Ag-Strewn ZnO Flowerlike Microstructures: Simple Synthesis and Versatile Applications
2015-03-18HANHanNIYonghongSHENGEnhongLIUAimin
HAN Han, NI Yong-hong, SHENG En-hong, LIU Ai-min
(1. College of Chemistry and Materials Science, Key Laboratory of Functional Molecular Solids of Ministry of Education, Anhui Laboratory of Molecule-Based Materials, Anhui Key Laboratory of Functional Molecular Solids, Anhui Normal University, Wuhu 241000, China; 2. College of Life Science, Anhui Normal University, Wuhu 241000, China)
Ag-Strewn ZnO Flowerlike Microstructures: Simple Synthesis and Versatile Applications
HAN Han1, NI Yong-hong1, SHENG En-hong1, LIU Ai-min2
(1. College of Chemistry and Materials Science, Key Laboratory of Functional Molecular Solids of Ministry of Education, Anhui Laboratory of Molecule-Based Materials, Anhui Key Laboratory of Functional Molecular Solids, Anhui Normal University, Wuhu 241000, China; 2. College of Life Science, Anhui Normal University, Wuhu 241000, China)
In this paper, Ag nanoparticles-strewn ZnO flowerlike microstructures were successfully synthesized via a two-step route. Porous ZnO flowerlike microstructures were firstly prepared by a simple hydrothermal process with subsequent calcination, employing Zn(NO3)2and urea as the starting reactants in the presence of L-Cysteine; Then, Ag nanoparticles-strewn ZnO flowerlike microstructures were obtained through a photoreduction method. The as-synthesized products were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), (high resolution) transmission electron microscopy (HRTEM/TEM), selected area electron diffraction (SAED), thermogravimetric analysis (TGA), Fourier transform infrared (FT-IR) spectra, UV-vis diffuse reflectance spectra (DRS) and photoluminescence spectra (PL). Experiments showed that the Ag nanoparticles-strewn ZnO flowerlike microstructures exhibited versatile applications in some fields including excellent catalytic capacities for the degradation of Rhodamine B (RhB) and methyl orange (MO), highly effective antimicrobial activities toward Escherichia coli and high electrocatalysis performance to hydrogen peroxide.
Ag-strewn ZnO microstructures; optical properties; versatile applications
Classification NO:O614.41+1 Document code:A Paper NO:1001-2443(2015)03-0217-11
Nanocomposites have been drawing remarkable attention because they often exhibit some novel/improved performances besides simultaneously bearing properties of components. For example, noble metal-ZnO hybrid nanostructures were reported to greatly improve the photocatalytic efficiency in the degradation of organic molecules compared to pure ZnO[1-5]. As important representatives of noble metals and semiconductors, Ag and ZnO are concentrated a great deal of research interest. The composite of Ag and ZnO is paid much attention, too. Many methods have been developed for preparation of Ag/ZnO hybrid nanostructures, including hydrothermal/solvothermal route[6,7], electrospinning method[8], biogenic process[9], microemulsion[10], refluxing synthesis[11], and hydrothermal-sonochemical approach[12]. Usually, the as-obtained Ag/ZnO hybrid nanostructures exhibit a few morphologies, such as Ag nanoparticles-strewn ZnO nanorods/nanofibers, Ag nanoparticles-strewn/coated ZnO nanoparticles, Ag nanoparticles-anchored ZnO microspheres, and worm-like Ag/ZnO core-shell heterostructural composites[6-13].
It was found that both ZnO and Ag exhibited good antibacterial activity[6,14-16]. Meanwhile, some transition-metal oxides and Ag nanostructures were found to be used as electrocatalysts for the detection of H2O2[17,18]. Therefore, it will be significant in the practical application to prepare Ag/ZnO nanocomposites with improved photocatalytic activity, excellent antibacterial ability and outstanding electrocatalytic capacity, simultaneously.
In the present work, we designed a two-step route to successfully obtain Ag nanoparticles-strewn porous ZnO flowerlike microstructures. 3D hierarchical zinc subcarbonate precursor was firstly synthesized via a facile hydrothermal process using zinc nitrate hexahydrate and urea as the starting reactants in the presence of L-Cysteine; and was further transferred into porous ZnO flowerlike microstructures in air at 300℃ for 2 h. Subsequently, small amounts of Ag nanoparticles were successfully strewn on the surfaces of the porous ZnO flowerlike microstructures through a simple photoreduction method. Experiments demonstrated that the as-obtained Ag/ZnO flowerlike microstructures exhibited excellent visible-light catalytic capacity for the degradation of Rhodamine B (RhB) and methyl orange (MO), highly-effective antimicrobial activities against Escherichia coli and high electrocatalysis performance to H2O2.
1 Experimental section
1.1 Materials
All reagents and chemicals were analytically pure, purchased from the Shanghai Chemical Company and used without further purification. Distilled water was used throughout the experiments.
1.2 Synthesis of flowerlike zinc subcarbonate precursor and conversion to porous ZnO microstructures
In a typical experimental procedure, 0.5 mmol Zn(NO3)2·6H2O, 5 mmol urea and 0.1 mmol L-Cysteine were dissolved in 30 mL distilled water under vigorous stirring at room temperature for 30 min. Then, the clear solution was transferred into a 40 mL Teflon-line autoclave, and maintained at 100℃ for 8 h. After cooling down to room temperature naturally, the white zinc subcarbonate precipitate was centrifuged, washed with distilled water and absolute ethanol several times, and dried in vacuum at 60℃ for 10 h. Finally, porous ZnO flowerlike microstructures were obtained by calcining zinc subcarbonate precursor in a tube furnace in air at 300℃ for 2 h.
1.3 Synthesis of Ag-strewn porous ZnO flowerlike microstructures
To prepare Ag/ZnO hybrid microstructures with the loaded amount of Ag nanoparticles of 1, 3, 5 and 7%, respectively, a certain amount of AgNO3was firstly dissolved into 20 mL distilled water. Then, under magnetic stirring 20 mg of the as-obtained porous ZnO was dispersed into the above AgNO3solution. After further stirring in the dark for another 5 min, the above mixed system was irradiated by a 450 W Xenon lamp for 10 min. The color of the system changed from yellowish to brown, indicating the formation of Ag/ZnO hybrid microstructures. Also, the color of the system gradually deepened with the increase of the loaded Ag amount. Finally, the dark precipitate was collected by centrifugation, washed with distilled water and absolute ethanol several times, and dried at 60℃.
1.4 Characterization
The X-ray powder diffraction (XRD) patterns were carried out on a Shimadzu XRD-6000 X-ray diffractometer equipped with Cu Kα radiation (λ=0.154060 nm), employing a scanning rate of 0.02s-1and 2θ ranges from 10° to 80°. Scanning electron microscopy (SEM) images and energy dispersive spectrometry (EDS) of the products were obtained on Hitachi S-4800 field emission scanning electron microscope, employing the accelerating voltage of 5 kV and 15 kV, respectively. High resolution transmission electron microscopy (HR/TEM) images and selected area electron diffraction patterns were recorded on a JEOL 2010 transmission electron microscope, employing an accelerating voltage of 200 kV. The TG-DTA of the precursor was completed on a differential thermal analyzer (Thermo Electron Company of America, SDT Q600). The FTIR spectra of the products were obtained on an IR Prestige-21 Infrared spectrometer (Shimadzu Corporation). The UV-vis diffuse-reflectance spectra were measured on a UV-vis-near-IR spectrometer. Photoluminescence (PL) spectra were recorded on a FLSP 920 with a Xe lamp at room temperature, employing the excitation wavelength of 330 nm from He-Cd laser.
1.5 Photocatalytic activity
To investigate the photocatalytic activities of Ag/ZnO hybrid microstructures, the organic dyes, Rhodamine B (RhB) and methyl orange (MO), were used as the pollution modes, and a 500W Xenon lamp with a cutoff filter at 420 nm as the visible light source. In a typical experiment, 20 mg of catalyst was firstly dispersed into a 50 mL RhB (or MO) solution with a concentration of 10 mg/L under the ultrasonication. Then, the system was continuously stirred in the dark for 30 min to establish an adsorption-desorption equilibrium between the catalyst and the dye. Finally, the system was irradiated under the visible light for desired durations (During irradiation, the system was ceaselessly stirred to maintain a suspension). The concentration change of the dye was monitored with a Metash 6100 UV-vis absorption spectrophotometer (Shanghai).
1.6 Antibacterial ability
In order to assess the antibacterial ability of the as-obtained Ag/ZnO hybrid microstructures, the inhibition of Escherichia coli (E. coli, 8099, a Gram negative bacterium) growth was employed as a case. All plates and materials were sterilized in an autoclave before the experiments. Firstly, 0.1mL of the diluted bacteria suspension was uniformly spread on the nutrient agar using a sterile glass rod. Then, they were coated by 0.1mL 10mg/L of the as-obtained products. Finally, the plates were cultured in an incubator at 37℃ for 24 h before the number of colonies on the plates was counted. All experiments were repeated at least three times.
1.7 Electrochemical Measurements
Electrochemical responses were performed on a CHI660-A electrochemical workstation (CH Instruments, Chenhua Corp., Shanghai, China) with a three-electrode system consisting of a saturated calomel electrode (SCE) as a reference electrode, a platinum wire as a counter electrode, and a bare or modified glassy carbon electrodes (GCE) as a working electrode, employing a scanning rate of 100 mV/s and a rest time of 2 s. To prepare glassy carbon electrodes modified by the products, 1 mg of the products was dispersed into the twice-distilled water under ultrasound. Then, 5 μL solutions were dropped onto the glassy carbon electrode and dried in air at room temperature.
2 Results and discussion
2.1 Characterization of structure and morphology of products

CO(NH2)2+3H2O→2NH3·H2O+CO2
(1)
(2)
(3)

Figure 1 (a, c) XRD pattern and EDS analysis of the precursor obtained by the hydrothermal route at 100℃ for 8 h. (b, d) XRD pattern and EDS analysis of the product obtained after calcining the above precursor at 300℃ for 2 h.
Since ZnCO3and Zn(OH)2have the close solubility, white zinc subcarbonate precipitate can be produced. In the present work, the XRD pattern of the white precipitate prepared at 100℃ for 8 h proved the formation of zinc subcarbonate. As shown in Fig.1a, all diffraction peaks can be indexed as monoclinic Zn5(OH)6(CO3)2by comparison with the standard data of JCPDS card files no.72-1100. However, the (002) peak exhibits stronger diffraction intensity than the (200) one, which is the strongest peak in the standard data. The above fact implies the oriented growth of the precursor. Fig.1c depicts EDS analysis of the precursor. The C, O, Zn and S peaks are visible. The former three elements should be attributed to zinc subcarbonate. The S peak should come from L-Cysteine.


Figure 2 (a) TG-DTA analyses of the precursor and (b) FTIR spectra of the precursor and ZnO obtained after calcining the precursor.
SEM observations found that the outlines of the samples before and after calcining at 300℃ for 2 h hardly changed. As shown in Fig.3a, the precursor consisted of abundant flowerlike microspheres, which were constructed by smooth nanosheets (see the inset in Fig.3a). After calcining at 300℃ for 2h, the product still maintained the shape of the precursor. However, after further enlargement the shape differences of two products were distinguishable. Figs.3b and 3c exhibit high-resolution SEM images of the precursor and ZnO. Obviously, the smooth nanosheets had converted into porous nanosheets. TEM observations proved the result of SEM. As shown in Fig.3d, the product obtained after calcining at 300℃ for 2 h still maintained the shape of the precursor. The SAED pattern given in the inset of Fig.3d presented the polycrystalline nature of the final product. The concentric circles could be indexed as the planes of (100), (101), (102), (110), (103) and (112) of hexagonal ZnO in turn (from inside to outside), which is in good agreement with the XRD result. Fig.3e depicts a typical HRTEM image of ZnO nanosheets. The d-spacing between neighbouring planes is measured to be -0.26 nm, which corresponds to the (002) plane of hexagonal ZnO. Moreover, a pore is labelled in Fig.3e, which confirms the porous structure of ZnO nanosheets.


Figure 3 (a) a low-magnification SEM image and (b) a high resolution SEM image of the precursor prepared under the hydrothermal conditions; (c) a high resolution SEM image, (d) TEM image and (e) HRTEM image of ZnO obtained after calcining the precursor at 300℃ for 2 h. The inset in (a) is a SEM image of single flowerlike precursor; The inset in (d) is a SAED pattern of ZnO.
Fig.4a depicts the XRD patterns of Ag/ZnO hybrid microstructures with loaded Ag amounts of 1, 3, 5 and 7%, respectively. A weak Ag peak at -38.2° was gradually visible with the increase of Ag amount. The successful loading of Ag on ZnO flowerlike microstructures could be proved by EDS analyses. As shown in Fig.4b, Ag peak could be easily detected in the 3% Ag-loaded product besides strong O and Zn peaks. Fig.4c gives a typical SEM image of Ag/ZnO hybrid microstructures with 7% Ag-loaded amount. Small Ag nanoparticles were successfully strewn on the surface of ZnO nanosheets without aggregation. Further evidence to form Ag nanoparticles-strewn ZnO microstructures came from TEM observations. As shown in Fig.4d, abundant spherical nanoparticles with the sizes of 5-17 nm are distributed on the surfaces of ZnO nanosheets. A HRTEM image of nanospheres is displayed in Fig.4e, from which the clear lattice fringes imply good crystallinity of nanospheres. The d-spacing of neighboring planes is measured to be -0.20 nm, corresponding to the (200) plane of Ag.

Figure 4 (a) XRD patterns of Ag/ZnO hybrid microstructures with various Ag-loaded amounts; (b) EDS analysis of Ag/ZnO hybrid microstructures with 3% Ag-loaded amount; (c) SEM image, (d) TEM image and (e) HRTEM image of Ag/ZnO hybrid microstructures with 7% Ag-loaded amount.
2.2 Optical properties
Fig.5a exhibits the UV-vis diffuse-reflectance spectra (DRS) of ZnO and Ag/ZnO hybrid microstructures. The absorption edges of Ag/ZnO hybrid microstructures present obvious red-shift against that of pure ZnO flowerlike microstructures. The corresponding band gap energies (Eg) are obtained in the light of the formula, αhν=A (hν-Eg)1/2, to be 3.03 (pure ZnO), 2.91 (1% Ag/ZnO), 2.87 (7% Ag/ZnO), 2.83 (5% Ag/ZnO) and 2.78 (3% Ag/ZnO). Where, α, A and hv are the absorption coefficient, the constant and the photon energy, respectively (see Fig.5b). Fig.5c depicts the PL emission spectra of ZnO flowerlike microstructures before and after Ag nanoparticles loading. Under the excitation of 330 nm light pure ZnO flowerlike microstructures showed a strong emission peak centred at -410 nm in the range from 350-550 nm. Compared with Lin’s report[8], the emission peak of ZnO red shifted -40 nm. This should be attributed to their different morphologies. After Ag nanoparticles loading, the intensity of the emission peak dramatically decreased, which should be attributed to the charge transfer from ZnO to Ag[8]. However, the emission intensity did not linearly decrease with the increase of Ag loading amount. Ag/ZnO hybrid microstructures with 3% Ag loading amount exhibited the lowest the emission intensity. Some groups also found the above similar experimental phenomenon and believed that the strong surface plasmon absorption of Ag nanoparticles at -410 nm caused the above phenomenon[8,22].

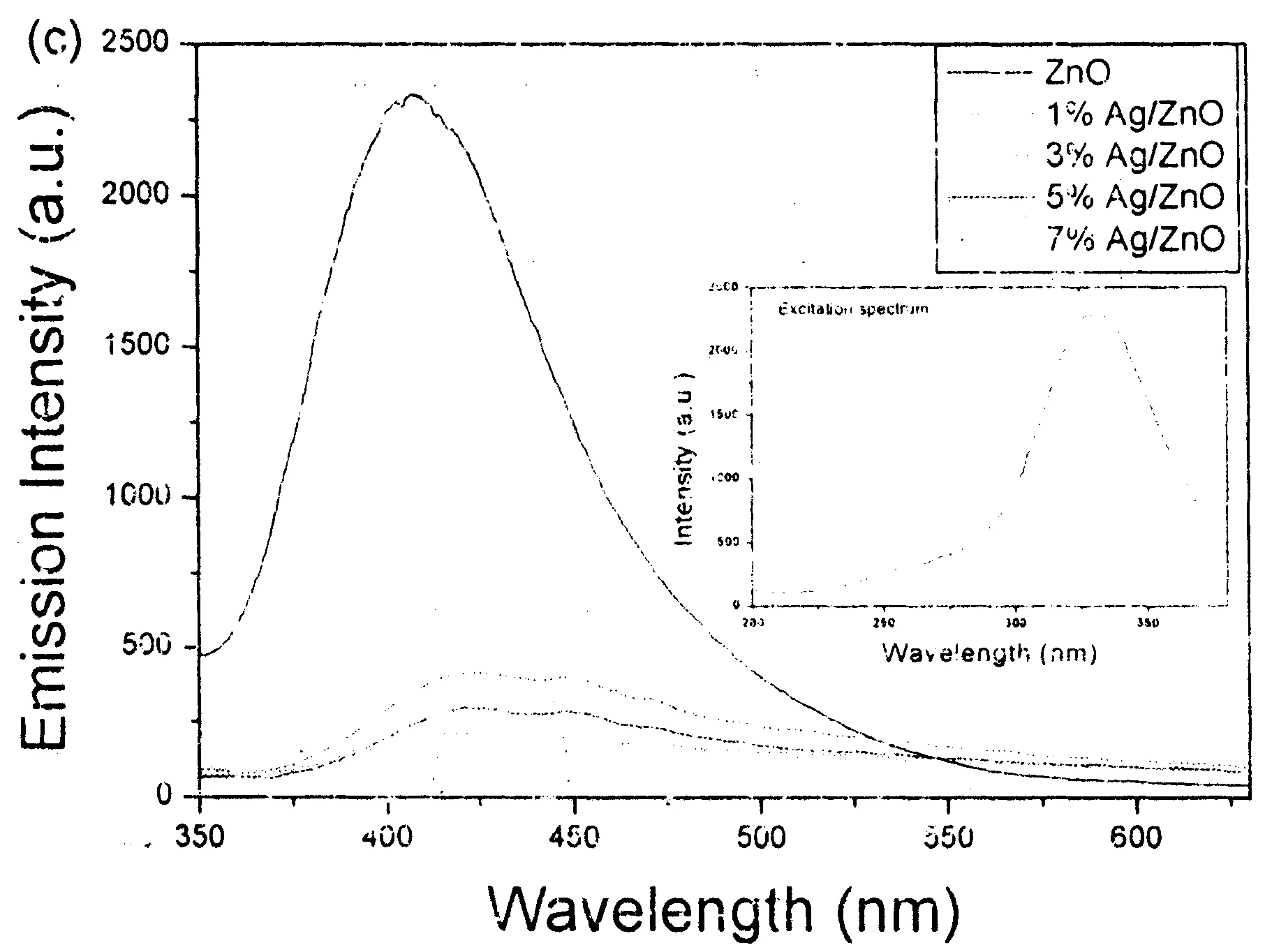
Figure 5 (a) UV-vis diffuse-reflectance spectra, (b) plots of (αhν)2 vs (hv) and (c) PL spectra of pure ZnO and Ag/ZnO hybrid microstructures with different Ag-loading contents. The inset in (c) is the excitation spectrum.
2.3 Photocatalytic activity

Ag-ZnO+h↔e-+h+
(4)
(5)
h++H2O→·OH+H+
(6)
h++OH-→·OH
(7)
(8)
2·HO2=H2O2+O2
(9)
Figs.6c and 6d depict the absorption spectra of RhB and MO solutions irradiated by the visible light for various durations in the presence of 20 mg 3% Ag/ZnO. RhB and MO solutions could be degraded with the prolonging of the irradiation time. After irradiating for 120 min, the degradation efficiency of RhB and MO reached nearly 100% and 80%, respectively. The color changes of RhB and MO solutions are separately displayed in the insets of Figs.6c and 6d.

Figure 6 The concentration-time curves of the organic dyes RhB (a) and MO (b) irradiated by the visible light in the presences of various catalysts of 20 mg; the absorption spectra of RhB (c) and MO (d) solutions after irradiated by the visible light for various durations under the presence of 20 mg 3% Ag/ZnO.
2.4 Antibacterial property
It was also found that the as-prepared Ag-nanoparticles-strewn ZnO microstructures presented prominent antibacterial performance. Fig.7a shows the histograms of the bacterial colony numbers of E. coli before and after introducing 0.1 mL 10 mg L-1ZnO flowerlike microstructures with various Ag loading contents. After E. coli has been cultured in an incubator at 37℃ for 24 h in the absence of ZnO or Ag/ZnO microstructures, the bacterial colony number was -250. When flowerlike ZnO microstructures were added, the bacterial colony number of E. coli decreased to -180 under the same culturing conditions, indicating that ZnO can restrain the propagation of E. coli. While Ag nanoparticles-strewn ZnO flowerlike microstructures were added into the system, the bacterial colony number further decreased. This proves that Ag/ZnO hybrid microstructures have stronger ability to restrain the propagation of E. coli than pure ZnO flowerlike microstructures. Also, with the increase of Ag nanoparticles loading content from 1% to 7%, the antibacterial capacity of the as-prepared product was further improved. Fig.7b clearly shows the antibacterial efficiencies of ZnO flowerlike microstructures with different Ag nanoparticles contents. Markedly, only 7% Ag nanoparticles contribute -75% antibacterial efficiency, which greatly reduces the use amount of Ag. It is very important for decreasing the cost in the practical application.

Figure 7 (a) The histograms of the bacterial colony numbers of E. coli in the presences of ZnO flowerlike microstructures with various Ag nanoparticles loading contents and (b) the antibacterial efficiencies of ZnO flowerlike microstructures with various Ag nanoparticles loading contents.
2.5 Electrochemical Measurements


Figure 8 (a) CV curves for 0.10 mM H2O2 in phosphate buffer (pH7.0) at bare and modified GCE (scan rate: 100 mV/s); (b) CV curves of 3 % Ag/ZnO/GCE in a series of H2O2 PBS solution; (c) the relation between the current and the concentration of H2O2.
Fig.8a shows the typical cyclic voltammogram (CV) curves of bare and modified GCE in 0.1 M N2-saturated PBS solution at pH7.0 in the presence of 0.10 mM H2O2. There were no obvious redox peaks observed at the bare GCE. However, all modified electrodes displayed exhibited increased current signal, implying that the as obtained ZnO or ZnO/Ag microstructures could promote the electron transfer between GCE and H2O2molecules. It was found that 3% Ag/ZnO microstructures had the largest current response toward H2O2sensing. Fig.8b shows the typical CV curves of 3% Ag/ZnO/GCE in PBS solutions with different concentrations of H2O2. Obviously, the current increases with the increase of concentration of H2O2, which implies possible application in the quantitative detection of H2O2. Fig.8c depicts the correlation between the current and the concentration of H2O2. A good linear relationship is readily visible. The sensitivity of the 3% Ag/ZnO was calculated to be -159.6 μA/mM.
3 Conclusion
In summary, versatile Ag nanoparticles-strewn porous ZnO flowerlike microstructures have been successfully prepared through a two-step route. It was found that the as-obtained Ag/ZnO hybrid microstructures presented outstanding visible-light photocatalytic capacity for the degradation of organic pollutants, highly effective antimicrobial activity toward Escherichia coli and high electrocatalytic ability to H2O2. Experiments showed that the amount of Ag nanoparticles loaded on the surfaces of porous ZnO nanosheets reduced the band gap of ZnO flowerlike microstructures. ZnO flowerlike microstructures with 3% Ag loading content presented the smallest band gap energy, the lowest PL emission peak, the strongest photocatalytic activity for the degradation of RhB and MO under the irradiation of the visible light, and the best electrocatalytic ability for the oxidization of H2O2. Meanwhile, Ag nanoparticles-strewn porous ZnO flowerlike microstructures exhibited stronger ability to restrain the propagation of E. coli than pure ZnO flowerlike microstructures. Also, with the increase of Ag nanoparticles loading content from 1% to 7%, the antibacterial capacity of the as-prepared product was further improved. The present work suggests that the as-obtained Ag/ZnO hybrid microstructures have promising applications as the visible-light photocatalyst, the antibacterial reagent for restraining the propagation of E. coli and the electrochemical sensor for the detection of H2O2.
[1] LI P, WEI Z, WU T, et al. Au-ZnO hybrid nanopyramids and their photocatalytic properties[J]. J Am Chem Soc, 2011, 133(15):5660-5663.
[2] HE W, KIM H K, WAMER W G, et al. Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity[J]. J Am Chem Soc, 2013,136(2):750-757.
[3] ZENG H, LIU P, CAI W, et al. Controllable Pt/ZnO porous nanocages with improved photocatalytic activity[J]. J Phys Chem C, 2008,112(49):19620-19624.
[4] YU C, YANG K, XIE Y, et al. Novel hollow Pt-ZnO nanocomposite microspheres with hierarchical structure and enhanced photocatalytic activity and stability[J]. Nanoscale, 2013,5(5):2142-2151.
[5] ZHENG Y, CHEN C, ZHAN Y, et al. Photocatalytic activity of Ag/ZnO heterostructure nanocatalyst: correlation between structure and property[J]. J Phys Chem C, 2008,112(29):10773-10777.
[6] LU W, LIU G, GAO S, et al. Tyrosine-assisted preparation of Ag/ZnO nanocomposites with enhanced photocatalytic performance and synergistic antibacterial activities[J]. Nanotechnology, 2008,19(44):445711-445716.
[7] ZHENG Y, ZHENG L, ZHAN Y, et al. Ag/ZnO heterostructure nanocrystals: synthesis, characterization, and photocatalysis[J]. Inorg Chem, 2007,46(17):6980-6986.
[8] LIN D, WU H, ZHANG R, et al. Enhanced photocatalysis of electrospun Ag-ZnO heterostructured nanofibers[J]. Chem Mater, 2009,21(15):3479-3484.
[9] ANSARI S A, KHAN M M, ANSARI M O, et al. Biogenic synthesis, photocatalytic, and photoelectrochemical performance of Ag-ZnO Nanocomposite[J]. J Phys Chem C, 2013,117(30):27023-27030.
[10] SATTER S S, HOQUE M, RAHMAN M M, et al. An approach towards synthesis and characterization of ZnO@ Ag core@ shell nanoparticles in water-in-oil microemulsion[J]. RSC Adv, 2014,4,20612-20615.
[11] GHOSH S, GOUDAR V S, PADMALEKHA K G, et al. ZnO/Ag nanohybrid: synthesis, characterization, synergistic antibacterial activity and its mechanism[J]. RSC Adv, 2012,2(3):930-940.
[12] LIU H R, SHAO G X, ZHAO J F, et al. Worm-like Ag/ZnO core-shell heterostructural composites: fabrication, characterization, and photocatalysis[J]. J Phys Chem C, 2012,116(30):16182-16190.
[13] WU Z, XU C, WU Y, et al. ZnO nanorods/Ag nanoparticles heterostructures with tunable Ag contents: A facile solution-phase synthesis and applications in photocatalysis[J]. CrystEngComm, 2013,15(30):5994-6002.
[14] YAMAMOTO O, NAKAKOSHI K, SASAMOTO T, et al. Adsorption and growth inhibition of bacteria on carbon materials containing zinc oxide[J]. Carbon, 2001,39(11):1643-1651.
[15] SAWAI J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay[J]. J Microbiol Methods, 2003,54(2):177-182.
[16] SHRIVASTAVA S, BERA T, ROY A, et al. Characterization of enhanced antibacterial effects of novel silver nanoparticles[J]. Nanotechnology, 2007,18(22):225103-225107.
[17] AZIZI S N, GHASEMI S, KAVIAN S. Synthesis and characterization of NaX nanozeolite using stem sweep as silica source and application of Ag-modified nanozeolite in electrocatalytic reduction of H2O2[J]. Bioelectron, 2014,62:1-7.
[18] HAN Y, ZHENG J, DONG S. A novel nonenzymatic hydrogen peroxide sensor based on Ag-MnO2-MWCNTs nanocomposites[J]. Electrochim Acta, 2013,90:35-43.
[19] ZHOU X F, HU Z L, FAN Y Q, et al. Microspheric organization of multilayered ZnO nanosheets with hierarchically porous structures[J]. J Phys Chem C, 2008, 112(31):11722-11728.
[20] LIU X, ZHANG J, WANG L, et al. 3D hierarchically porous ZnO structures and their functionalization by Au nanoparticles for gas sensors[J]. J Mater Chem, 2011,21(2):349-356.
[21] SONG R Q, XU A W, DENG B, et al. From layered basic zinc acetate nanobelts to hierarchical zinc oxide nanostructures and porous zinc oxide nanobelts[J]. Adv Funct Mater, 2007,17(2):296-306.
[22] HE R, QIAN X, YIN J, et al. Preparation of polychrome silver nanoparticles in different solvents[J]. J Mater Chem, 2002,12(12):3783-3786.
韩寒,倪永红,盛恩宏,等.花状Ag/ZnO微纳米结构:简单的合成和多功能应用[J].安徽师范大学学报:自然科学版,2015,38(3):217-227.
花状Ag/ZnO微纳米结构:简单的合成和多功能应用
韩寒1, 倪永红1, 盛恩宏1, 刘爱民2
(1.安徽师范大学 化学与材料科学学院,教育部功能性分子固体重点实验室,安徽分子材料实验室,安徽省功能性分子固体重点实验室,安徽 芜湖 241000;2.安徽师范大学 生命科学学院,安徽 芜湖 241000)
设计了一条两步路线成功合成了Ag纳米粒子点缀的ZnO花状微结构(Ag/ZnO).首先以六水合硝酸锌、尿素和L-半胱氨酸为反应原料,100°C下水热反应8h,合成出花状碱式碳酸锌前驱体,并经空气中高温煅烧得到多孔ZnO花状微结构;然后,通过光还原硝酸银得到了Ag/ZnO花状微结构.所得产物用X-射线衍射仪、扫描电子显微镜、透射电子显微镜、热重分析仪、红外光谱、紫外-可见漫反射光谱和荧光光谱等进行系统的表征.研究显示,所得的Ag/ZnO花状微结构在光催化、灭菌和电催化等领域都具有很好的应用.
Ag/ZnO花状微结构;光学性能;多用途
10.14182/J.cnki.1001-2443.2015.03.002
Foundation item:National Natural Science Foundation of China (21171005); Key Foundation of Chinese Ministry of Education (210098).
Received date:2015-03-10
Author's brief:HAN Han(1990-), Female, Master. Research direction: Micro/nano-materials; Corresponding author: NI Yonghong(1969-), male, Professor, Doctor. Research direction: controlled synthesis, characterization and performances of functional micro/nano-materials.
