labeled and separated. After one week of mating,unpregnancy rats were abandoned. Then, 27 pregnant rats were achieved and they were equally randomized into control group,GDM group and intervention group.
Once the rats were identified as fertilization, rats in GDM group and intervention group were fasting for 12 h. Then, rats in both groups received the single intraperitoneal injection of fresh 2% streptozotocin solution (prepared with citrate buffer, 0.1 mol/L, pH 4.4) at dose of 45 mg/kg, while rats in control group received equal volume of citrate buffer. Seven days after streptozotocin injection,rats with high blood glucose levels in the range of 13.9-22.2 mmol/L were considered as DM model rats. After the model was established successfully, rats of intervention group were administered orally solution containing 200 mg/kg/d okra extract according to the reported studies[14,11]. All rats were fed a standard diet and provided with water ad libitum.
On the 19th day of pregnancy, all rats were received intraperitoneal anesthesia by 10% chloral hydrate (300-350 mg/kg) for taking blood from heart, the fetus was also collected through laparotomy for recording the fetus weight and placental weight.
2.2. Collection of serum and tissues and detection of biochemical indexes
The serum samples were collected after the centrifugation at 3 000 rpm and 4 ℃ for 10 min and reserved in fridge at -20 ℃. Then, the total cholesterol (TC), triglyceride (TG), glycosylated haemoglobin A1c (HbA1c), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels in serum were detected by the automatic biochemical analyzer. Fasting insulin (FINS) and free fatty acid (FFA) were measured with ELISA kits. Fasting blood glucose(FBG) level was determined by glucose oxidase method. HbA1c level was determined by glycated hemoglobin meter.
After taking blood from heart, the liver and pancreas tissues were weighed and homogenized by adding the 0.1 M phosphate buffer. Then, the tissue homogenate was centrifuged for 10 min and the supernatant was collected for detecting the levels of superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx) and glutathione (GSH). In order to detect the secretory function of pancreatic islet β-cells and endogenous insulin level in blood, serum C-peptide (CP) content was detected by125I-radioimmunoassay method. In order to know the glycogen metabolism of pregnant rats, content of hepatic glycogen in liver tissue was determined by anthrone method.
2.3. Data statistics
SPSS 18.0 software was used to perform data analysis and each index was showed as mean±SD. Pairwise comparisons between groups were performed by LSD-t test. Multiple comparisons of means were performed by One-Way ANOVA. When P<0.05, the difference was considered as statistically significant.
3. Results
3.1. Body weight of pregnant rats and fetal rats
Figure 1 showed that the body weight of rats in each group was no difference before pregnancy, but the prenatal body weight of rats in GDM group was lower than the other groups (P<0.05). The fetal rats weight and placenta weight in GDM group were also lower than those in control group and intervention group (P<0.05). Moreover,there was no difference in all of the above indexes between controlgroup and intervention group (P>0.05).
3.2. Biochemical indexes of each group in late stage of pregnancy
Through the comparisons of the blood glucose and serum lipids levels in each group, we found that except the serum CP content,each biochemical index in GDM group differed significantly with that in control group and that in intervention group (P<0.05). However, after the treatment, each index in intervention group improved significantly, especially the FFA, FBG, HDL, FINS and hepatic glycogen levels showed no difference as compared with control group (P>0.05, Table 1). These results indicated that okra extract could obviously enhance the insulin sensitivity of GDM rats and improve IR, thus stabilizing the blood glucose level.
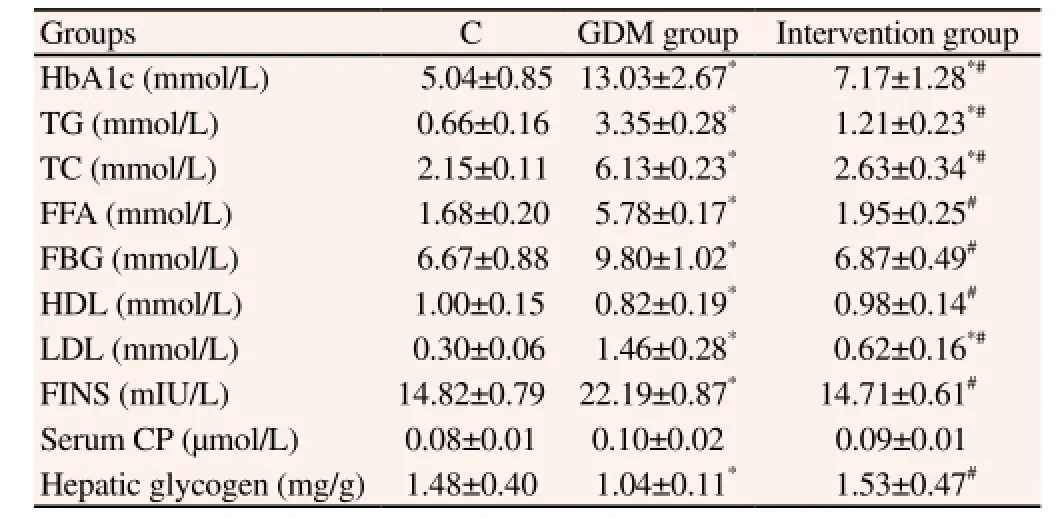
Table 1 Comparisons of biochemical indexes in each group.
3.3. Antioxidant enzymes levels in liver and pancreas tissues in late stage of pregnancy
Figure 2 showed that the antioxidant enzymes (SOD, GPx, catalase and GSH) levels in liver and pancreas tissues of GDM group were lower than the other groups (P<0.05); after the treatment of okra extract, the antioxidant enzymes levels in liver and pancreas tissues were equivalent to those of control group (P>0.05).
4. Discussion
GDM is regarded as the early stage of T2DM in some published reports, and IR is one of the common pathogenesis of GDM and T2DM. In the study, we treat GDM rats with okra extract and the resulted showed serum glucose and lipids levels as well as IR improved significantly after the treatment. Meanwhile, after the treatment of okra extract, the antioxidant enzymes levels of GDM rats showed no difference with normal pregnant rats.
Generally, hyperglycemia often causes the increase of free radicals in body, the glucose oxidation, non-enzymatic glycation of proteins and subsequent degradation of glycated proteins are responsible for the formation of oxygen free radicals in patients with diabetes[15]. Antioxidant enzymes in the body, such as SOD,glutathione peroxidase, catalase, vitamin A, C and E, are natural substances which can scavenge free radicals and prevent their deleterious effects[16]. Okra is one of the most important vegetables in the mallow family; it constitutes a combination of flavonoids,polysaccharides, vitamins and mineral salts. And the plant has a wide range of medicinal values and has been used to treat many diseases. Okra polysaccharide possesses anticomplementary and hypoglycemic activity in normal mice[17]. In this study, compared to GDM group, antioxidant enzymes levels of intervention group increased significantly, this may be due to the hypoglycemic activity of okra in GDM rats and then reduced the extent and level of glucose oxidation, thereby reducing superoxide anion formation and restoring antioxidant enzymes levels. Samarji and Balbsaa[18]certified that Nigela sativa and olive oils returned catalase and arysulfatase B activities back near to normal by fixing their catalytic properties, they believed that diabetes induces significant alterations of the catalytic characters of arysulfatases and some oils decrease this alteration through an antioxidant-mediated effect. Zhao and Hu evaluated the efficacy and safety of -lipoic acid in the treatment of aged T2DM complicated with acute cerebral infraction, and their results showed that after application of -lipoic acid, NIHSS score was significantly decreased ,while efficiency improved significantly,indicating that -lipoic acid could prevent brain cells injury caused by the increasing oxygen free radicals and lipid peroxidation in cerebral ischemia-reperfusion period, helping to promote the symptomamelioration and function recovery[19].
Oxidative stress was closely related to DM and its complications,Baynes et al[20] pointed out that oxidative stress increased obviously at the late stage of DM patients, Collier et al[21] also found the LDL oxidation and the obvious increase of easily oxidized substances. In the study, because of rich in antioxidative substances, oxidative stress in GDM rats was restrained after the administration of okra extract[22]. The activity of SOD indirectly reflects the body's ability to eliminate oxygen free radicals, and giving GDM rats the treatment of okra extract could avoid the excessive consumption of SOD and other antioxidant enzymes, which is beneficial to timely and efficiently eliminate the reactive oxygen species (ROS) in body. In regard to ROS, as a signal molecule, it is similar to the second messengers and could activate many redox sensitive signal paths,and then causes the phosphorylation of insulin receptor and insulin receptor substrate in the insulin signal paths, thus leading to the reduced activity of downstream signal molecules and the weakened insulin-sensitizing effect, and eventually IR occurred[23]. In this study, because of the eliminating ROS effect of okra extract and/ or endogenous antioxidant enzymes, IR was weakened and insulinsensitizing effect was enhanced, this may be the cause of the blood glucose level in rats of intervention group could be regulated and improve.
In summary, because of rich in antioxidative substances, the endogenous antioxidant enzymes could not be excessive consumed,which efficiently inhibited the oxidative stress and IR, and consequently blood glucose level of GDM rats was regulated.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care 2007; 30(4): 878-883.
[2] Lappsa M. GSK3β is increased in adipose tissue and skeletal muscle from women with gestational diabetes where it regulates the inflammatory response. PLoS One 2014; 9(12): e115854.
[3] Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007; 30(Suppl 2): S141-146.
[4] Catalano PM, Nizielski SE, Shao J, Preston L, Qiao L, Friedman JE. Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: relationship to FFA during pregnancy. Am J Physiol Endocrinol Metab 2002; 282(3): E522-533.
[5] Colomiere M, Permezel M, Lappas M. Diabetes and obesity during pregnancy after insulin signalling and glucose transporter expression in maternal skeletal muscle and subcutaneous adipose tissue. J Mol Endocrinol 2010; 44(4): 213-223.
[6] Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants(Council of Industrial and Scientific Research). New Delhi; 1956, p.1-133.
[7] Moïse MM, Benjamin LM, Doris TM, Dalida KN, Augustin NO. Role of Mediterranean diet, tropical vegetables rich in antioxidants, and sunlight exposure in blindness, cataract and glaucoma among African type 2 diabetics. Int J Ophthalmol 2012; 5(2): 231-237.
[8] Taniguchi-Fukatsu A, Yamanaka-Okumura H, Naniwa-Kuroki Y, Nishida Y, Yamamoto H, Taketani Y, et al. Natto and viscous vegetables in a Japanese-style breakfast improved insulin sensitivity, lipid metabolism and oxidative stress in overweight subjects with impaired glucose tolerance. Br J Nutr 2012; 107(8): 1184-1191.
[9] Tongjaroenbuangam W, Ruksee N, Chantiratikul P, Pakdeenarong N,Kongbuntad W, Govitrapong P. Neuroprotective effects of quercetin, rutin and okra (Abelmoschus esculentus Linn.) in dexamethasone-treated mice. Neurochem Int 2011; 59(5):677-685.
[10] Ai G, Liu Q, Hua W, Huang Z, Wang D. Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic: In vitro and in vivo studies. J Ethnopharmacol 2013; 146(3): 794-802.
[11] Zhou L, An XF, Teng SC, Liu JS, Shang WB, Zhang AH, et al. Pretreatment with the total flavones glycosides of Flos Abelmoschus manihot and hyperoside prevents glomerular podocyte apoptosis in streptozotocin-induced diabetic nephropathy. J Med Food 2012; 15(5):461-468
[12] Fan S, Zhang Y, Sun Q, Yu L, Li M, Zheng B, et al. Extract of okra lowers blood glucose and serum lipids in high-fat diet-induced obese C57BL/6 mice. J Nutr Biochem 2014; 25(7): 702-709.
[13] Sabitha V, Ramachandran S, Naveen KR, Panneerselvam K. Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. in streptozotocin-induced diabetic rats. J Pharm Bioallied Sci 2011; 3(3):397-402.
[14] Alqasoumi SI. ‘Okra' Hibiscus esclentus L.: A study of its hepatoprotective activity. Saudi Pharm J 2012; 20(2): 135-141.
[15] Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress and antioxidants: a review. J Biochem Mol Toxicol 2003; 17(1): 24-38.
[16] Sabitha V, Ramachandran S, Naveen KR, Panneerselvam K. Investigation of in vivo antioxidant property of Abelmoschus esculentus (L) moench. Fruit seed and peel powders in streptozotocin-induced diabetic rats. J Ayurveda Integr Med 2012; 3(4): 188-193.
[17] Tomoda M, Schimizu N, Gonda R, Kanari M, Yamada H, Hikino H. Anticomplementary and hypoglycemic activity of okra and Hibiscus mucilages. Carbohydr Res 1989; 190(2): 323-328.
[18] Samarji R, Balbaa M. Anti-diabetic activity of different oils through their effect on arysulfatases. J Diabetes Metab Disord 2014; 13(1): 116.
[19] Zhao L, Hu FX. -lipoic acid treatment of aged type 2 diabetes mellitus complicated with acute cerebral infarction. Eur Rev Med Pharmacol Sci 2014; 18(23): 3715-3719.
[20] Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 1991; 40(4): 405-412.
[21] Collier A, Rumley A, Rumley AG, Paterson JR, Leach JP, Lowe GD, et al. Free radical activity and hemostatic factors in NIDDM patients with and without microalbuminuria. Diabetes 1992; 41(8): 909-913.
[22] Hu L, Yu W, Li Y, Prasad N, Tang Z. Antioxidant activity of extract and its major constituents from okra seed on rat hepatocytes injured by carbon tetrachloride. Biomed Res Int 2014; 2014: 341291.
[23] Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stressactivated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003; 52(1): 1-8.
15 September 2015
Li-Chen Guo, Department of Gynaecology and Obstetrics,Central Hospital of Shanghai Jiading District ,No.1 Chengbei Road, Jiading District,Shanghai, China.
E-mail:lichen_guo@126.com
猜你喜欢
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Immunomodulatory effect of garlic oil extract on Schistosoma mansoni infected mice
- Larvicidal activity, inhibition effect on development, histopathological alteration and morphological aberration induced by seaweed extracts in Aedes aegypti (Diptera: Culicidae)
- Human ocular dirofilariasis due to Dirofilaria repens in Sri Lanka
- Childhood brucellosis: Review of 317 cases
- Effect of cyclophosphamide on fungal infection in SLE mice detected by fluorescent quantitative PCR
- Effect of low intensity pulsed ultrasound on expression of TIMP-2 in serum and expression of mmp-13 in articular cartilage of rabbits with knee osteoarthritis
