色素细胞谱系及其发育调控研究进展
2015-04-09文胜等
文胜等
摘 要 色素干细胞是一种来源于神经嵴并能够分化产生色素细胞的干细胞.了解色素细胞谱系分化和发育的生物学规律,掌握体色形成的分子调控机制,可为临床治疗以及观赏动物的人工培育提供理论基础.概述了动物色素细胞谱系来源和组成,色素干细胞分化发育的分子调控研究进展.
关键词 色素细胞;色素干细胞;mitf基因
中图分类号 Q954;S965 文献标识码 A 文章编号 1000-2537(2014)06-0024-05
Abstract Pigment stem cells originate from the neural crest and differentiate into variants of chromatophores. It can provide theoretical support to the pathogenesis of diseases and the cultivation of ornamental animals to reveal the molecular mechanism in the development of pigment cell lineage and obtain the body color formation. This paper focuses on reviewing the advances in the research of the pigment cell lineage and the mechanism of chromatophore development, which are associated with the sources of the pigment cell lineage in animals and the regulation of gene on its development.
Key words chromatophore; pigment stem cell; mitf gene
色素细胞是动物体内含有生物色素的一类特化细胞,通过其色素系统选择性地吸收特定波长的光而产生颜色,从而形成动物体的体色.体色在保护个体免受天敌或紫外线伤害、信息交流和生理调节等方面有着重要生物学作用.本文概述了动物色素细胞谱系来源和组成,色素干细胞分化发育的分子调控相关研究进展.
1 色素细胞及色素细胞谱系
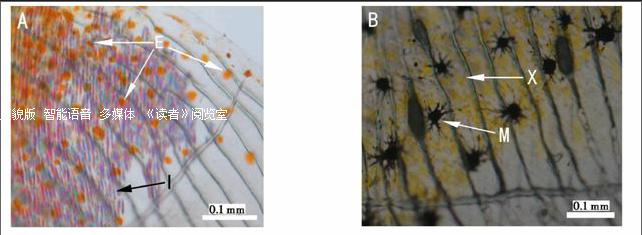
根据所含色素的不同,动物色素细胞主要有黑色素细胞、黄色素细胞、红色素细胞、虹彩细胞等4种基本类型 (图1,彩图见封三).黑色素细胞(melanophore)内含大量由酪氨酸、多巴胺等氧化聚合在体内合成的黑色素,能够吸收特定波长的入射光而使身体呈现黑色.红色素细胞(erythrophore)和黄色素细胞(xanthophore)形态非常相似,都含有胡萝卜素与蝶啶的色素体.黄色素细胞和红色素细胞与动物的黄、橙、红体色有关,一般认为黄色素细胞拥有大量带黄色的蝶酸色素,而红色素细胞则含有较多呈红色和橙色的类胡萝卜素.虹彩细胞(iridophore)也称为鸟粪素细胞(guanophore)或白色素细胞(leucophore),其呈色物质主要是与水结合成晶体形式的鸟嘌呤,通过反射特定波长的光表现为白色、蓝色和紫红3种色彩结晶体.上述几种色素细胞类型的数量和分布、反光结晶体的能力强弱等,决定了动物体的颜色和斑纹[1-4].黑色素细胞是动物体内存在最广泛且研究得最多的色素细胞类型,而其他几种非黑色素细胞则主要在体色鲜艳的低等脊椎动物,如鱼类、两栖类中较为常见.也正是由于与高等羊膜动物在色素细胞类型方面的差异,色彩斑斓的鱼类已成为色素细胞谱系研究的重要材料.
对于色素细胞谱系的研究,主要集中于探究色素细胞是怎样起源、迁移和分化,最后形成一定形状的体纹.人们想知道色素细胞是怎样按照特定的时序到达特定的部位形成特定的器官,这涉及到发育生物学的根本问题.神经嵴则是胚胎发育中短暂出现的过渡性结构,是由背部外胚层分化的位于神经管和表皮之间的细胞带.色素细胞的前体细胞称为色素胚或色素母细胞(chromatoblast),是在胚胎发生时期,由神经嵴细胞发展而来的.色素母细胞具有分化为成黑色素细胞(melanoblast)、成黄色素细胞(xanthoblast)和成虹彩细胞(iridoblast)等色素干细胞的发育潜能.在斑马鱼中,黑色素细胞谱系的特化约在受精后24 h就开始发生;孵化出膜后第3天的斑马鱼胚胎中,就已经观察到黑色素细胞、黄色素细胞与虹彩细胞;出膜2~4周后, 幼体黑色素细胞由成体黑色素细胞取代[5].斑马鱼胚胎发育期出现的黑色素细胞一般认为都是由神经嵴细胞直接分化形成,出膜后的黑色素细胞则是由黑色素干细胞(melanocyte stem cell)发育而来[6].
2 色素干细胞的发现及研究现状
色素细胞的数目和组成直接由色素干细胞控制.色素干细胞的最早出现时间是从神经嵴发生色素细胞谱系的特化开始,随后它们发生分化、增殖、迁移,最终定位于表皮基底膜之下或者毛发着生的毛囊龛中[6].成体色素细胞的更新依赖于真皮干细胞.早在1954年,Goodrich等就发现,在鱼类黄色素细胞局部受损的区域,原本并不存在的黑色素细胞在此出现,这也就暗示了受损组织中有色素干细胞存在[7].但由于缺乏对黑色素细胞的标记方法,未能区分是原本存在的黑色素细胞的迁移还是非色素化的前体细胞的分化导致新的黑色素细胞的产生.在斑马鱼中已经证实,大多数再生鳍条的黑色素细胞来源于非色素化的前体细胞[8].人们从小鼠和人类均分离得到了皮肤干细胞,并在体外进行培养[9-10].其中Li等人获得的人类真皮干细胞不仅能够表达神经嵴的标签基因NGFRp75和nestin,而且还能表达在胚胎干细胞中高表达的OCT4;这些真皮干细胞虽然不表达黑色素细胞的标签基因,但却具有分化产生有功能的黑色素细胞的潜能,这与人类胚胎干细胞向黑色素干细胞的分化条件是相同的[10].目前很多研究已经证实,在动物的真皮、毛囊中存在黑色素干细胞(melanocyte stem cell, MSC)[11-12].Lin等2013年首次在鸟类皮肤下方的圆筒形羽囊底部精确找到黑色素干细胞,并成功揭示出色素干细胞导致鸟类羽毛拥有独特而又复杂的黑白图案并随着个体的生长保持动态平衡[13].
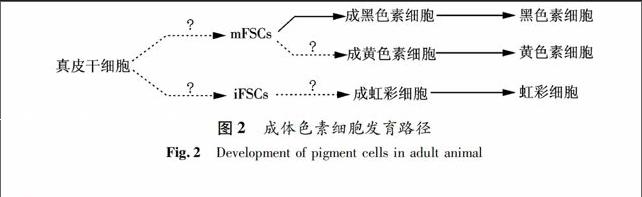
人类肤色和发色主要是由两种黑色素(黑色或褐色的真黑素Eumelanin和红色的棕黑素Pheomelanin)的含量不同所导致的.基于临床研究的需要,目前对黑色素干细胞的研究较为深入,而有关黄色素干细胞等几种非黑色素干细胞的研究资料则十分有限.研究显示,斑马鱼胚胎成黑色素细胞与成虹彩细胞的标签基因有显著性的重叠,但是与成黄色素细胞的不同.该结果表明黑色素细胞与虹彩细胞可能来源于共同的前体细胞,而黄色素细胞则可能来自于不同的发育路径[14].同时Lister等人也证实,在斑马鱼突变体中,成黑色素细胞能够改变原来的细胞谱系命运转变成为成虹彩细胞[15].然而,利用谱系特异的转座子对斑马鱼早期胚胎进行标记发现,来自于真皮干细胞的mFSCs (the same melanocyte-producing founding stem cells)具有分化为黑色素干细胞的发育潜能,同时成体黄色素细胞可能和黑色素细胞一样来自于相同的前体干细胞mFSCs;而虹彩细胞则可能由另一种前体细胞iFSCs(iridophores arise from a distinct founding stem cell)产生(如图2所示)[8].目前对于色素干细胞的分化发育途径还没有一致性的结论.
3 色素细胞谱系发育的分子调控机制
神经嵴细胞分化产生色素细胞以及成体体色模式的发育和维持,涉及到非常严格而复杂的分子调控.在大规模ENU诱导突变和筛选过程中,发现了大量的体色突变斑马鱼品系,这些突变体正在成为研究人类体色多样性和体色失常的强有力工具.这些突变的基因,功能涉及黑色素细胞的早期决定(如sox10, mitf)、迁移(如kit)、黑色素的合成(如tyr, dct)等等[14,16-18].
Mitf(microphthalmia transcription factor)基因是黑色素细胞谱系中已知最早的特异性的标签基因[19-20].在哺乳动物中MITF至少有6个异构体[21],其中MitfA,MitfD,MitfH是视网膜色素上皮(retinal pigment epithelium, RPE)发育必须的,而MitfM只在黑色素细胞发育过程中起作用[22-23].人类mitf基因突变可以导致Waardenburg 综合症,引起皮肤黑色素细胞缺失和皮肤斑驳样色素减退、听觉神经性耳聋[24].mitfa和mitfb是鱼类中分离到的mitf基因的两个亚型[15,25].青鳉中也获得了mitfa和mitfb的同源基因[26].mitfa基因对鱼类体色的黑色素细胞发育起着重要调控作用,但不影响眼色素的发育;而mitfb基因则只参与眼色素上皮细胞的发育[25-27].
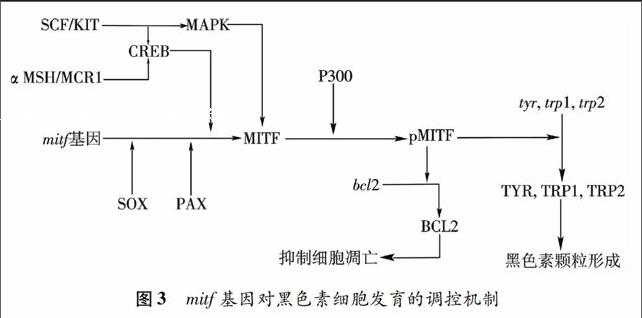
MITF在生物体内能调控黑色素合成关键限制酶——酪氨酸酶基因(tyr)的表达,影响黑色素细胞的分化[28].在 mitfavc7/ BRAFV600E斑马鱼的黑色素瘤模型中,通过温敏控制MITF表达水平,发现低剂量MITF能诱导黑色素瘤;若完全抑制MITF表达,则导致黑色素瘤的消退[29].MITF通过影响tbx2、cdk2、p16和p21等细胞周期调控基因, 控制黑色素细胞的生长[22,30-31].mitf基因的表达受到CREB、SOX10和PAX3等信号通路的调控(图3),SOX10通过结合到mitf的调节序列中,激活mitf的转录,是黑素瘤细胞维持正常发育和生存潜势的主要调控基因[32].研究表明,Ednrb信号通路对神经嵴来源的黑色素前体细胞的增殖、存活以及黑色素细胞的分化是必须的,G蛋白偶联Ednrd受体及其配体Et3与胚胎色素细胞及幼体色素细胞向成体色素细胞的转变有关[33-34].
黄色素细胞谱系和虹彩细胞谱系的标签基因已经成功获得了鉴定.Odenthal在斑马鱼中筛选了多个与黄色素细胞的形成和迁徙相关的基因[35].除了较早所知的成黄色素细胞的标签基因csf1 (colony stimulating factor-1) [36],人们又发现pax家族中的pax7在黄色素细胞谱系中特异性表达,认为pax3与pax7共同调控由神经嵴向黄色细胞的发育[37].Lopes报道了一个虹彩细胞缺失的斑马鱼突变体Shady(shd),经鉴定,shd 的白细胞酪氨酸激酶Ltk(leukocyte tyrosine kinase)同源基因发生了突变 [38].ltk基因在成黑色素细胞和成黄色素细胞中不表达,可以作为虹彩细胞的前体细胞的基因标签.此外,foxd3可能作为黑色素细胞谱系与虹彩细胞谱系之间的转换开关,与mitf基因共同参与了对黑色素细胞谱系和虹彩细胞谱系的分化发育调控 [14].
4 色素细胞谱系研究展望
目前,对色素细胞谱系,尤其是黑色素细胞谱系的定位、分化发育及其相关基因调控机制已经有了较深入的了解.鱼类及其他含有多种色素细胞类型的低等脊椎动物的色素表型较羊膜动物复杂,涉及3种或更多色素细胞的有序组合,红/黄色素细胞谱系、虹彩细胞谱系的分化调控及其与黑色素细胞谱系的发育关联尚待进一步研究.
Ohta等利用Yamanaka诱导体系的SOX2、OCT3/4、KLF4和c-MYC,成功将人皮肤成纤维细胞诱导为iPS细胞.同时通过添加Wnt3a, SCF和ET-3对获得的iPS细胞进一步诱导分化后,检测到TYR、TYRP1等黑色素细胞标记分子的表达,并且观察到培养细胞中黑色素体的存在,表明从iPS细胞诱导产生了黑色素细胞[39].通过体外培养iPS细胞产生色素细胞这一体外诱导分化体系的建立,一方面为再生组织或器官提供临床材料来源,另一方面可以作为研究色素细胞分化发育机制的体外模型.
参考文献:
[1] 陈 桢. 金鱼家化史与品种形成的因素[J]. 动物学报, 1954,6(2):89-116.
[2] MATSUMOTO J, OBIKA M. Morphological and biochemical characterization of goldfish erythrophores and their pterinosomes[J]. J Cell Biol, 1968,39(2):233-250.
[3] MATSUMOTO J. Studies on fine structure and cytochemical properties of erythrophores in swordtail, Xiphophorus helleri, with special reference to their pigment granules (Pterinosomes) [J]. J Cell Biol, 1965,27(3):493-504.
[4] OSHIMA N, KASAI A. Iridophores involved in generation of skin color in the zebrafish brachydanio rerio[J]. Forma, 2002,17(2):91-101.
[5] RAWLS J F, MELLGREN E M, JOHNSON S L. How the zebrafish gets its stripes[J]. Dev Biol, 2001,240(2):301-314.
[6] HULTMAN K A, JOHNSON S L. Differential contribution of direct developing and stem cell-derived melanocytes to the zebrafish larval pigment pattern[J]. Dev Biol, 2010,337(2):425-431.
[7] GOODRICH H B, MARZULLO C M, BRONSON W H. An analysisof the formation of color patterns in two fresh-water fish [J]. J Exp Zool, 1954,125(3):487-505.
[8] TU S, JOHNSON S L. Clonal analyses reveal roles of organ founding stem cells, melanocyte stem cells and melanoblasts in establishment, growth and regeneration of the adult zebrafish fin[J]. Development, 2010,137(23):3931-3939.
[9] WONG C E, PARATORE C, DOURS-ZIMMERMANN M T, et al. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin[J]. J Cell Biol, 2006,175(6):1005-1015.
[10] LI L, FUKUNAGA-KALABIS M, YU H, et al. Human dermal stem cells differentiate into functional epidermal melanocytes[J]. J Cell Sci, 2010,123(6):853-860.
[11] KUNISADA T, YOSHIDA H, YAMAZAKI H, et al. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors[J]. Development, 1998,125(15):2915-2923.
[12] SCHMIDT ULLRICH R, PAUS R. Molecular principles of hair follicle induction and morphogenesis[J]. Bioessays, 2005,27(3):247-261.
[13] LIN S J, FOLEY J, JIANG T X, et al. Topology of feather melanocyte progenitor niche allows complex pigment patterns to emerge[J].Science, 2013,340(6139):1442-1445.
[14] CURRAN K, JAMES A, LISTER B. Interplay between Foxd3 and Mitf regulates cell fate plasticity in the zebrafish neural crest[J]. Dev Biol, 2010,344(1):107-118.
[15] LISTER J A, ROBERTSON C P, LEPAGE T, et al. Nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate[J]. Development, 1999,126(17):3757-3767.
[16] EMMA R, GREENHILL, ANDREA ROCCO, et al. An iterative genetic and dynamical modelling approach identifies novel features of the gene regulatory network underlying melanocyte development[J]. PLoS Genet, 2011,7(9):e1002265.
[17] OSHIMA N, NAKAMARU N, ARAKI S, et al. Comparative analyses of Pigment-aggregating and dispersing actions of MCH on fish chromatophores[J]. Comp Biochem Phys C, 2001,129(2):75-84.
[18] GUO H R, BING H, ZHANG S C, et al. Biochemical and histochemical activities of tyrosinase in the skins of normal and albino turbot scophthalmus maximus[J]. Fish Physiol Biochem, 2003,29(1):67-76.
[19] GODING C R. Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage[J]. Gene Dev, 2000,14(14):1712-1728.
[20] LEVY C, KHALED M, FISHER D E. MITF master regulator of melanocyte development and melanoma oncogene[J]. Trends Mol Med, 2006,12(9):406-414.
[21] FUSE N, YASUMOTO K, TAKEDA K, et al. Molecular cloning of cDNA encoding a novel microphthalmia-associated transcription factor isoform with a distinct amino-terminus[J]. Biochem J, 1999,126(6):1043-1051.
[22] KOLUDROVIC D, DAVIDSON I. MITF, the Janus transcription factor of melanoma [J]. Future Oncol, 2013(2):235-244.
[23] OBOKI K, MORII E, KATAOKA T R, et al. Isoforms of mi transcription factor preferentially expressed in cultured mast cells of mice[J]. Bioch Bioph Res Co, 2002,290(4):1250-1254.
[24] TASSABEHJI M, NEWTON V E, READ A P. Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene[J]. Nat Genet, 1994,8(3):251-255.
[25] ALTSCHMIED J, DELFGAAUW J, WILDE B, et al. Subfunctionalization of duplicate mitf genes associated with differential degeneration of alternative exons in fish[J]. Genetics, 2002,161(1):259-267.
[26] LI M, ZHU F, HONG Y, et al. Differential evolution of duplicated medakafish mitf genes[J]. Int J Biol Sci, 2013,9(5):496-508.
[27] LI M, ZHU F, HONG N, et al. Alternative transcription generates multiple Mitf isoforms with different expression patterns and activities in medaka[J]. Pigm Cell Melanoma R, 2014,27(1):48-58.
[28] FANG D, SETALURI V. Role of microphthalmia transcription factor in regulation of melanocyte differentiation marker TRP-1[J]. Bioch Bioph Res Co, 1999,256(3):657-663.
[29] LISTER J A, CAPPER A, ZENG Z, et al. A conditional zebrafish MITF mutation reveals MITF levels are critical for melanoma promotion vs. regressionin vivo[J]. J Invest Dermatol, 2014,134(1):133-140.
[30] CURRAN K, LISTER J A, KUNKEL G R, et al. Interplay between Foxd3 and Mitf regulates cell fate plasticity in the zebrafish neural crest[J]. Dev Biol, 2010,344(1):107-118.
[31] KELSH R N, INOUE C, MOMOI A, et al. The tomita collection of medaka pigmentation mutants as a resource for understanding neural crest cell development[J]. Mech Dev, 2004,121(7-8):841-859.
[32] SHAKHOVA O, ZINGG D, SCHAEFER S M, et al. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma[J]. Nat Cell Biol, 2012,14(8):882-890.
[33] LAHAV R. Endothelin receptor B is required for the expansion of melanocyte precursors and malignant melanoma[J]. Int J Dev Biol, 2005,49(2-3):173-180.
[34] LEE H O, LEVORSE J M, SHIN M K. The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors[J]. Dev Biol, 2003,259(1):162-175.
[35] ODENTHAL J, ROSSNAGEL K, HAFFTER P, et al. Mutations affecting xanthophore pigmentation in the zebrafish, Danio rerio[J]. Development, 1996,123(1):391-398.
[36] PARICHY D M, RANSOM D G, PAW B, et al. An orthologue of the kit-related gene fms is required for development of neural crest-derived xanthophores and a subpopulation of adult melanocytes in the zebrafish, danio rerio[J]. Development, 2000,127(14):3031-3044.
[37] JAMES E, MINCHIN N, SIMON M HUGHES. Sequential actions of Pax3 and Pax7 drive xanthophore development in zebrafish neural crest[J]. Dev Biol, 2008,317(2):508-522.
[38] LOPES S S, YANG X, MLLER J, et al. Leukocyte tyrosine kinase functions in pigment cell development[J]. PLoS Genet, 2008,4(3):e1000026.
[39] OHTA S, IMAIZUMI Y, OKADA Y, et al. Generation of human melanocytes from induced pluripotent stem cells[J]. PloS one, 2011,6(1):e16182.
(编辑 王 健)
