Bi3+敏化Sr1.8Eu0.8-xV1.2P0.8O8的发光性质
2015-02-19李瀚博郑赣鸿李勇强戴振翔聂笑笑
李瀚博,郑赣鸿,李勇强,戴振翔,聂笑笑
(安徽大学 物理与材料科学学院,安徽省信息材料与器件重点实验室, 安徽 合肥 230039)
Bi3+敏化Sr1.8Eu0.8-xV1.2P0.8O8的发光性质
李瀚博,郑赣鸿*,李勇强,戴振翔,聂笑笑
(安徽大学 物理与材料科学学院,安徽省信息材料与器件重点实验室, 安徽 合肥230039)
摘要:采用固相法制备Sr1.8Eu0.8-BispanV1.2P0.8O8(0≤x≤0.7)系列粉末样品.研究样品中的Bi和Eu的相互作用,并讨论相关机理.用X射线衍射和荧光分光光度计对样品的结构和光学性质进行研究,结果表明:x=0.30时,样品的发光强度最强.
关键词:发光材料;敏化剂;Bi-Eu相互作用
0Introduction
The white light-emitting diode (w-LED) has been extensively investigated due to its advantages such as high reliability, high luminescent efficiency, long lifetime, low energy consumption, safety and its environment-friendly characteristic. It is expected to be the fourth generation light sources replacing the incandescent and fluorescent lamps[1-3]. Recently, Sr3V2O8and rare-earth (Re=Eu, Sm, Nb, Yb, etc) doped orthovanadates materials have attracted increasing interests due to their great potential application in luminescent[4-5]. Furthermore, Eu3+ion is an especially important activator for red phosphors, which has been extensively studied for years, because the visible emission of Eu3+ion is 4f shell is insensitive to the influence of the surroundings due to the shielding effect of 5s and 5p electron. More importantly, as reported in our previous work the luminescence of Eu3+ions can be well sensitized by the isolated VO43-group in Sr3V2O8matrix[6-7]. These advantages have impelled more and more researchers to study experimental synthesis process and characterization of rare earth-doped Sr3V2O8phosphor. At the same time, Bi3+ions can be used as the sensitizer of Eu3+luminescence through energy transfer from Bi to Eu in these systems such as YVO4:Bi3+,Eu3+[8], Y2O3:Bi3+,Eu3+[9], CaWO4:Bi3+,Eu3+[10], Y(Gd)BO3:Bi3+,Eu3+[11], which can also strengthen and broaden ultraviolet excitation bands[12]. In the current work, the effect of Bi on the Sr1.8Eu0.8V1.2P0.8O8phosphors has been investigated. Our results show that the incorporation of some Bi3+ions is favorable for the photoluminescence of Sr1.8Eu0.8V1.2P0.8O8.
1Experimental
1.1 Sample preparation
Sr1.8Eu0.8-xBixV1.2P0.8O8(x=0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, and 0.7) were prepared by the traditional solid state reaction method. The initiative materials were SrCO3(99.99%), Eu2O3(99.99%), Bi2O3(99.0%), NH4VO3(99.0%),(NH4)2HPO4(99.0%). The initial materials were mixed together in an agate mortar and finely ground. The obtained mixture was heated in an alumina crucible (in air) at 680 ℃ for 10 h. After being naturally cooled and carefully ground, these mixtures were pressed into tablets. Then the obtained mixtures were heated in an alumina crucible (in air) at 850 ℃ for 10 h. Finally, the obtained mixtures were heated in an alumina crucible (in air) at 1 000 ℃ for 12 h.
1.2 Characterization of Samples
The crystal structure of the phosphor powders was characterized by X-ray diffraction (XRD) analysis. In the process of XRD analysis, one X-ray diffractometer DX-2000 SSC with CuKαirradiation (λ=0.154 06 nm) is used, with operating voltage being 36 kV and the operating current being 25 mA. The activation and emission spectra were measured on a FL fluorescence spectrophotometer (F-4500). The weight of every sample was equal. All these operations were carried out at room temperature.
2Results and Discussion
2.1 The crystal structure of Sr1.8Eu0.8-xBixV1.2P0.8O8 (0≤x≤0.7)
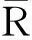
2.2 The photoluminescence properties of the Sr1.8Eu0.8-xBixV1.2P0.8O8 (0≤x≤0.7)
Fig.2a presents that the emission spectra in case ofx=0 under excitation at 365 nm. The four emission peaks locating at 594, 617, 656, and 702 nm are assigned to the transitions from the metastable orbital singlet state5D0to the spin-orbital states7Fj(j=1,2,3,4) of Eu3+. The5D0→7F1transition is magnetic-dipole-allowed and its intensity is almost independent of the local environment around Eu3+ions. The5D0→7F3transition exhibits a mixed magnetic dipole and electric dipole character[13]. The5D0→7F4is electric dipole transition. The5D0→7F2transition is electric-dipole-allowed due to an admixture of opposite parity 4fn-15d states by an odd parity crystal-field component[14-15]. Therefore, its intensity is sensitive to the local structure around Eu3+ions. When the Eu3+activator ion is located at a low-symmetry local site (without an inversion center), the electric dipole transition is often dominated in the emission spectra[16]. In addition, the results provide a piece of information as following:Sr3P2O7has not optical activity in our studying wavelength region, because we could not observe a distinct emission band.
The emission spectra of the Sr1.8Eu0.8-xBixV1.2P0.8O8samples withx=0.1, 0.2, 0.3, 0.4, 0.5, 0.6 and 0.7 are exhibited in Fig.2b and 2c. From this figure, it is found that the spectra are similar with that in the case ofx=0. However, the relative emission intensity of the four characteristic peaks of Eu3+is varied with the doped Bi3+content, especially for 617 nm. It is also found that, when Bi doped content increases fromx=0 to 0.3, the maximum emission intensity (at 617 nm) is enhanced by 33 times. The intensity of5D0→7F2(617nm) transitions is enhanced with Bi doping, which proves that there exists an efficient energy transfer process between Bi3+and Eu3+ion. In our previous studies, it has reported that in Sr3-3xEu2xV1.2P0.8O8(0 On the other hand, as we all know, acting as one sensitizer, Bi3+absorbs the excitation energy and then transfers the energy to an activator (a luminescent center).As a result, the emission intensity increases. There are electric mulitpole-multipole interaction and exchange interaction between sensitizer (S) and activator (A). Postulating that the dipole-dipole interaction plays an important role in the energy between Bi3+and Eu3+ion, the probability of energy transfer is given by the following equation[6] (1) wherePSAis the probability of energy transfer,QAis the absorption cross section ofA,Ris the distance betweenSandA,nis the refraction index of host lattice,τsis the radiative decay time ofS. On the other hand, (ε/x1/2εc)4is the local electric field,xis the dielectric constant. The integral represents the overlap between the normalizedSemission andAexcitation band. The distance in which the energy transfer betweenSandAcan occur is defined asRc.Considering structural factors,Rccan be described by the following equation (2) whereXcis the Bi3+ion concentration andNis the number of Bi3+ions in the unit cell. In this case, as Bi3+concentration increases, the distance between Bi3+and Eu3+ion decreases, subsequently the energy transition probability increases and consequently red light by Eu3+increases. However, when Bi3+ion content is more than 0.30, the emission intensity decreases, in other words, the optimal Bi3+doped content in our samples is 0.30. This can be understood as follows. One is the decrease of Eu3+ions with Bi3+addition in Sr1.8Eu0.8-xBixV1.2P0.8O8, which directly results in the luminescent intensity lower. On the other hand, when Bi content is more than 0.30, the distance between Bi and Bi would be near enough to cause one efficient energy transfer process between Bi ions. Such an energy transfer process may reduce the probability of energy transfer from Bi to Eu. As a result, the sensitized effectiveness of Bi on the Eu emission intensity is reduced, which leads to the strengthening of emission intensity limits. In particular, in case ofx=0.6 and 0.7 the emission intensity is even much lower than that of thex=0 sample as shown in Fig.2c. Additionally, the effect of structural change on a radiation transition of Eu3+ion by Bi3+addition due to the difference between Bi3+and Eu3+ions may be considered. For clarity, the emission intensity at 617 nm vs Bi concentration of Sr1.8Eu0.8-xBixV1.2P0.8O8sample is shown in Fig.3. With increasing the Bi concentrationx, the Eu3+emission intensity is found to increase gradually and reach the maximum atx=0.3. As discussed above, the energy transfer between Bi and Eu plays an important role in the emission of the Sr1.8Eu0.8-xBixV1.2P0.8O8(0≤x≤0.7) systems. In our present samples, with Bi concentration increasing, the distance between Bi and Eu ion decreases correspondingly, the energy transition probability increases subsequently, and thus red light by Eu is enhanced. However, in the case of the amount of Bi being bigger than 0.3, the distance between Bi and Bi ions may be close enough to cause the direct Bi-Bi energy transfer. Such a direct energy transfer process results in the lower energy transfer between Bi-Eu. Therefore, the emission intensity decreases as shown in Fig.3. Fig.4a presents the excitation spectra of Sr1.8Eu0.8V1.2P0.8O8sintered at 1 000 ℃ for monitoring the5D0→7F2transition of Eu3+. In the short wavelength region from 200 to 300 nm, it exhibits a shoulder peak at about 272 nm(marked as arrow). This is assigned to the excitation of the Sr3V2O8host crystal due to O2--V5+charge transfer, followed by energy to Eu3+. This peak is also overlapped by the excitation peak due to O2--Eu3+charge transfer around 260 nm[17]. Another peak located at 318 nm(7F0→5H6) is likely to the f-f transitions within Eu3+4f6configuration. In the longer wavelength region, the excitation band exhibits some weaker narrow bands, resulting from the f-f transitions within Eu3+4f6electron configuration. In this region, the main peaks at 318, 385, 398, 467, and 538 nm correspond to the electron transitions from the7F1ground state to5L7,5L6,5D2, and5D1, consistent with spectral characterizations observed in previous work[18-19]. Fig.4b and 4c present the excitation spectra of the samples Sr1.8Eu0.8-xBixV1.2P0.8O8withx=0.1, 0.2, 0.3, 0.4, 0.5, 0.6 and 0.7. They show the same excitation profiles as thex=0 sample. With Bi doping, the Eu content decreases, the shoulder at 272 nm is weaker and gradually disappears. At the same time, the excitation peak at 318 nm becomes stronger, even for 0.1≤x≤0.7 samples, only one larger peak near 315 nm is observed. As discussed before, the peak located at 318 nm comes from the f-f transition within Eu3+4f6configuration. The excitation intensity at 315 nm is enhanced with Bi doping shows the sensitization effect of the Bi3+ion on the Eu3+emission plays an important role in the excitation region of the V-O components. On the other hand, the phenomenon is ascribed to the appearance of the charge transition from Bi3+to V5+with Bi doping. With increasing Bi concentration from 0.1 to 0.3, this charge transition becomes stronger and brings about the increase of excitation intensity, shown in Fig.4a. With increasing the Bi concentrationx, the excitation intensity at 318 nm increases gradually and then decreases. The strongest excitation intensity is achieved in case ofx=0.3 sample. This phenomenon is attributed to the decrease of Eu content with Bi doping. In addition, forx=0.6 and 0.7 samples, another broad excitation band with a peak at 341 nm is also found. Generally speaking, this broad excitation is attributed to the1A1→1T1transition of VO43-and the1S0→3P1transition of Bi3+in YVO4:Eu, Bi[8]. In this case, 40% VO43-has been replaced by PO43-, therefore, the contribution of1A1→1T1transition of VO43-may be not obvious. And only when the Bi content reaches some certain contentx=0.6 and 0.7, this peak at 341 nm appears. The result is corresponding with the disc ussion in emission part. That is to say, with Bi doping, the distance between Bi and Bi ions may be close enough to cause the direct Bi-Bi energy transfer. Such a direct energy transfer process results in the lower energy transfer between Bi-Eu. 3Conclusions We have synthesized the Sr1.8Eu0.8-xBixV1.2P0.8O8(0≤x≤0.7) phosphors by the conventional solid-state reaction and investigated their ultraviolet activation and emission spectra. The host crystal structure is found to vary little with Bi doping. The emission spectra of the phosphors under excitation at 365 nm exhibit four emission peaks located at 594, 617, 656, and 702 nm. The emission intensity enhances with increasing Bi. It is ascribed to the two following reasons, one is the decrease of Eu content with Bi doping and make Eu-Eu aggregation destroy. The other is the energy transfer between Eu3+and Bi3+. However, with increasing Bi concentration further, the decrease of Eu content makes the emission intensity lower. At the same time, the direct Bi-Bi energy transfer occurs and makes the energy transfer between Bi-Eu lower with Bi doping. As a result, the emission intensity is lower correspondingly. The peak at 318 nm in the excitation spectra is assigned to the f-f transition within Eu3+4f6configuration. The f-f transitions within Eu3+4f6electron configuration also lead to the other three peaks in the longer wavelength region. The excitation intensity increases with Bi doping,however,with Bi concentration increasing further,the Eu concentration decreases, Eu excitation intensity is lowered in Sr1.8Eu0.8-xBixV1.2P0.8O3phosphors. Additionally, with Bi content reaches 0.6 and 0.7, the broad excitation band with a peak at 341 nm appears which is attributed to1S0→3P1transition of Bi3+. References: [1]Schubert E F, Kim J K.Solid-state light sources getting smart[J].Science, 2005,308:1274-1278. [2]Hoppe H A.Recent developments in the field of inorganic phosphors[J]. Chem Int Ed, 2009, 48:3572-3578. [3]Li P L, Wang Z J, Yang Z P, et al. Luminescent characteristics of LiSrBO3:M (M=Eu3+, Sm3+, Tb3+, Ce3+, Dy3+) phosphor for white light-emitting diode[J]. Mater Res Bull,2009,44:2068-2071. [4]Rao B V, Jang K W, Lee H S,et al. Synthesis and photoluminescence characterization of RE3+(=Eu3+, Dy3+)-activated Ca3La(VO4)3phosphors for white light-emitting diodes[J].J Alloys and Compds, 2010,49:251-255. [5]Lin H Y, Chang W F, Chu S Y, et al. Luminescence of (Ca,Sr)3(VO4)2:Pr3+, Eu3+phosphor for use in CuPc-based solar cells and white light-emitting diodes[J].J Lumin, 2013,133:194-199. [6]Cao S, Ma Y Q, Yang K, et al. The effect of PO4doping on the luminescent properties of Sr3-3zEu2zV2-xPxO8[J]. Opt Materials,2010,32:1256-1260. [7]Zhu W L, Ma Y Q, Zhai C, et al. Photoluminescence properties of Sr3(VO4)2, Sr2Y2/3-yEuy(VO4)2and Sr2Y2/3-zSmz(VO4)2[J]. Opt Materials, 2011,33:1162-1166. [8]Chen L, Chen K J, Lin C C, et al. Combinatorial approach to the development of a single mass YVO4:Bi3+,Eu3+phosphor with red and green dual colors for high color rendering white light-emitting diodes[J]. J Comb Chem, 2010,12:587-594. [9]Ju G F, Hun Y H, Chen L, et al, Luminescence properties of Y2O3:Bi3+, Ln3+(Ln=Sm, Eu, Dy, Er, Ho) and the sensitization of Ln3+by Bi3+[J]. J Lumin, 2012,132:1853-1859. [10]Pode R B, Dhoble S J. Photoluminescence in CaWO4:Bi3+, Eu3+material[J]. Phys State Sol (b),1997,203:571-577. [11]Zeng X, Kim S J,Jang S H, et al.Luminescent properties of (Y,Gd)BO3:Bi3+,RE3+(RE=Eu, Tb) phosphor under VUV/UV excitation[J]. J Lumin, 2006,121:1-6. [12]Takeshita A, Nikkura A, Nikkura S, et al. Low-temperature wet chemical synthesis and photoluminescence properties of YVO4:Bi3+, Eu3+nanophosphors[J].J Lumin, 2008,128:1515-1522. [13]Ray S, Pramanik Q.Optical properties of nanocrystalline Y2O3:Eu3+[J]. J Appl Phys,2005,94:094-312. [14]Judd B. Optical absorption intensities of rare-earth Ions[J].Phys Rev,1962,127:750. [15]Ofelt G. Intensities of crystal spectra of rare-earth Ions[J].J Chem Phys,1962,37:511-513. [16]Yu W, Wang Z, Wang Z, et al. Fabrication, patterning, and optical properties of nanocrystalline YVO4:A (A=Eu3+, Dy3+, Sm3+, Er3+) phosphor films via sol-sel soft lithography[J]. Chem Mater,2002,14:2224-2231. [17]Wang Y H, Zuo Y Y, Gao H, et al. Luminescence properties of nanocrystalline YVO4:Eu3+under UV and VUV excitation[J].Mater Res Bull,2006,41:2147-2150. [18]Li Y H, Hong G Y. Synthesis and luminescence properties of nanocrystalline YVO4:Eu3+[J]. J Solid State Chem, 2005,178:645-649. [19]Riwotzki K, Haase M. Engineering a molecular model for water phase equilibrium over a wide temperature range[J]. J Phys Chem B,1998,102:1029-1035. (责任编辑郑小虎) 通信作者 作者简介:曹向阳(1989-),男,安徽亳州人,安徽大学硕士研究生;*郭建友(),安徽大学教授,博士生导师,E-mail:jianyou@ahu.edu.cn. 基金项目:国家自然科学基金资助项目(11175001) 收稿日期:2014-06-12 doi:10.3969/j.issn.1000-2162.2015.02.009
