Netrin-1 overexpression in bone marrow mesenchymal stem cells promotes functional recovery in a rat model of peripheral nerve injury
2015-02-14XianjinKeQianLiLiXuYingZhangDongmeiLiJianhuaMaXiaomingMao
Xianjin Ke,Qian Li,Li Xu,Ying Zhang,Dongmei Li,Jianhua Ma,Xiaoming Mao
1Department of Neurology,Affiliated Hospital of Jiangsu University,Zhenjiang,Jiangsu 212001,China;2Department of Endocrinology,Nanjing First Hospital,Nanjing Medical University,Nanjing,Jiangsu 210006,China.
Netrin-1 overexpression in bone marrow mesenchymal stem cells promotes functional recovery in a rat model of peripheral nerve injury
Xianjin Ke1,△,Qian Li2,△,✉,Li Xu1,Ying Zhang2,Dongmei Li2,Jianhua Ma2,Xiaoming Mao2
1Department of Neurology,Affiliated Hospital of Jiangsu University,Zhenjiang,Jiangsu 212001,China;
2Department of Endocrinology,Nanjing First Hospital,Nanjing Medical University,Nanjing,Jiangsu 210006,China.
Transplantation of bone marrow mesenchymal stem cells(BMSCs)has been developed as a new method of treating diseases of the peripheral nervous system.While netrin-1 is a critical molecule for axonal path finding and nerve growth,it may also affect vascular network formation.Here,we investigated the effect of transplanting BMSCs that produce netrin-1 in a rat model of sciatic nerve crush injury.We introduced a sciatic nerve crush injury,and then injected 1×106BMSCs infected by a recombinant adenovirus expressing netrin-1 Ad5-Netrin-1-EGFP or culture medium into the injured part in the next day.At day 7,14 and 28 after injection,we measured motor nerve conduction and detected mRNA expressions of netrin-1 receptors UNC5B and Deleted in Colorectal Cancer(DCC), and neurotrophic factors brain-derived neurotrophic factor(BDNF)and nerve growth factor(NGF)by real-time PCR.We also detected protein expressions of BDNF and NGF by Western blotting assays and examined BMSCs that incorporated into myelin and vascellum.The results showed that BMSCs infected by Ad5-Netrin-1-EGFP significantly improved the function of the sciatic nerve,and led to increased expression of BDNF and NGF(P<0.05).Moreover,28 days after injury,more Schwann cells were found in BMSCs infected by Ad5-Netrin-1-EGFP compared to control BMSCs.In conclusion,transplantation of BMSCs that produce netrin-1 improved the function of the sciatic nerve after injury.This method may be a new treatment of nerve injury.
bone marrow mesenchymal stem cells,netrin-1,UNC5B,Deleted in Colorectal Cancer,brain-derived neurotrophic factor,nerve growth factor
Introduction
In humans and other animals,injury of nerves of the peripheral nervous system(PNS)that innervate the body and viscera significantly decreases the function of innervated tissues.Experimental models of sciatic nerve injury or sciatic axotomy have proven to be invaluable in understanding the consequences of nerve injury and test strategies that may improve its functional consequences. Sciatic nerve injury has been used for evaluating efficacy of therapeutic strategies aimed at promoting nerve regeneration and functional recovery.Recently,stem cell transplantation has increasingly been used for treating nerve injury[1-2].Schwann cells are peripheral glial cells that form myelin in the PNS and can support nerve regeneration in both PNS and the central nervous system(CNS). Transplantation of Schwann cells promotes the recoveryfrom nerve injury[3],but may result in immunological rejection when donor cells are not from the host animal. Furthermore,it is difficult to harvest and expand Schwann cells in numbers that suffice for cell-based therapy during the time window when nerve recovery may occur.
BMSCs are multi-potential stem cells with relatively low immunogenicity.They differentiate into many types of cells,such as osteoblasts,adipocytes and endothelial cells[4].They also develop into neural lineages,such as neurons and astrocytes both in vivo and in vitro[5-7].BMSCs can be induced to differentiate into Schwann cells by sequentially treating the cells with several reagents and trophic factors:firstly with β-mercaptoethanol,followed by retinoic acid,and then culturing in the presence of forskolin(a reagent known to increase the level of intracellular cyclic adenosine monophosphate),basic fibroblast growth factor,platelet-derived growth factor-AA,and heregulin-β1[8-10]. BMSCs are easily accessible through aspiration of the bone marrow from patients(which also pose no serious ethical problem)and they can be quickly expanded for auto-transplantation.
While transplantation of BMSCs promotes regeneration of peripheral nerves and alleviates the sciatic nerve injury,several characteristics of transplanted cells remain problematic:their vitality,age,homing and low survival.Here,we speculated that transplanted BMSCs combined with neuronal guidance factor netrin-1 may provide a novel therapeutic alternative to recovery from sciatic nerve injury.Netrins are a family of secreted proteins that direct the migration of neuronal cells and axon growth cones during neural development[11].Netrins function either as attractants or as repellents that bind to their classical receptors UNC5H or Deleted in Colorectal Cancer(DCC)[12]. Netrin-1 is an important survival factor that acts via DCC and UNC5H[13],and it also acts as an angiogenic factor and induces brain neovascularization[14-16]. Netrin-1 induces proliferation of Schwann cells through UNC5B receptor[17].
Taking into account the biological functions of BMSCs and netrin-1,we constructed a recombinant adenovirus netrin-1 vector(Ad5-netrin-1-EGFP)to examine the efficacy of a therapy that combined netrin-1 with BMSC transplantation in a model of sciatic nerve injury.We demonstrated that BMSCs can differentiate into Schwann and endothelial cells.We found that netrin-1 increased the level of NGF and BDNF.All these results showed that transplantation of BMSCs in fected with Ad5-Netrin-1 enhanced the recovery of sciatic nerve crushed injury.
Materials and methods
Adenovirus vector construction and production
We completed the construction of the Netrin-1 recombinant adenovirus in 3 steps.Firstly,Netrin-1 cDNA was cloned by RT-PCR and then subcloned into shuttle vector pDC316-CMV,which carries the reporter gene EGFP.Secondly,after identification by nuclease digestion analysis and sequencing analysis,we transfected this newly constructed plasmid pDC316-Netrin-1 together with adenovirus-packaging plasmid pBHGlox_E1.3Cre into human embryonic kidney cells HEK293 by using Lipofectamine 2000.Based on homologous recombination of 2 plasmids within HEK293 cells,the recombinant adenovirus vector carrying Netrin-1,Ad5-Netrin-1-EGFP was created. Thirdly,Ad5-Netrin-1-EGFP was subsequently identified by PCR,purified using repeated plaque passages, proliferated by freezing and melting with HEK293 cells,and titrate dusing 50% Tissue Culture Infective Dose(TCID50).BMSCs of Sprague-Dawley rats aged from 3-4 weeks were primary cultured and infected with A d5-Netrin-1-EGFP. Expression of netrin-1 was detected by Western blotting assays.
Isolation and cultivation of BMSCs
BMSC expansion was performed as described previously[18].In brief,we sacrificed male Sprague-Dawley and harvested the bone marrow by flushing the cavity of the femurs and tibias with phosphate buffer saline(PBS).Bone marrow cells were introduced into 100-mm dishes and cultured in L-DMEM (Hyclon,USA).A small number of cells developed visible symmetric colonies by 5 to 7 days.Non-adherent hematopoietic cells were removed and the medium was replaced.Adherent and spindle-shaped BMSC population was expanded to over 50 million cells after 4-5 passages.Quality of BMSCs was checked by flowcytometry.BMSCs were stained with CD 105-PE, CD73-PE,CD90-FITC,CD34-PE and CD45-PE,and data was acquired by a Beckman Coulter flow cytometer.BMSCs were identified as CD105,CD73, CD90 triple-negative,and CD105,CD106 double-positive cells(data not shown).
Sciatic nerve crush injury model establishment
Male Sprague-Dawley rats weighing 200-250 g were used in this study(Shanghai Laboratory Animal Center,Chinese Academy Sciences).A total of 30 rats were anesthetized by intraperitoneally injecting 10%chloral hydrate(300 mg/kg).For each rat,the left hindquarter was carefully shaved.The sciatic nerve was crushed 8 mm proximal to its trifurcation with hemostatic forceps for 30 seconds.The crush site was marked with a 9-0 suture,and then a 3-mm injured region was generated.The muscle was closed with 5-0 nylon sutures,and the skin was closed with interrupted 3-0 nylon sutures[19].The study protocol was approved by the local institutional review board at the authors’affiliated institutions and animal studies were carried out in accordance with the established institutional guidelines regarding animal care and use. Animal welfare and the experimental procedures were carried out strictly in accordance with the Guide for Care and Use of Laboratory Animals(National Institutes of Health of USA,1996).
Transplantation of BMSCs to the injured sciatic nerve
Rats were divided randomly into 3 groups(n=12 rats per group).The control group received 0.05 mL L-DMEM.The BMSC group received 0.05 mL LDMEM with 1×106BMSCs infected by Ad5-EGFP.The BMSC+Netrin-1 group received 0.05 mL L-DMEM with 1×106BMSCs infected by Ad5-Netrin-1-EGFP.In the last two groups,rats were injected with BMSCs into the injured site.Prior to transplantation,BMSCs were infected with Ad5-EGFP or Ad5-Netrin-1-EGFP.After GFP expression in BMSCs was confirmed by fluorescence microscopy, cells were transplanted as an artificial graft.
Sciatic nerve function
Behavior analysis was performed by gait pattern assessment of the lower limb,and a piece of white paper(A4 paper mm)was placed in the bottom of a corridor.The hind feet of each rat were dipped into black ink,and then they were allowed to walk down the corridor leaving their footprints on the paper. Walking track analysis of each rat was performed at day 7,14 and 28 after the operation.
The sciatic nerve function index(SFI)was calculated using several measurements as inputs,including PL(third toe to heel),TS(first to fifth toe)and ITS (second to fourth toe).They were measured on the experimental(E)sides(EPL,ETS and EITS,respectively)and the contralateral normal(N)sides(NPL, NTS and NITS,respectively).SFI was calculated as follow s:SFI=13.3×(EIT-NIT)/NIT+109×(ETSNTS)/NTS+(-38.3×(EPL-NPL)/NPL)-8.8[20].SFI oscillates around 0 for normal nerve function,whereas SFI around-100 represents total dysfunction.
Measurement of motor nerve conduction velocity
Measurement of motor nerve conduction velocity (MNCV)was measured 28 days after operation. Temperature was maintained between 37°C and 37.5°C in air-conditioned room.During the procedure, an electromyograph(Tonsbakken 16-18 DK-2740 Skovlunde,Denmark)was performed to record the electrical activity of muscles.Briefly,after general anesthesia was induced by intraperitoneal injection of 10% chloral hydrate(300 mg/kg)in rats,the left sciatic nerve was exposed and directly stimulated at the distal end of the proximal sciatic nerve segment by using bipolar hook-shaped electrodes.M wave was recorded by electromyogram at the first interosseous muscle via unipolar needle electrodes.A ground was placed on a muscle between the 2 electrodes.Next,the distal nerve was directly stimulated at the Achilles tendon and the M wave was also recorded.MNCV was calculated by dividing the distance between 2 stimulating points by time interval.It was measured 3 times for each rat.MNCV of the right sciatic nerve(contralateral intact side)was also measured.
Quantitative real-time RT-PCR
Total RNA was extracted from the injured portion of the nerve(the distal nerve to the repair or crush site was harvested)(n=4)using TRIzo l reagent (Invitrogen,Carlsbad,CA,USA)as recommended by the manufacturer.Aliquots of total RNA(2 μg)were reversely transcribed to cDNA using a reverse reaction kit(TaKaRa,Osaka,Japan)as recommended.Primer sequences were used for UNC5B:(sense)5′-CGACCCTAAAAGCCGCCCC-3′and(antisense) 5′-GGGATCTTGTCGGCAGAGTCC-3′;DCC (sense)5′-ACATCCGACGTTCGGCTTT-3′and (antisense)5′-TGATTTTCCCATTGGCTTCC-3′; BDNF:(sense)5′-CCATAAGGACGCGGACTTGTAC-3′and(antisense)5′-GAGGAGGCTCCAAAGGCACTT-3′;NGF:(sense)5′-GGACGCAGCTTTCTATCCTGG-3′and(antisense)5′-CCCTCTGGGACATTGCTATCTG-3′;β-actin (sense)5′-ACTATCGGCAATGAGCGGTTC-3′and (antisense)5′-AGAGCCACCAATCCACACAGA-3′. The target cDNAs were quantified using a real-time RT-PCR kit(SYBR Green Real time PCR master mix;TaKaRa).PCR was carried out in triplicate on a ABI Prism7500 sequence detection system(Applied Biosystems,Foster City,CA,USA)at 95°C for 30 seconds,40 cycles at 95°C for 15 seconds,34 seconds at 58°C to 65°C for different primer sets and 1 minute for 60°C.To confirm the specificity of amplification,PCR products from each primer pair were analyzed by melting curve analysis.Expression of mRNA was evaluated by threshold cycle(CT)values.CTvalues were normalized by the expression level of β-action.The relative amount of mRNA specific to every target gene was calculated using the 2-ΔΔCTmethod,where Ct=(CtTarget-Ct)-(Ct-Ct)[21].
ActinT TimeX Target ActinT Time0
Western blotting assay
Samples containing 50 μg of total protein from the injured nerve were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis(SDSPAGE)and electroblotted ontonitrocellulose membranes(Bio-Rad),and then blocked with 5% defatted milk for 2 hours at 37°C.The membranes with the transferred proteins were incubated with rabbit anti-BDNF and anti-NGF(1:500,Santa Cruz Biotechno logy, Santa Cruz,CA,USA)primary antibodies,followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin(IgG)(1:2,000)as the secondary antibody.Chemiluminescence reaction was carried out with an ECL kit(Pierce)for 1 minute, followed by exposure to a Kodak X-Omat radiographic film.Similar procedures were carried out with anti-βactin(1:500;Santa Cruz Technology)antibody.
Immunofluorescent microscopy
Immunofluorescence double staining on another set of rats(n=4)was performed to assess whether the implanted BMSCs had been incorporated in the myelin and vascellum.fouurteen and 28 days after implanting BMSCs transfected with virus or L-DMEM,the injured nerves were gathered,frozen and sectioned at 5 μm thickness.Survival of BMSCs in vivo was confirmed by identification of EGFP+spots under fluorescent microscopy.The differentiation of BMSCs into endothelial or Schwann cells was respectively determined by immunofluorescence double staining.We used the following antibody sets:(I)primary antibodies:rabbit anti-rat CD31(Santa Cruz Biotehnology) and anti-S100 beta antibody(Abcam);(II)secondary antibody goat anti-rabbit conjugated to Cy3 fluorophore(IgG.Abcam).Endothelial or Schwann cells differentiated were confirmed by identification of the double stained cells by both EGFP and CY3 under a laser scan copolymerization microscope.
Statistical analysis
Data from independent experiments were shown as mean±standard deviation(SD).The groups were compared using the two-tailed Student's t-test or ANOVA. P<0.05 was considered statistically significant.

Fig.1 Netrin-1 and GFP are expressed in transfected BMSCs.A:Netrin-1 protein expression in BMSCs after Ad5-EGFPNetrin-1 successful transfection.Netrin-1 is at 67 kDa.β-actin acted as the internal control.Lane 1:Ad5-EGFP;Lane 2:Ad5-Netrin-1-EGFP(50 particles/cell);Lane 3:Ad5-Netrin-1-EGFP(100 particles/cell).B: BMSCs infected with Ad5-EGFP-Netrin-1 at 24h,EGFP is expressed in most BMSCs(Scale bars=50 μm).
Results
Adenovirus vector mediated-netrin-1 expression in BMSCs
To determine whether Ad5-EGFP-Netrin-1 sustained netrin-1 expression,we examined netrin-1 expression in BMSCs infected with Ad5-Netrin-1-EGFP at different multiplicities of infection.After infection of BMSCs with Ad5-Netrin-1-EGFP, netrin-1 was detected by Western blotting assay. EGFP-expression of BMSCs was examined by fluorescence microscopy(Fig.1A-B).
Function of the sciatic nerve
One week after surgery,we found that the gait of rats in the 3 groups was unstable.The left limb was obviously pulled,and footprint was unclear.Thus,measurements were not exact,and data were not included in the statistical analysis.From 2 to 4 weeks,the function of the sciatic nerve recovered gradually.The recovery,as reflected by SFI changes,in the transplantation group was superior to that in the control group.At days 14 and 28,SFI was -23.69±3.17 and-15.45±3.19 in the BMSC/Netrin-1 group,respectively,which was higher than that in the BMSC(-33.81±0.74,-26.24±7.16)and control (-53.06±1.65,-40.98±1.96)groups(P<0.05). However,no significant differences were found betweenthe BMSC/Netrin-1(-35.49±4.30)and BMSC(-43.35±5.37)group at day 7(P>0.05),although both groups were significantly different from the control group (-63.20±1.61,P<0.01).The SFI changes of each group were significantly different at day 7,14 and 28(all P<0.01,Fig.2).
Electrophysiological study
In the electrophysiological study,at 4 weeks after surgery,the mean MNCV was(13.75±0.78)m/s, (17.26±0.92)m/s,and(22.29±1.19)m/s in the control group,the BMSCs group and the BMSCs/Netrin-1 group,respectively.The difference was statistically significant(Fig.3).
Differential expression of netrin-1 receptors and neurotrophic factors in normal and injured nerve
Expressions of UNC5B,DCC,BDNF and NGF were at low levels in the normal nerve,and UNC5B and DCC,the receptors for netrin-1,were significantly up-regulated at day 7 and 14 compared with the normal group,but they were significantly lower at day 28 than at day 7 and 14.At day 7,there were no significant differences among the groups(all P>0.05).The expression of BDNF and NGF had a similar tendency. BDNF levels were the highest at day 7,while NGF levels were the highest at day 14.There were significant differences among the groups(all P<0.05).At day 28,they returned to normal with no significant differences among the groups(all P>0.05).The mRNA expressions of UNC5B,DCC,BDNF and NGF in each group had significant differenceat day 7,14 and 28 (all P<0.05)(Fig.4A-D).
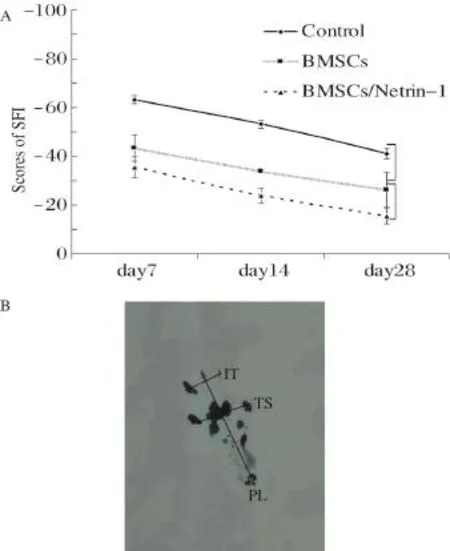
Fig.2 The function of the sciatic nerve was evaluated.A: Neurobehavioral evaluation.A representative illustration of the sciatic nerve function index(SFI)from day 7 to 28 after surgery in the three treatment groups is shown.B:The sketch map of SFI was shown.
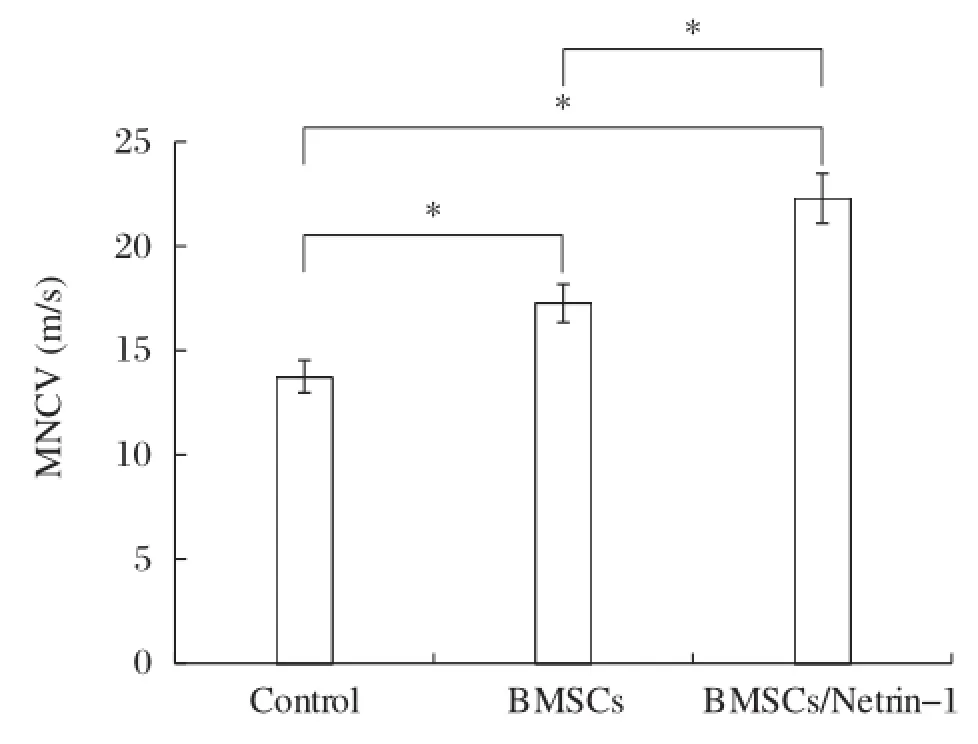
Fig.3 Bar graphs demonstrated the motor nerve conduction velocity(MNCV)in 4 weeks after transplantation.*P<0.05.
The expression of BDNF and NGF in the injured nerve
In injured nerves,BDNF and NGF protein levels changed similarly to the time-course of mRNA changes.The highest level of BDNF was observed at day 7 and 14 for NGF.There were significant differences among the groups (all P<0.05).At day 28,the protein levels decreased and approached normal levels,with no significant differences among the groups(all P>0.05).BDNF level in the BMSC/Netrin-1 group had significant difference at day 7,14 and 28.In the BMSC group,and there was a significant difference between day 28 and day 7 or day 14 (P<0.05),but no significant difference was found between day 7 and day 14(P>0.05).NGF level in the BMSC/ Netrin-1and BMSCs groups had significant difference among day 7,14 and 28.BDNF and NGF levels in the control group have no significant difference among day 7,14 and 28(all P>0.05,Fig.5A-B).
Assessment of BMSCs incorporated in the myelin and vascellum
EGFP-labeled BMSCs transplanted in the injured nerve were observed using fluorescent microscopy. BMSCs were detected by their expression of GFP on day 7,14 and 28,which declined over time.We foundthat some implanted BMSCs were located in the nerve during recovery and expressed the Schwann cell marker S100 beta.At day 14 or 28,treatment with either BMSCs alone(335.6±34.08 relative density/mm2)or BMSCs/Netrin-1(472.6±23.60 relative density/mm2) significantly enhanced the expression of Schwann cells as compared tonon-treatment(P<0.05).Furthermore, treatment with BMSCs/Netrin-1 showed a higher expression than BMSCs alone(P<0.05).At day 7, treatment with either BMSCs alone(114.8±13.77 relative density/mm2)or BMSCs/Netrin-1(122.8± 15.50 relative density/mm2)was higher than the control group(P<0.05),but there was no difference between them(P>0.05).The number of Schwann cells induced by BMSCs in the BMSC/Netrin-1 group was higher than that in the BMSC group(Fig.6A-B).The expressions of Schwann cells in the BMSCs and BMSC/ Netrin-1 group showed significant difference among day 7,14 and 28(all P<0.05).We also observed some double stained cells in the surface of the injured nerve at day 14,suggesting that some transplanted BMSCs were involved in vessel formation(Fig.6C).
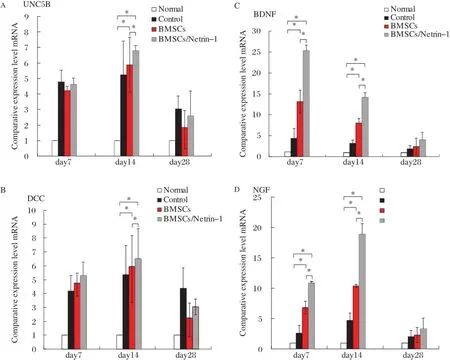
Fig.4 mRNA levels of Netrin-1 receptors and neurotrophic factors changes in the injured nerve.Quantitative RT-PCR analysis of mRNA expression of UNC5B(A),DCC(B),BDNF(C)and NGF(D)after rat sciatic nerve crush injury.*P<0.05.
Discussion
Sciatic nerve crush is the most commonly studied nerve injury and has been used to test numerous neuroregenerative therapeutic modalities[22-25].Crush injury of the peripheral nerve breaks the neurites,endoneurium,perineurium and the surface of microvessels. The broken parts may recover if a micro-environment that promotes neural regeneration is restored.While transplantation of BMSCs or Schwann cells in peripheral nerve injury is known to promote recovery[10],we showed that transplantation of BMSCs that were infected with netrin-1 had distinct advantages over previously tried strategies.We designed our experiment by utilizing the abilities of both cells and molecules topromote neural regeneration.BMSCs and netrin-1 were used to rescue nerves after injury.We assessed their efficacy by measuring the time-dependent change of several biochemical,cellular,electrophysiological and physiological parameters,and found that the combined therapy was significantly more effective than transplanting BMSCs alone.We showed that nerve conduction and gait were both significantly improved.These changes may be due to the differentiation of transplanted cells into Schwann cell myelin and the incorporation of some transplanted cells into the perineural vasculature.
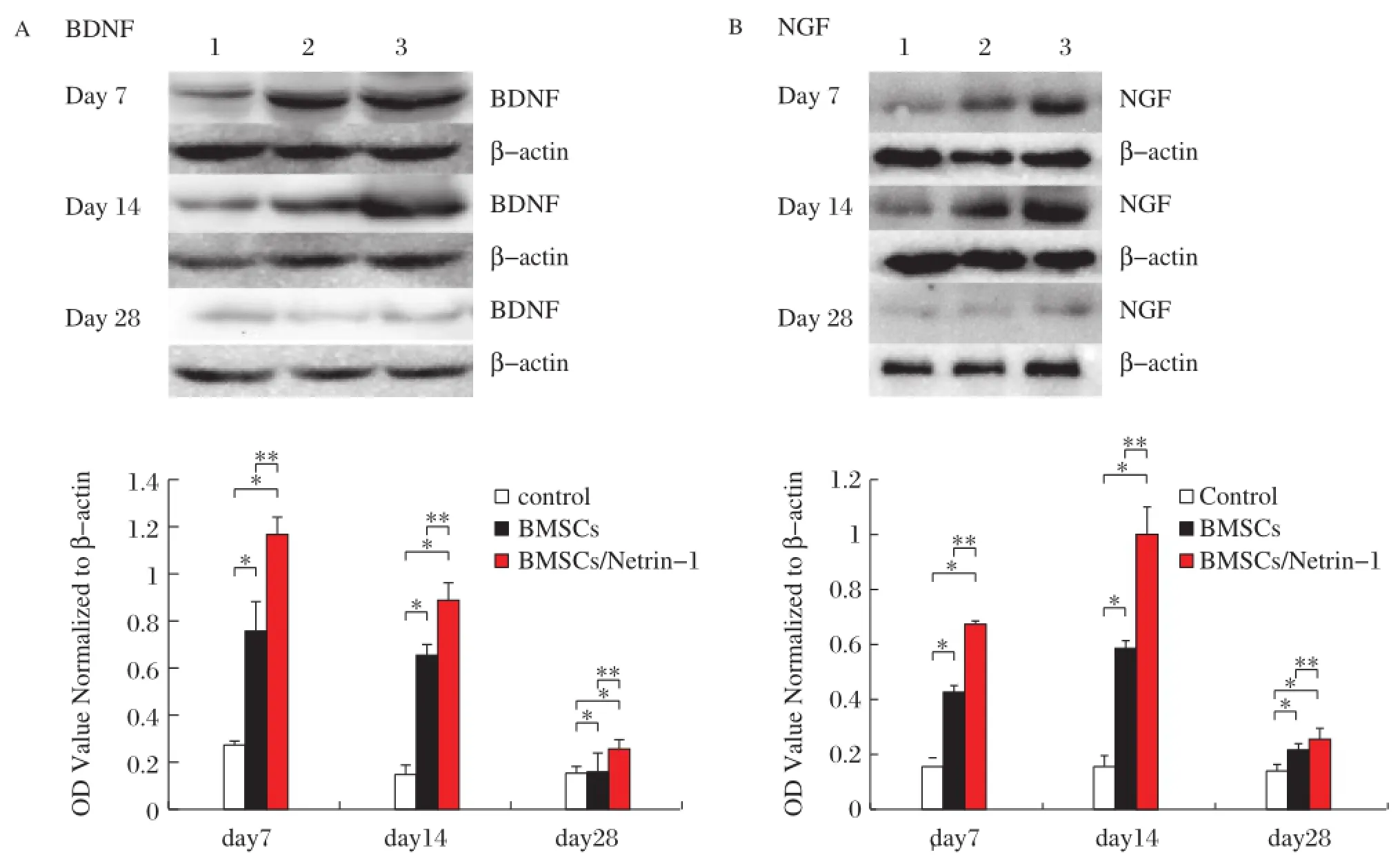
Fig.5 Prote in levels of neurotrophic factors change after nerve injury.Expression of BDNF(A)and NGF(B)protein at d7,d14 and d28 after surgery.Representative Western blot of protein with β-actin used as an internal control from nerve treated with control medium,Null-BMSCs,and Netrin-1-BMSCs.Values are means±SD of data from 6 separate samples.*P<0.05.
BMSCs produce a variety of cytokines,such as neurotrophic factor and arteriogenic cytokines.Several groups found that BMSCs produced VEGF,bFGF,PDGF,NGF and GDNF,NGF,BDNF,as well as GDNF and NT-3. Chen et al.[26-27]found that NGF,BDNF,VEGF and bFGF were secreted by BMSCs in the CNS.We also demonstrated in vitro by RT-PCR that BMSCs expressed VEGF,bFGF,NGF and BDNF.In the present experiment,we detected that BDNF and NGF expressions were increased at the end of injured sciatic nerve.These neurotrophic factors can improve nerve recovery and neovascularization.The expression of neurotrophic factors increased transiently after nerve injury and returned to almost normal levels after 4 weeks.Notably,at the peak of the transient increase,the expression of neurotrophic factors was significantly increased after transplantation of BMSCs infected with a netrin-1 virus compared to controls or BMSCs alone.
BMSCs may differentiate into Schwann cells after treatment with trophic factors[28].In our study,after transplantation of EGFP-labeled BMSCs into injured sciatic nerves,we found that some BMSCs differentiated into Schwann cells expressing S-100 protein and EGFP.EGFP-labeled BMSCs were still observed 4 weeks later.The number of surviving EGFP-labeled BMSCs and induced Schwann cells in the group of transplantated BMSCs infected with Ad5-Netrin-1-EGFP was higher than in the other two groups.The results suggest that netrin-1 may improve the viability of BMSCs.During the development of the nervous system,netrin-1 not only plays a role of a guidance cue,but also acts as a survival factor together with its receptors UNC5H and DCC.Thus,netrin-1 bound to UNC5B activates GTPase PIKE-L,triggers the activation of Pldlns-3-OH kinase signaling,which prevents the proapoptotic activity of UNC5B and enhances neuronal survival and regeneration[29-30].Netrin-1 also prevents endothelial cell apoptosis,probably by blocking the proapoptotic effect of receptor UNC5B and the down-stream signaling effector death associated protein kinase[31].DCC may promote direct caspase-3 activation by interacting with caspase-9 in the absence of netrin-1, which can result in apoptotic cell death[32].When unbound to its ligand,DCC and UNC5H are cleaved by caspases and then activate a cell death program.We also found that BMSCs expressed UNC5B and DCC by RT-PCR(data not shown),and that UNC5B and DCC were expressed at higher levels in injured nerve than in normal nerve.Although netrin-1 is detectable in crushed nerves,its level is very low[33].Thus,we have constructed a novel Ad5-Netrin-1-EGFP which enables netrin-1 expression for a longer time by transplantation of BMSCs infected with Ad5-Netrin-1-EGFP.Netrin-1 might induce proliferation of BMSCs induced or remaining Schwann cells through UNC5B receptor[17,29].
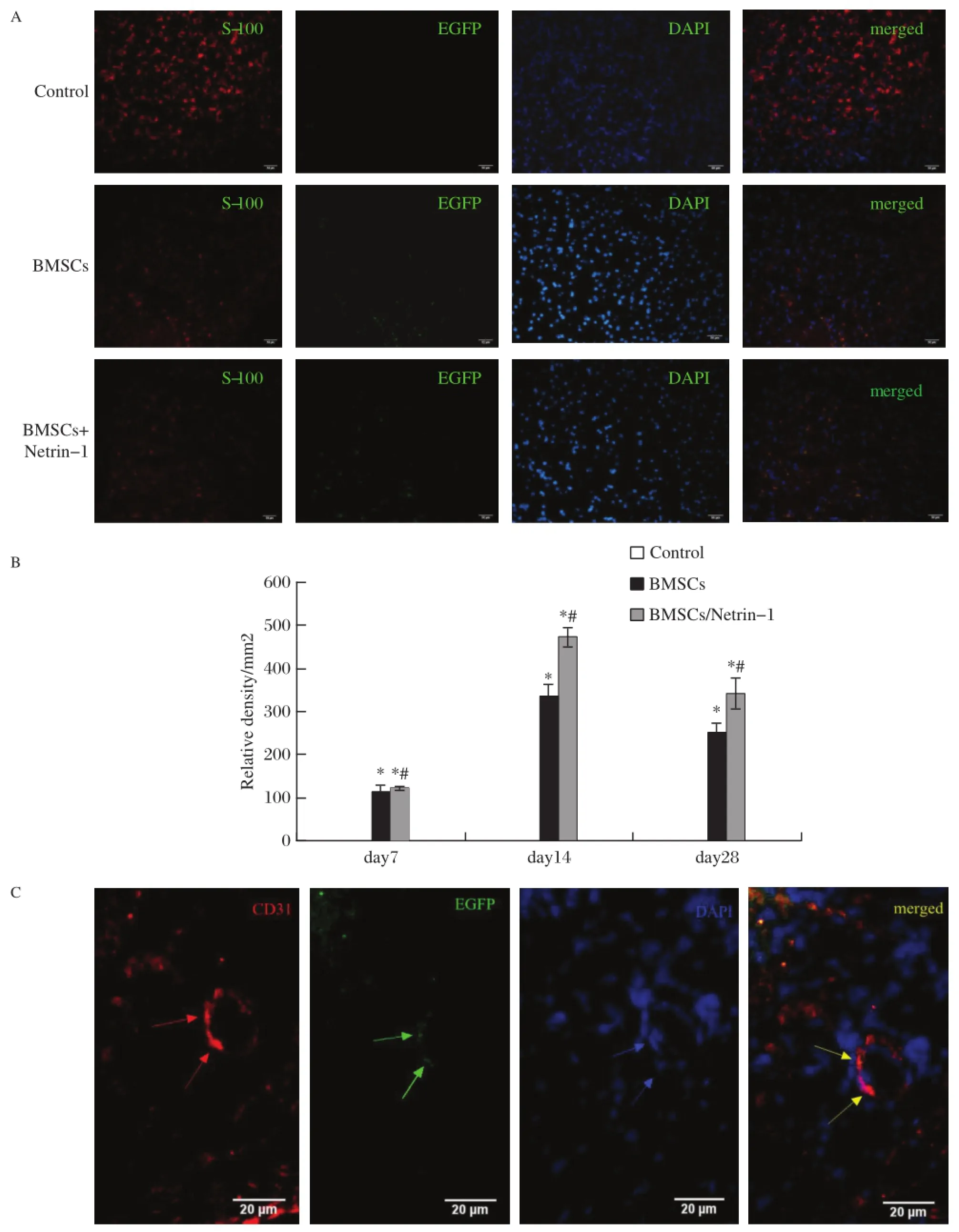
Fig.6 Transplanted BMSCs are integrated into the vasculature or become Schwann cells.A:Determination of neurofilament.The nerve tissues were retrieved 14 days after injury and were subjected to immunohistochemistry with antibody against neurofilament in the four treatment groups.The NF-positive axons(red)are ensheathed by GFP-positive BMSCs(green),forming myelinated nerve fibers.Nuclear marker in blue.Scale bars=50 μm.B:The quantitative analysis of relative density of neurofilament,*P<0.05.C:Arteriogenesis induced by GFP-positive transplanted BMSCs at 14 days post implantation.Red fluorescence signified CD31,whereas green for GFP,blue for DAPI and yellow for merged.Bars=20 mm.
Netrin-1 is not only a neural guidance factor,but also an angiogenic factor since it stimulates the proliferation and migration of endothelial cells,and promotes blood vessel formation in the CNS[14,34].In our previous study, we reported that netrin-1 promoted angiogensis and capillary density,which improved function of ischemic limbs[35].To determine whether the effects of treatment with BMSCs expressing netrin-1 that we show here depend critically on netrin-1 signaling,we analyzed the efficacy and speed of recovery after nerve injury in mice in which Dcc of Unc5b(orboth)were conditionally inactivated in glia or neurons or blood vessels.Alternatively, the use of Netrin-1-/-BMSCs may indicate that if netrin-1 alone suffices to the recovery of injury.Furthermore, we used this BMSC cell transplantation strategy after nerve injury to examine the effectiveness of other factors,such as NGF and BDNF in both wild type mice and mice carrying mutations that inactivate the signal transduction pathways normally activated by the factors we test.Nerve regeneration in a crush experimental model was not complete and serious neurological deficits remained at the time point of 4 weeks.At the cut off point of 4 weeks[36-38],there were significant differences between the groups either in neurological deficits and electrophysiological parameters,or in nerve myelination which are all used as important indicators to evaluate any treatment of nerve regeneration.In the transectional model,the suture technique and materials regarded as inflammation provoking agents are used, and these factors may compromise the interpretation of inflammatory response and affect the anti-apoptosis of netrin-1.Thus,for the sake of simplicity,we used the crush model to investigate the effects of netrin-1 on the survival of transplanted BMSCs in this study.
In conclusion,a new method of transplantation of BMSCs infected with Ad5-Netrin-1-EGFP has more advantages than BMSCs alone for the therapy of peripheral nerve injury.BMSCs can produce VEGF,bFGF, PDGF,NGF and BDNF.The VEGF gene is hypoxia-responsible,and since crush injury to the sciatic nerve can affect neural microcirculation and capillary occlusion, which creates a hypoxia milieu that may induce VEGF production by BMSCs.Furthermore,netrin-1 may protect the survival of BMSCs,Schwann cells,and endothelial cells.It also can promote the proliferation of Schwann cells.As netrin-1 is also an angiogenic factor,it promotes new vessel formation via endothelial cells induced by BMSCs,primarily on the surface of the sciatic nerve, ameliorating neural microcirculation.Netrin-1 is superior in restoring nerve conduction velocity,possibly due to its potent effects on both endothelial and neural biology.The results of this study indicate that BMSCs combined with netrin-1 improve the rehabilitation of neural function.The mechanism should be elucidated in the future work.This may be a novel direction for research on nerve injury and regeneration.
Acknowledgments
This study was supported by grants from Jiangsu Planned Projects for Postdoctoral Research Funds, Nanjing Medical Technology Development Project (No.ZKX08014),Nanjing Medical Science and Technique Development Foundation,National Natural Science Foundation of China(No.81200594)
[1] Cuevas P,Carceller F,Dujovny M,et al.Peripheral nerve regeneration by bone marrow stromal cells[J].Neurol Res, 2002,24(7):634-638.
[2] Mimura T,Dezawa M,Kanno H,et al.Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived schwann cells in adult rats[J].J Neurosurg, 2004,101(5):806-812.
[3] Bryan DJ,Wang KK,Chakalis-Haley DP.Effect of schwann cells in the enhancement of peripheral-nerve regeneration[J].J Reconstr Microsurg,1996,12(7):439-436.
[4] Pittenger MF,Mosca JD,McIntosh KR.Human mesenchymal stem cells:Progenitor cells for cartilage,bone,fat and stroma[J].Curr Top Microbiol Immunol,2000, 251:3-11.
[5] Garcia R,Aguiar J,Alberti E,et al.Bone marrow stromal cells produce nerve growth factor and glial cell line-derived neurotrophic factors[J].Biochem Biophys Res Commun,2004,316(3):753-754.
[6] Kinnaird T,Stabile E,Burnett MS,et al.Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms[J].Circ Res,2004,94(5):678-685.
[7] Oliveira JT,Almeida FM,Biancalana A,et al.Mesenchymal stem cells in a polycaprolactone conduit enhance median-nerve regeneration,prevent decrease of creatinephosphokinase levels in muscle,and improve functional recovery in mice[J].Neuroscience,2010,170(4):1295-1303.
[8] Dezawa M,Takahashi I,Esaki M,et al.Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells[J].Eur J Neurosci,2001,14(1):1771-1776.
[9] Gutierrez-Fernandez M,Rodriguez-Frutos B,Alvarez-Grech J,et al.Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats[J].Neuroscience,2011,175:394-405.
[10]Shimizu S,Kitada M,Ishikawa H,et al.Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with schwann cell property[J]. Biochem Biophys Res Commun,2007,359(4):915-920.
[11]de Castro F.Chemotropic molecules:Guides for axonal path-finding and cell migration during CNS development[J]. News Physiol Sci,2003,18:130-136.
[12]Hong K,Hinck L,Nishiyama M,et al.A ligand-gated association between cytoplasmic domains of unc5 and dcc family receptors converts netrin-induced growth cone attraction to repulsion[J].Cell,1999,97(7):927-941.
[13]Llambi F,Causeret F,Bloch-Gallego E,et al.Netrin-1 acts as a survival factor via its receptors UNC5H and DCC[J]. EMBO J,2001,20(11):2715-2722.
[14]Fan Y,Shen F,Chen Y,et al.Overexpression of netrin-1 induces neovascularization in the adult mouse brain[J].J Cereb Blood Flow Metab,2008,28(9):1543-1551.
[15]Lu H,Wang Y,He X,et al.Netrin-1 hyperexpression in mouse brain promotes angiogenesis and long-term neurological recovery after transient focal ischemia[J].Stroke, 2012,43(3):838-843.
[16]Park KW,Crouse D,Lee M,et al.The axonal attractant netrin-1 is an angiogenic factor[J].Proc Natl Acad Sci U S A,2004,101(46):16210-16215.
[17]Lee HK,Seo IA,Seo E,et al.Netrin-1 induces proliferation of Schwann cells through Unc5b receptor[J]. Biochem Biophys Res Commun,2007,362(4):1057-1062.
[18]Zhang L,Chan C.Isolation and enrichment of rat mesenchymal stem cells(mscs)and separation of single-colony derived mscs.J Vis Exp,2010.
[19]Bridge PM,Ball DJ,Mackinnon SE,et al.Nerve crush injuries-a model for axonotmesis[J].Exp Neurol,1994, 127(2):284-290.
[20]Bain JR,Mackinnon SE,Hunter DA.Functional evaluation of complete sciatic,peroneal,and posterior tibial nerve lesions in the rat[J].Plast Reconstr Surg,1989, 83(1):129-138.
[21]Schmittgen TD,Zakrajsek BA,Mills AG,et al. Quantitative reverse transcription-polymerase chain reaction to study mrna decay:Comparison of endpoint and real-time methods.Anal Biochem,2000,285(2):194-204.
[22]Akassoglou K,Yu WM,Akpinar P,et al.Fibrin inhibits peripheral nerve remyelination by regulating schwann cell differentiation[J].Neuron,2002,33(6):861-875.
[23]Le N,Nagarajan R,Wang JY,et al.Analysis of congenital hypomyelinating egr2lo/lo nerves identifies sox2 as an inhibitor of schwann cell differentiation and myelination[J]. Proc Natl Acad Sci U S A,2005,102(7):2596-2601.
[24]Myckatyn TM,Mackinnon SE,Hunter DA,et al.A novel model for the study of peripheral-nerve regeneration following common nerve injury paradigms[J].J Reconstr Microsurg,2004,20(7):533-544.
[25]Schiaveto de Souza A,da Silva CA,Del Bel EA. Methodological evaluation to analyze functional recovery after sciatic nerve in jury[J].J Neurotrauma,2004, 21(5):627-635.
[26]Chen Q,Long Y,Yuan X,et al.Protective effects of bone marrow stromal cell transplantation in injured rodent brain:Synthesis of neurotrophic factors[J].J Neurosci Res,2005,80(5):611-619.
[27]Chen X,Katakowski M,Li Y,et al.Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts:Growth factor production[J].J Neurosci Res, 2002,69(5):687-691.
[28]Zurita M,Vaquero J,Oya S,et al.Schwann cells induce neuronal differentiation of bone marrow stromal cells[J]. Neuroreport,2005,16(5):505-508.
[29]Jaminet P,Kohler D,Rahmanian-Schwarz A,et al. Expression patterns and functional evaluation of the unc5b receptor during the early phase of peripheral nerve regeneration using the mouse median nerve model[J]. Microsurgery,2013,33(3):216-222.
[30]Tang X,Jang SW,Okada M,et al.Netrin-1 mediates neuronal survival through pike-l interaction with the dependence receptor unc5b[J].Nat Cell Biol,2008,10(6):698-706.
[31]Castets M,Coissieux MM,Delloye-Bourgeois C,et al. Inhibition of endothelial cell apoptosis by netrin-1 during angiogenesis[J].Dev Cell,2009,16(4):614-620.
[32]Forcet C,Ye X,Granger L,et al.The dependence receptor DCC(deleted in colorectal cancer)defines an alternative mechanism for caspase activation[J].Proc Natl Acad Sci U S A,2001,98(6):3416-3421.
[33]Madison RD,Zomorodi A,Robinson GA.Netrin-1 and peripheral nerve regeneration in the adult rat[J].Exp Neurol,2000,161(2):563-570.
[34]Shimizu A,Nakayama H,Wang P,et al.Netrin-1 promotes glioblastoma cell invasiveness and angiogenesis by multiple pathways including activation of rhoa,cathepsin b,and camp-response element-binding protein[J].J Biol Chem,2013,288(4):2210-2222.
[35]Li Q,Yao D,Ma J,et al.Transplantation of mscs in combination with netrin-1 improves neoangiogenesis in a rat model of hind limb ischemia[J].J Surg Res,2011,166(1): 162-169.
[36]Pan HC,Chen CJ,Cheng FC,et al.Combination of g-csf administration and human amniotic fluid mesenchymal stem cell transplantation promotes peripheral nerve regeneration[J].Neurochem Res,2009,34(3):518-527.
[37]Pan HC,Cheng FC,Chen CJ,et al.Post-injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells[J].J Clin Neurosci,2007,14(11):1089-1098.
[38]Pan HC,Cheng FC,Chen CJ,et al.Dietary supplement with fermented soybeans,natto,improved the neurobehavioral deficits after sciatic nerve injury in rats[J].Neurol Res,2009,31(5):441-452.
△These two authors contributed equally to this study.
✉Corresponding author:Dr.Qian Li,Department of Endocrinology, Nanjing First Hospital,Nanjing Medical University,68 Changle Road, Nanjing,Jiangsu 210006,China.Tel:86-25-52887080,E-mail:shygu@ njmu.edu.cn.
Received 16 May 2014,Revised 01 October 2014,Accepted 08 April 2015,Epub 30 July 2015
R745,Document code:A
The authors reported no conflict of interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20140076
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Internal radiation therapy:a neglected aspect of nuclear medicine in the molecular era
- Hypercholesterolemia,low density lipoprotein receptor and proprotein convertase subtilisin/kexin-type 9
- Endothelin-1-induced mini-stroke in the dorsal hippocampus or lateral amygdala results in deficits in learning and memory
- Acute effect of aspartame-induced oxidative stress in Wistar albino rat brain
- Differential mRNA expression profiling of oral squamous cell carcinoma by high-throughput RNA sequencing
- Diacerein protects against iodoacetate-induced osteoarthritis in the femorotibial joints of rats
