氰根桥联CrⅢ-CuⅡ一维配合物{[Cu(cyclam)][Cr(bpb)(CN)2]2·2H2O}n的合成、结构与磁性
2015-06-01杨代胜许丽华张丽芳倪中海王文峰
杨代胜 许丽华 陈 会 张丽芳 倪中海*, 王文峰,2
(1中国矿业大学化工学院,徐州221116) (2中国矿业大学资源学院,徐州221116)
氰根桥联CrⅢ-CuⅡ一维配合物{[Cu(cyclam)][Cr(bpb)(CN)2]2·2H2O}n的合成、结构与磁性
杨代胜1许丽华1陈 会1张丽芳1倪中海*,1王文峰1,2
(1中国矿业大学化工学院,徐州221116) (2中国矿业大学资源学院,徐州221116)
基于构筑单元K[Cr(bpb)(CN)2]和[Cu(cyclam)](ClO4)合成了一个氰根桥联的CrⅢ-CuⅡ一维化合物{[Cu(cyclam)][Cr(bpb)(CN)2]2· 2H2O}n[cyclam=1,4,8,11-四氮杂环十四烷;bpb2-=1,2-二(2-吡啶甲酰胺基)苯](1),并通过X-衍射单晶分析表征其结构特征。结果表明:化合物1是由氰根桥联的2种不同金属组成的聚合物,其结构属于三斜晶系,P1空间群,a=0.9667 3(19)nm,b=1.345 1(3) nm,c=1.382 0(3)nm,α=77.12(3)°,β=76.93(3)°,γ=82.02(3)°,V=1.699 1(6)nm3,Z=2,Dc=1.567 g·cm-3,μ=1.086 mm-1,F(000)=828,R1=0.0413,wR2=0.1200。磁性研究表明:配合物1中的CrⅢ离子和CuⅡ离子之间存在弱的铁磁耦合作用。
氰根桥联;晶体结构;磁性;杂金属
0 Introduction
In the past decades,heterometallic complexes as one of the most known molecular-based magnetic materials have attracted much attention because of theirfascinatingstructuralfeaturesandexcellentmagneticproperties,thusfar,anumberof heterometallic complexes molecular-based magnetic materials have been synthesized and characterized structurallyandmagnetically[1-4].Amongthose complexes,cyanide group plays unique roles in design and assembly of heterobimetallic even heterotrimetallic speciesbecauseofitsasymmetriccharacter[5-11]. Cyanide-bridgedheterometalliccomplexeswhich magnetic exchange interaction occur through cyanide bridge have made great contributions for the building of magneto-structural relationships and the elucidation of the nature of magnetic coupling[12-16].
The number and position of cyanide group and the charge of cyanide-containing building blocks are very significant factors for building cyanide-bridged complexes with fascinating structural and excellent magnetic properties[17-20].However,thedesignand synthesis of the stable and suitable cyanide-containing building blocks are still a challenge.Compared with cyanide-bridged heterometallic FeⅢ-M(M=CuⅡ,NiⅡ, CoⅡ,MnⅡ,MnⅢet al.)complexes,CrⅢ-M are still limited due to the shortage of stable and suitable cyanide-containing building blocks[21-23].In our group, we have focused our efforts on the design and synthesis of new cyanide-containing building blocks and the assembly of new cyanide-bridged complexes based on them in the past several years[24-26].Recently, we synthesized a one-dimensional cyanide-bridged CrⅢ-CuⅡcomplex{[Cu(cyclam)][Cr(bpb)(CN)2]2·2H2O}n(1) by using building block K[Cr(bpb)(CN)2].Herein,we report its synthesis,crystal structure and magnetic properties.
1 Experimental
1.1 Materials and physical measurements
Elemental analyses(C,H and N)were carried out on an Elementary Vario EL instrument.The infrared spectra of solid samples on KBr pellets were recorded on a Nicolet 7199B FT/IR spectrophotometer in the region of 4 000~400 cm-1.Magnetic properties measurements on crystal samples were carried out on a Quantum Design MPMS SQUID magnetometer.The experimental susceptibilities were corrected for the diamagnetism estimated based on Pascals tables.
All chemicals and solvents were purchased from commercial sources and used without further handing. The precursors[Cu(cyclam)](ClO4)2[27]and K[Cr(bpb) (CN)2][28]werepreparedaccordingtoliterature methods.
1.2 Preparation of complex 1
Dark red block single crystals of complex 1 were prepared at room temperature by carefully mixing a purple 50%aqueous methanol solution(10 mL)of[Cu (cyclam)](ClO4)2(0.1 mmol,46.3 mg)and a red methanol solution(5 mL)ofK[Cr(bpb)(CN)2](0.1 mmol,45.9 mg).The single crystals were carefully collected after about three days.Yield:0.040 g (49.9%).Anal.Calcd.(%)for CuCrC30H38N10O7Cl:C: 44.95;H:4.78;N:17.47.Found(%):C:44.88;H: 4.79;N:17.38.Selected IR frequencies(KBr disk, cm-1):2 225(m,νC≡N),1 622(s,νC=O),1 089(vs,νO-Cl).
1.3 X-ray data collection and structure refinement
The structure of complex 1 was solved by direct methods with the SHELXS-97 program[29]and refined by full-matrix least-squares methods on F2with the SHELXS-97[30].The diffraction data were collected at 123 K on a Bruker Smart ApexⅡCCD diffractometer equipped with a graphite-monochromatized Mo Kα radiation(λ=0.071 073 nm).Anisotropic thermal parameters were used for the non-hydrogen atoms and isotropic parameters for the hydrogen atoms.Hydrogen atoms were added geometrically and refined using a ridingmodel.Imageswerecreatedbyusing DIAMONDprogram.Crystallographicdataand structure refinement parameters are listed in Table 1, and selected bond lengths and angles of complex 1 are listed in Table 2.
CCDC:972349.
2 Results and discussion
2.1 Crystal structure of complex 1
Single crystal X-ray diffraction analysis reveals that the structure of complex 1 is a one-dimensional cationic polymer[Cu(cyclam)][Cr(bpb)(CN)2]+with free ClO4-ascounteranions.Thewavelikechainiscomprised of[Cr(bpb)(CN)2]-units and[Cu(cyclam)]2+fragments which are alternatively linked by cyanide group.Intheone-dimensionalchain,each[Cu (cyclam)]2+group is linked to two[Cr(bpb)(CN)2]-ions in the trans positions.The labeling scheme of the chain-like structure of complex 1 is shown in Fig.1 .

Table1 Crystal data and structure refinement parameters for complex 1
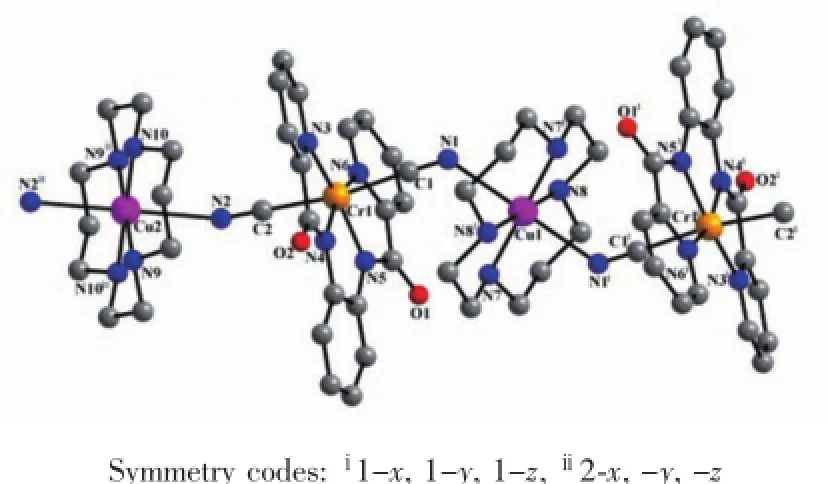
Fig.1 One-dimensional crystal structure of complex 1
The CrⅢcenter is hexacoordinated with two bridging cyanide nitrogen atoms and four coplanar nitrogen atoms of bpb2-ligand,which forms a slightly distorted octahedron.The average bond distances of Cr-N(amide)and Cr-N(pyridine)are 0.197 11(19)and 0.208 95(18)nm,respectively.Apparently,the average bond lengths of Cr-N(amide)are shorter than Cr-N (pyridine)in complex 1,which is in agreement with the fact that the deprotonated amide group is a very strong σ-donor.The average bond distance of Cr-C is 0.209 9(2)nm.The angles of Cr1-C1-N1 and Cr1-C2-N2 are almost linear with the value of 176.86(19)° and 176.99(18)°,respectively.Besides,the C1-Cr1-C2 bond angle with the value of 171.99(8)°is also nearly linear.
There are two independent CuⅡcenters in the one-dimensional chain of complex 1,each CuⅡcenter is also hexacoordinated with six nitrogen atoms which come from two bridging cyanides in the axial position and cyclam ligand in the equatorial plane,forming an elongated octahedron.The in-plane Cu-N bond lengths span from 0.200 49(17)to 0.204 86(18)nm,while the apicalCu-Nbondlengthsarerelativelylong (0.248 94(18)nm for Cu1-N1 and 0.255 28(18)nm for Cu2-N2)due to Jahn-Teller effect of CuⅡion.The angles of the in-plane N-Cu-N range from 85.70(8)°to 94.30(8)°,and the angles of Cu1-N1-C1 and Cu2-N2-C2 are 130.87(8)°and 146.93(8)°,respectively.The distances of the intramolecular CuⅡand CrⅢcenters separated through bridging cyanide are 0.521 03(17) nm for Cr1…Cu1 and 0.554 15(18)nm for Cr1…Cu2.

Fig.2 Supermolecular structure for complex 1,formed by hydrogen-bond interactions:(a)along the a axis;(b)along the b axis

Table2 Selected bond distances(nm)and bond angles(°)of complex 1
The cell packing diagram (Fig.2) of complex 1shows the existence of interchain hydrogen bonds between the oxygen atoms from the free water molecules,bpb2-ligands and ClO4-anions and the nitrogen atoms from cyclam ligands, and these hydrogen bonds link the chains into the threedimensional supermolecular structure.
2.2 Magnetic property of complex 1
The magnetic susceptibilities of complex 1 were measured in the 2~300 K temperature range under an applied field of 2 000 Oe,which is plotted as χmT-T and χm-1-Tin Fig.3 ,where χmisthemagnetic susceptibility per CuⅢCrⅡunit.At 300 K,the value of χmT for complex 1 is 2.27 emu·K·mol-1,which is almost equal to the anticipated spin-only value(2.25 emu·K·mol-1)of the isolate system composed by CrⅢ(S=3/2)ion and CuⅡ(S=1/2)ion based on g=2.00. Withthetemperaturedecreasing,theχmTvalue increase slowly until about 50 K,and then,they increase rapidly to the maximum value of 3.07 emu· K·mol-1.The χmT curve shows that the ferromagnetic interaction between CrⅢand CuⅡions through cyanide group is weak ferromagnetic for complex 1.The magnetic susceptibilities of complex 1 obey the Curie-Weiss law in the whole temperature range with the Weiss constant θ=1.70(1)K and Curie constant C= 2.26(1)emu·K·mol-1.The positive Weiss constant also supports the overall ferromagnetic interaction.
The field(H)dependence of magnetization(M) for complex 1 was measured in the field range from 0 to 50 kOe at T=2 K as shown in Fig.4 .The field dependence of the magnetization of complex 1 is almost in agreement with their corresponding Brillouin curve for the S=2 spin state with g=2.00.However, the magnetization data are higher than the Brillouin curve deduced from an isolated CrⅢspin(S=3/2)and CuⅡspin(S=1/2)with g=2.00,further demonstratingthattheslightferromagneticcouplingexistsin complex 1.
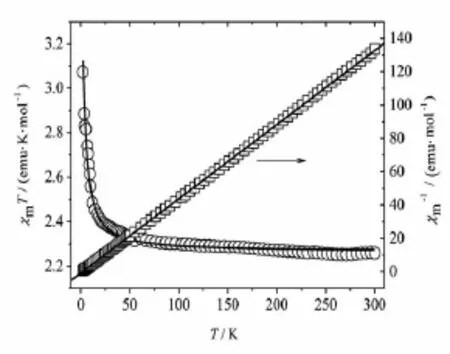
Fig.3 Temperature dependences of χmT for complex 1 measured under an applied field of 2 000 Oe

Fig.4 Field dependence of magnetization at T=2 K for complex 1
To evaluate the magnetic coupling strength between the different metal ions of cyanide-bridged one-dimensional heterometallic complexes, the magnetic susceptibilities of this one-dimensional complex has been simulated by using MAGPACKprogram[31-33].According to this method and on the basis of the crystal structure of complex 1 as discussed above, the one-dimensional complex 1 can be considered as a isotropic Heisenberg chain containing alternating spins 1/2 and 3/2 with two magnetic exchange interactionJ1and J2(J1vs J2in Scheme 1).In this infinite single chain of complex 1, the magnetic susceptibilities can be simulated and calculated rationally and satisfactorily based on a closed eight members ring cluster model consisting of alternating spins 1/2 and 3/2 with two magnetic exchange interactions J1and J2.

Scheme 1Magnetic fitting model for complex 1
Accordingtothismodel,themagnetic susceptibilities in the 2~300 K temperature range for complex1wassimulated,givingthebest-fit parameters J1=0.72(1)cm-1,J2=0.38(1)cm-1,g=2.006 and R=∑(χobsdT-χaldT)2/∑(χobsdT)2=8.8×10-5.The relatively small magnetic constants based on this model reveal that the magnetic coupling between CrⅢand CuⅡions in complex 1 is weak ferromagnetic,which is reasonable and can be compared with those of most cyanide-bridged CrⅢ-CuⅡcomplexes[34-35].
3 Conclusions
In summary,a cyanide-bridged CrⅢ-CuⅡcomplex has been synthesized based on[Cu(cyclam)](ClO4)2and K[Cr(bpb)(CN)2]building blocks.Single crystal X-ray diffraction analyses show that complex 1 has onedimensional structure.Magnetic investigations indicate that the complex 1 exhibits a weak ferromagnetic coupling between CrⅢand CuⅡions through the cyanide bridge,and the magnetic property is fitted based on a suitable theoretical model.
[1]Gatteschi D,Sessoli R.Angew.Chem.,2003,115:278-309
[2]Tang J K,Si S F,Wang L Y,et al.Inorg.Chem.Commun.,2002,5:1012-1015
[3]Ni Z H,Kou H Z,Zhang L F,et al.Angew.Chem.Int.Ed., 2005,44:7742-7745
[4]Caneschi A,Gatteschi D,Renard J P,et al.Inorg.Chem., 1989,28:1976-1980
[5]Brechin E K,Boskovic C,Wernsdorfer W,et al.J.Am.Chem. Soc.,2002,33:9710-9711
[6]Gianneschi N C,Masar M S,Mirkin C A.Acc.Chem.Res., 2005,38:825-837
[7]Tabares L C,Navarro J A R,Salas J M.J.Am.Chem.Soc., 2001,123:383-387
[8]Zhang K L,Chen W,Xu Y,et al.Polyhedron,2001,20:2033-2036
[9]Mironov V S,Chibotaru L F,Ceulemans A J.J.Am.Chem. Soc.,2003,125:9750-9760
[10]Visinescu D,Toma L M,Lloret F,et al.Dalton Trans., 2008,31:4103-4105
[11]Berlinguette C P.Angew.Chem.,2003,42:1523-1526
[12]Shen W Z,Chen X Y,Cheng P,et al.Z.Anorg.Allg.Chem., 2003,629:591-594
[13]Luo J,Hong M,Weng J,et al.Inorg.Chim.Acta,2002, 329:59-65
[14]Kou H Z,Liao D Z,Jiang Z H.Inorg.Chem.Commun.,2000, 3:151-154
[15]Mehrotra R C,Singh A,Sogani S.Chem.Soc.Rev.,1994, 23:215-225
[16]Shi Z L,Lin N.Chem.Phys.,2010,11:97-107
[17]Mock M T,Kieber M T,Popescu C V,et al.Inorg.Chim. Acta,2009,362:4553-4562
[18]Zhang D P,Zhang L F,Zhao Z D,et al.Inorg.Chim.Acta, 2011,377:165-169
[19]Li G L,Ni Z H,Cheng W Q,et al.Inorg.Chem.Commun., 2013,31:58-61
[20]Liu C M,Zhang D Q,Zhu D B.Inorg.Chem.,2009,48: 4980-4987
[21]Hu X,Zeng Y F,Chen Z,et al.Cryst.Growth Des.,2009,9: 421-426
[22]Stamatatos T C,Dionyssopoulou S,Efthymiou G,et al.Inorg. Chem.,2005,44:3374-3376
[23]Lan Y Q,Li S L,Fu Y M,et al.Dalton Trans.,2008,31: 6796-6807
[24]Ni Z H,Kou H Z,Zhao Y H,et al.Inorg.Chem.,2005,44: 2050-2059
[25]MIAO Bao-Xi(苗宝喜),LI Guo-Lin(李国玲),ZHAO Yun (赵云),et al.Chinese J.Inorg.Chem.(无机化学学报), 2013,29(11):2470-2474
[26]Li G L,Nie J,Chen H,et al.Inorg.Chem.Commun.,2012, 19:66-69
[27]Kolinski R A,Korybut-Daszkiewicz B.Inorg.Chim.Acta, 1975,14:237-245
[28]Chen H,Miao B X,Ni Z H,et al.Inorg.Chim.Acta, 2013,404:34-40
[29]Sheldrick G M.Acta Crystallogr.,2008,A64:112-122
[30]Sheldrick G M.SHELXL-97,Program for Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[31]Borrás-Almenar J J,Clemente-Juan J M,Coronado E,et al. Inorg.Chem.,1999,38:6081-6088
[32]Borrás-Almenar J J,Clemente-Juan J M,Coronado E,et al. J.Comput.Chem.,2001,22:985-991
[33]Zhang D P,Wang H L,Ni Z H,et al.Inorg.Chem., 2009,48:5488-5496
[34]Bieńko A,Suracka K,Mroziński J,et al.Polyhedron,2010, 12:2546-2552
[35]Parker R J,Lu K D,Batten S R,et al.J.Chem.Soc.Dalton Trans.,2002,19:3723-3730
Synthesis,Crystal Structure and Magnetic Properties of a One-Dimensional Cyanide-Bridged CrⅢ-CuⅡComplex{[Cu(cyclam)][Cr(bpb)(CN)2]2·2H2O}n
YANG Dai-Sheng1XU Li-Hua1CHEN Hui1ZHANG Li-Fang1NI Zhong-Hai*,1WANG Wen-Feng1,2
(1School of Chemical Engineering and Technology,China University of Mining and Technology,Xuzhou,Jiangsu 221116,China)
(2School of Resources and Geosciences,China University of Mining and Technology,Xuzhou,Jiangsu 221116,China)
A one-dimensional cyanide-bridged CrⅢ-CuⅡcomplex{[Cu(cyclam)][Cr(bpb)(CN)2]2·2H2O}n(cyclam=1, 4,8,11-tetraazacyclotetradecane,bpb2-=1,2-bis(pyridine-2-carboxamido)-benzenate)(1)has been synthesized by the reaction of building blocks[Cu(cyclam)](ClO4)2and K[Cr(bpb)(CN)2].Single crystal X-ray diffraction analysis reveals that complex 1 crystallizes in triclinic space group P1 with a=0.966 73(19)nm,b=1.345 1(3)nm,c= 1.382 0(3)nm,α=77.12(3)°,β=76.93(3)°,γ=82.02(3)°,V=1.699 1(6)nm3,Z=2,Dc=1.567 g·cm-3,μ=1.086 mm-1, F(000)=828,R1=0.041 3,wR2=0.120 0,the structure of complex 1 is a type of polymer with two different metal centers which are alternatively linked by cyanide group.Magnetic investigations indicate that complex 1 exhibits a weak ferromagnetic coupling between CrⅢand CuⅡcenters through the cyanide bridge.CCDC:972349.
crystal structure;cyanide-bridged;magnetic property;heterometallic
O614.61+1;O614.121
A
1001-4861(2015)03-0565-06
10.11862/CJIC.2015.089
2014-09-18。收修改稿日期:2015-01-10。
江苏省高校优势学科建设工程项目和中央高校基本科研业务费专项资金资助项目(No.2014ZDPY25)。*
。E-mail:nizhonghai@cumt.edu.cn
