Internal radiation therapy:a neglected aspect of nuclear medicine in the molecular era
2015-02-14YansongLin
Yansong Lin
Internal radiation therapy:a neglected aspect of nuclear medicine in the molecular era
Yansong Lin✉
Department of Nuclear Medicine,Peking Union Medical College Hospital,Beijing 100730,China.
With increasing evidence,internal radiation therapy,also known as brachytherapy,has become a neglected aspect of nuclear medicine in the molecular era.In this paper,recent developments regarding internal radiation therapy,including developments in radioiodine-131(131I)and thyroid,radioimmunotherapy(RIT)for non-Hodgkin lymphoma(NHL),and radiopharmaceuticals for bone metastases.Relevant differences and status of their applications in China were mentioned as well.These molecular mediated internal radiation therapies are gaining increasing importance by providing palliative and curative treatments for an increasing number of diseases and becoming one of the important parts of molecular nuclear medicine.
brachytherapy,radioisotope,neoplasm,radioiodine,hyperthyroidism
Introduction
Internal radiation therapy,also known as brachytherapy,is a form of treatment where a source of radiation is introduced inside the body.Most radionuclides used in brachytherapy emit beta particles which have a low range of tissue penetration.A few emit auger electrons and alpha particles,and several also emit gamma rays and X-rays during their decay.The radiation only travels a short distance so there is less risk of damaging nearby normal tissues.Most internal radiation therapies locate to tissues or a certain lesion by specifically binding through molecular targets such as metabolic pathways,a ligand-receptor or a specific antibody for its target antigen.The most successful application of metabolic pathway is the use of radionuclide iodine-131(131I)for the treatment of thyroid diseases, including benign hyperthyroid conditions and thyroid carcinoma.Although sodium iodide symporter (NIS),the specific target of radioiodine,was cloned in 1996,it is amazing that radioiodine therapy had set up a paradigm of molecular medicine more than half a century earlier when radioiodine was first clinically used in 1946.
In this review,131I treatment of benign and malignant disorders of the thyroid is reviewed first and then other representative targeted radiotherapies are reviewed as well.
Radioiodine-131(131I)and thyroid
131I decays at a physical half-life of 8.1 days with both beta and gamma emissions.The principal γ-ray of 364 keV enables thyroid function imaging or post-131I therapy imaging to see the distribution of131I with a gamma camera.A principal β-particle has a maximum energy of 0.61 MeV,an average energy of 0.192 MeV,a mean range in tissue of 0.4 mm,and is the radiation effector which has a potential role ininternal radiation therapy of131I.The particular target of radioiodine in thyroid disease is sodium iodide symporter(NIS),which is an intrinsic plasma membrane protein that mediates active iodide transport into the thyroid gland.NIS is modulated by many factors; thyrotropin(TSH)activates NIS via the cAMP pathway in a protein synthesis dependent manner[1],while iodide[2],thyroglobulin(Tg)[3],estradiol[4]and cytokines[5],such as tumor necrosis factor(TNF)-α,TNF-β,interferon(IFN)-γ,interleukin(IL)-1α,IL-1β,and IL-6 inhibit NIS by decreasing its expression at the mRNA or protein level.NIS can be expressed up to 140x in Graves’hyperthyroid tissue than that in normal thyroid gland[6].While NIS is expressed in differentiated thyroid cancer(DTC),which is primarily comprised of papillary,follicular thyroid cancer and Hurthle cell carcinomas,it can also be expressed at a low level or even over expressed as non-targeted NIS. Thus,131I therapy could be used to ablate residual thyroid to prevent recurrence and to destroy recurrent or metastatic foci of DTC.Common indications for therapy of thyroid diseases with131I primarily include,but are not limited to,benign diseases such as certain types of Graves’hyperthyroidism and toxic nodular hyperthyroidism[7],thyroid remnant ablation,and recurrent or metastatic disease treatment for DTC after near-total thyroidectomy.
Patients who are candidates for131I treatment should have a special preparation before therapy,such as discontinuation of iodide containing medications and preparations which could potentially affect the ability of thyroid tissue to accumulate iodide.The nuclear physician who performs this treatment must explain the procedure,treatment,complications,side effects, therapeutic alternatives,and radiation protection to patients.
Radioiodine treatment in Graves’hyperthyroidism
Graves’disease(GD)hyperthyroidism is caused by thyrotropin receptor antibody(TRAb),a thyroid-stimulating antibody which binds to and activates the thyrotropin receptor on thyroid cells.TRAb not only causes thyroid hypersecretion but also hypertrophy and hyperplasia of the thyroid follicles.In addition to hyperthyroidism,GD may affect the eyes(Graves’ophthalmopathy,GO)and the skin(localized dermopathy or myxedema)as a result of autoimmune reaction.So far the ideal treatment for GD,which would correct the autoimmune responses in the thyroid and orbits,thereby restoring thyroid function and resulting in the disappearance of ophthalmopathy,is not available.Once GD hyperthyroid has been identified, the patient and physician must choose among three effective and relatively safe initial treatment options to restore the thyroid function:131I therapy(radioactive iodine), antithyroid drugs(ATD),or thyroidectomy.In the United States,131I had been the therapy most preferred by physicians.However,according to a recent survey which compared findings from a 1991 survey,fewer U.S.(59.7 vs.69%)and European(13.3%vs.25%) clinicians would use RAI therapy,with a mild shift away from RAI and toward ATDs in patients with uncomplicated GD[8].This may be due to the concern of causing new or worsening of GO after131I therapy[9].In China, the preferred m ode of therapy in uncomplicated Graves’hyperthyroidism is ATDs,131I and surgery, according to recent questionnaire survey conducted by Chinese Nuclear Medicine Society,and only 32% of clinicians prefer choosing131I as an initial therapy;among the worries of131I therapy,78% of clinicians worried about hypothyroidism and 14% worried about infertility while 8% worried about carcinogenesis[10].
Among factors that may impact patient preference, choosing131I therapy for GD hyperthyroidism means that the patient would likely place a higher value on definitive control of hyperthyroidism,the avoidance of traumatic surgery,and the potential side effects (including agranulocytosis,thrombocytopenia,acute hepatic necrosis,cholestatic hepatitis,etc.) of antithyroid medications,while placing a lower value on the need for lifelong thyroid hormone replacement,rapid resolution of hyperthyroidism,and potential worsening or development of GO[9].131I contraindications include pregnancy,lactation,co-existing thyroid cancer,inability to comply with radiation safety guidelines,and females planning a pregnancy within 4-6 months.It might be the first choice for individuals with comorbidities that may increase surgical risk,patients with previously operated or externally irradiated necks,or patients who lack access to a skilled thyroid surgeon or have contraindications to ATD use.
131I is administered orally in solution or as a capsule.It is rapidly incorporated into the thyroid,produces thyroiditis and fibrosis via its β emissions, and results in euthyroidism or hypothyroidism within 6-18 weeks.The long-term quality of life (QOL)following131I treatment for GD was found to be the same as that of the other two options in a randomized clinical study.
In the United States and European countries,the goal of131I is to control hyperthyroidism by rendering the patient with either euthyroid or hypothyroid with sufficient radiation deposited in the thyroid.In China,the goal of131I treatment is to strive for rendering euthyroid state or,the least severe extent ofhypothyroidism as possible.This may be due to the fact that most Chinese patients are afraid of hypothyroidism with lifelong levothyroxine replacement therapy.Hence,a calculated dose regime is the choice for most Chinese nuclear medicine physicians and the dose of131I administered is less than the American Thyroid Association(ATA)guideline recommendation (70-120 μCi vs.150-300 μCi per gram of thyroid estimated from either ultrasound or gamma camera imaging)accordingly.In a randomized prospective study from China[11],researchers tried to find an optimal dosing strategy for131I treatment of Graves’hyperthyroidism and attempted to achieve euthyroidism.Individual131I activity was modulated according to a clinical scoring system,including age,duration of disease,complications,severity of hyperthyroidism,use of ATDs,and gland characteristics.A fter a 12-year follow-up,one group of patients who received 50-110 μCi per gram of thyroid tissue was identified as the study arm that received the optimum131I dose,with 71.8% of the patients maintaining a euthyroid status,5.8% remaining hyperthyroid,and 22.3% becoming hypothyroid by the end of the study;the recurrence rate was 13.6%.These results are convincing that hypothyroidism could be reduced to some extent after an individualized dose modulated with the particular patient clinical score.
131I has been used to treat hyperthyroidism for six decades and is well-tolerated with rare complications, except for those related to ophthalmopathy[9].Little evidence can be observed regarding the association of131I with birth defects,infertility or overall cancer incidence.Thyroid storm occurs only rarely following the administration of radioactive iodine.Compared with the other two methods,131I therapy of Graves’hyperthyroidism proves to be more cost-effective with an 80% single remission rate and an overall effective rate above 95%,a 1%-4% recurrence rate,and the inefficacy rate of about 2%-4%[12].So far,there is still an uncertainty and controversy about the optimal dose, required follow-up,and radiation safety of131I therapy on Graves’hyperthyroidism,owing to the lack of wellorganized multicenter randomized studies.
The role of radioactive iodine-131(RAI) treatment in differentiated thyroid cancer(DTC)
Postsurgical re-evaluation of risk of recurrence in DTC
An escalating incidence of thyroid cancer worldwide during the last three decades has been reported[13].As one of the major adjuvant post-surgery treatments, ablation of the small amount of normal thyroid remaining after total thyroidectomy may facilitate both initial staging,by identifying previously undiagnosed disease in post RAI whole body scan(-WBS),and surveillance for early recurrence based on serum Tg measurement and/or RAI WBS.Meanwhile,the potential tumoricidal effect of RAI on persistent thyroid cancer cells in patients at risk of recurrence or disease specific mortality cannot be neglected.Moreover,131I therapy remains an effective modality for RAI-avid metastatic DTC. Before RA I treatment,a postsurgical assessment should be done.
The AJCC/International Union against Cancer (AJCC/UICC)classification system,based on pTNM parameters and age in DTC,allows accurate identification of the majority of patients at low-risk of mortality (T1-3,M 0 patients)and the higher-risk minority(T4 and M 1patients)to determine the appropriate intensity of management strategy and follow-up.For the assessment of DTC recurrence risk,a three level stratification has been formulated by American Thyroid Association(ATA)guidelines(Table 1).However, controversy remains in the definition of ‘high risk’’mentioned in this table,as‘thyroiglobulinemia is out of proportion to what is seen on Rx-WBS’.This meansthat some micrometastatic patients with normal radiologic findings would be identified as high risk only after the first RAI ablation therapy,which has no impact on the dose decision to ablate(Fig.1). Recent studies suggested that pre-ablative stimulated Tg(ps-Tg)might be considered as a predictive marker for distant metastasis of DTC[14-15],which implies that suspicious high ps-Tg could be regarded as one of the indices for high risk classification even without Rx-WBS(Fig.2).Moreover,in accordance with the above evidence the recently published‘‘Chinese Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer’emphasized high ps-Tg level as an important pre-assessment marker and regarded it as one of the high recurrence risk indices before RAI treatment and recommendation for high dose131I.

Table 1 There-level stratification of recurrence risk by ATA guideline(2009)
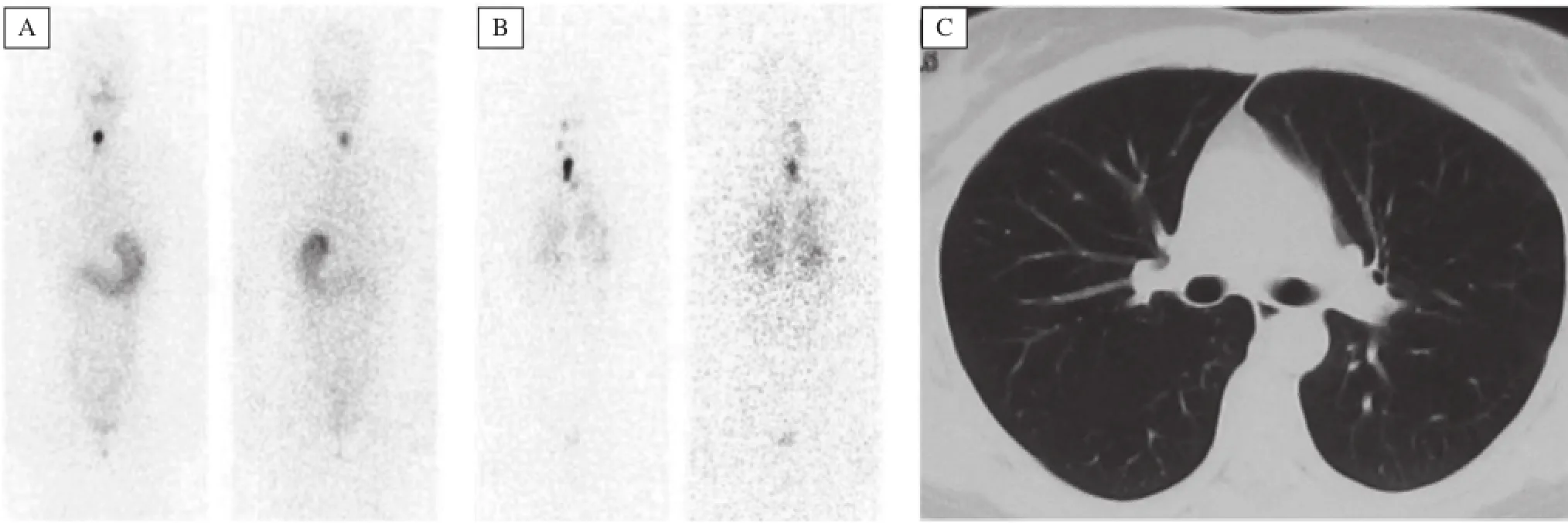
Fig.1 Diffuse pulmonary micrometastases(B)identified by post radioactive iodine-131(RAI)treatment scintigraphy with normal diagnostic131I scan(A)and chest CT(C).Cited from Lin Y et al.Clin Nucl Med,2011;36:1102-1105[14]with permission
RAI dose management in DTC
To date,the optimal131I activity for remnant thyroid ablation and metastatic disease remains controversial. Though there is a trend toward higher ablation rates with higher activities,two randomized multicenter studies indicated that low dose131I(1110 MBq, 30 mCi)provides an equally satisfactory ablative outcome,compared with high dose(≥3700 M Bq, 100 mCi),in low or intermediate risk patients for the purpose of convenient follow-up with Tg and diagnostic131I-WBS[16,17].For patients with suspicious residual microscopic disease or more aggressive tumor histology or high recurrence risk,a high dose (≥3700 MBq) of131I is recommended for the first RAI treatment for the purpose of reducing the risk of recurrence and/or mortality.The use of123I or131I with modern SPECT/CT or124I PET/CT-based dosimetry may facilitate whole-body and lesional dosimetry determination,131I therapeutic dose management will eventually evolve fromempiric fixed amounts to quantitative tumor dosimetry.
Low dose131I has numerous advantages,compared with high dose.It induces fewer radioiodine related adverse effects or secondary tumors,does not require hospitalization,and is low cost.Recently,Tuttle et al.[18]proposed the concept of reassessment and emphasized the evaluation of response to therapies to modify the initial risk stratification predicted by ATA staging system.Therefore,it is worth arguing the necessity of using high dose in those high risk patients with undetectable ps-Tg,which means excellent prognosis and potential to be free of131I treatment[19].In China,fear of causing radioactive adverse effects suchas secondary tumors and infertility are the major worries in using131I in DTC from the standpoint of both patients and clinicians other than nuclear medicine specialists.A countrywide,rather uneven use of131I may be due to a shortage of nuclear medicine and uneven recognition of the role of131I among surgeons and endocrinologists. Thus,appropriate use and avoiding an unnecessary dose of131I in DTC patients in China remains the primary subject of the Chinese Nuclear Medicine Society.
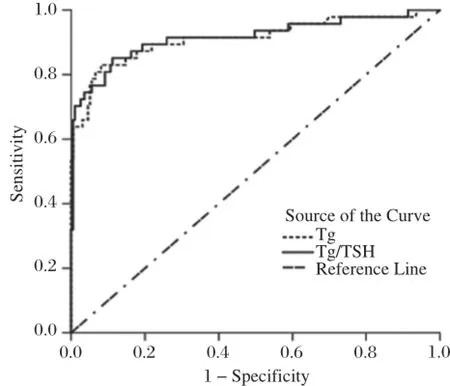
Fig.2 The ROC curve of accuracy of ps-Tg and Tg/TSH in distinguishing distant metastases from normal condition.Cited from Lin Y et al.Clin Nucl Med,2011;36:1102-1105[14]with permission.
Cox regression revealed that131I remnant ablation in patients with uptake only in the thyroid bed and no evidence of residual tumor could decrease the risk of recurrence,distant metastases and cancer related mortality with a hazard ratio of 0.8,0.6 and 0.5,respectively[20].131I up take in the metastatic lesions is a significant prognostic factor for survival.In a long-term retrospective study of 444 DTC patients with distant metastases,Schlumberger et al.found that131I treatment is highly effective in younger patients with131I uptake and with small metastases and patients with131I uptake in metastatic foci had a higher survival rate than those without;the survival rate was 56%vs.10% in 10 years, and 45%vs.6% at 15 years[21].
Dedifferentiation and re-differentiation therapy of DTC
In well-differentiated thyroid cancer(WDTC),during the natural process of the disease,2%-5% patients lose their differentiated phenotype and dedifferentiate to a more aggressive type,which is defined as dedifferentiation.Dedifferentiated thyroid cancers(dDTC) usually present with RAI resistance and are prone to be more aggressive and more malignant,with a median survival of less than 5 years[22].Early identification of d DTC is the key point for subsequent management. In metastatic patients with elevating Tg level and negative radioiodine uptake,dDTC should be suspected and18FDG PET/CT imaging should be considered for further localization.However,it is important to realize that18FDG trapping is not tumor-specific.Malignant thyroid tumors and some benign thyroid diseases,cervical lymph nodes with inflammation,or other inflamed tissues could show high18FDG uptake.At present,in China,SPECT is more widely available than PET and most patients cannot afford the expense of18FDG PET for diagnosis instead of treatment.A recent study indicated that dDTC lesions can be traced using angiogenesis imaging such as99mTc-RGD imaging(Fig.3),thus providing a new approach for diagnosis and localization of dDTC.Meanwhile,the highly neovascularized lesions confirmed by angiogenesis imaging may afford an alternative therapeutic target and monitor the efficacy of certain anti-angiogenetic therapy[23].
As conventional treatment options are of very limited benefit for d DTC patients,the management of d DTC remains a significant clinical concern.Redifferentiation therapy is one of the prospects,in conjunction with the probability of re-use RAI,which is defined as a treatment to inhibit tumor growth and regain thyroid-specific functions,thus restoring radioiodine uptake and increasing the response to RA I therapy.Various agents,such as retinoid acid[24], thiazolidinedione[25],histone acetylation[26],and methylation inhibitor[27]have demonstrated a promising effect on these RAI-refractory patients.
It has been reported that approximately 39% of RAI-refractory thyroid cancers have BRAFV600Emutation[28], which is involved in mitogen-activated protein kinase (MAPK)signaling.The MAPK pathway is triggered by extracellular mitogenic stimuli that activate a receptor tyrosine kinase(RTK)in the cell membrane,then to RAS,RAF(such as BRAFV600E),MEK and ERK.ERK is activated by phosphorylation(P)and enters the nucleus where it regulates the balance betweentumor-promoting genes and thyroid-specific genes. Constitutive activation by BRAFV600Emutation stimulates the signaling of the MAPK pathway,consequently causing the silencing of the thyroid specific gene and reducing the expression of NIS[28],while simultaneously upregulating tumor-promoting genes and leading to dedifferentiation and poor prognosis[29]. When MAPK activation was switched of for its downstream signaling was inhibited by MAPK kinase inhibitors,the tumors would regain the ability to trap radioiodine[30].
Recently,selumetinib(AZD6244,ARRY-142886), a selective MEK 1 and MEK 2 inhibitor,has shown the ability to restore radioiodine incorporation RAI-refractory thyroid cancers in several clinical trials[31]. In one well-conducted clinical trial[31],selumetinib increased the uptake of iodine-124 in 12/20 patients (4/9 patients with BRAF mutations and 5/5 patients with NRAS mutations)(Fig.4).Of the 12 patients who restored uptake of RAI,8/12 patients reached the dosimetric threshold for radioiodine therapy,including all 5 patients with NRAS mutations.This study provides a pro of of principle that MEK inhibitors can induce iodine up take and retention in thyroid tumors.In another phase II study of selumetinib[32],patients with BRAFV600Emutation(12 of 26 evaluated,46%)had an obviously longer median progression-free survival (PFS)compared with patients with BRAF wildtype tumors(33 vs.11 weeks,respectively).These two studiessuggest that selumetinib may have more effectiveness in the case of BRAFV600Eor RAS mutation.A randomized,double-blinded study to compare the complete remission rate following a 5-week course of selumetinib or placebo and single dose adjuvant radioactive iodine therapy is ongoing.Although it remains a challenge to manage RAI-refractory advanced or metastatic thyroid cancer,re-differentiation agents may serve as a potential regimen for such patients.We suppose that a combination regimen of MAPK kinase inhibitors and other agents on a re-differentiation therapy with RAI may achieve more promising outcomes.
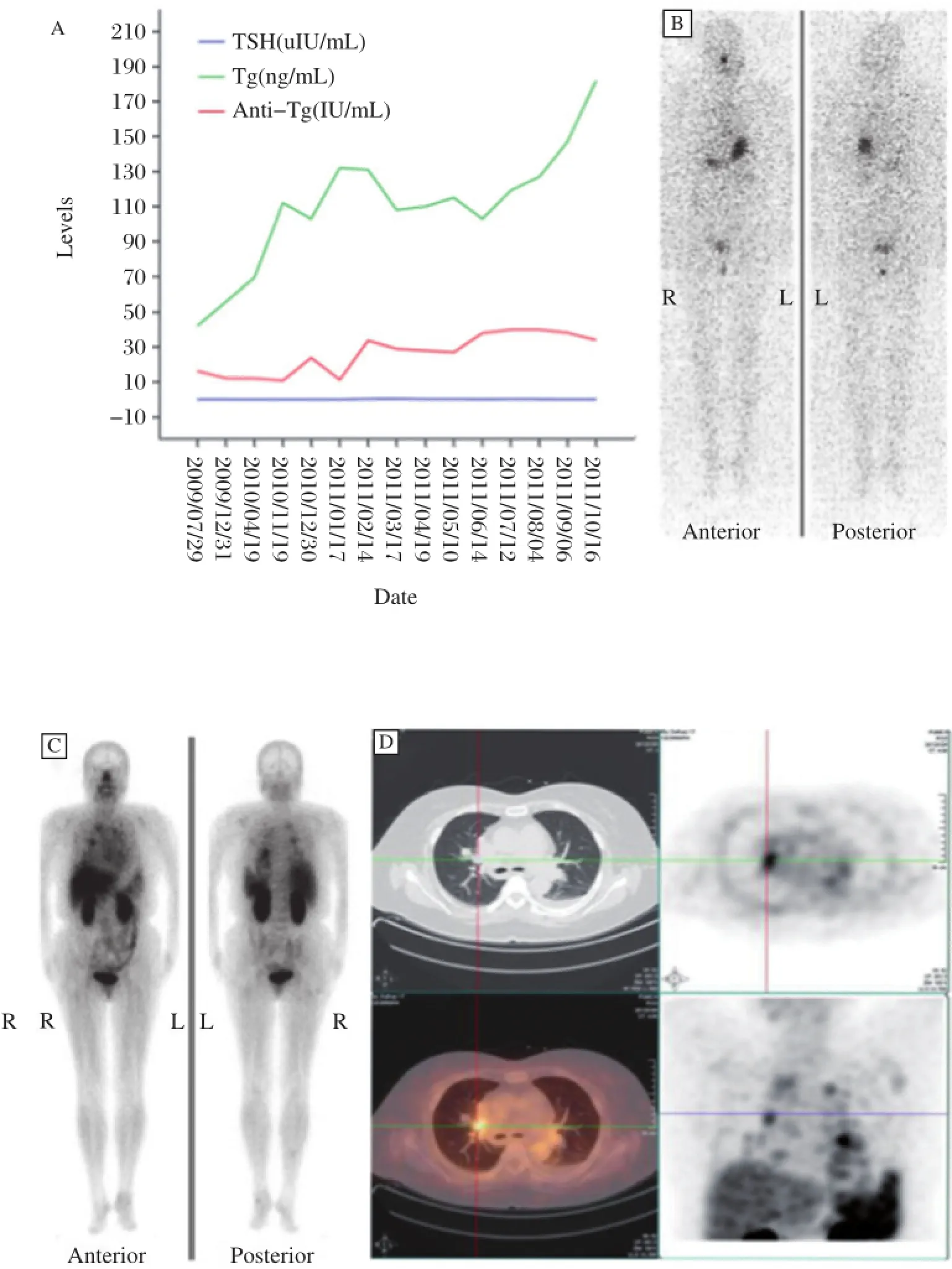
Fig.3 Elevated levels of unstimulated Tg(ng/mL)and Tg-Ab(IU/mL)with corresponding TSH(A),131I-negative image with TSH stimulation(TSH>30 u IU/mL)(B),and99mTc-RGD-positive planar(C)and SPECT images(D).Cited from Zhao D.et al.J Nucl Med,2012;53:1872-1877 with permission.
Radioimmunotherapy for non-Hodgk in lymphoma
Radioimmunotherapy(RIT)refers to the therapeutic use of monoclonal antibodies conjugated via a chelator to a radioactive nuclide.The therapeutic effect is achieved through both the action of the immunoglobu-lin as well as the radiotherapeutic effect of the attached radioisotope.RIT has been shown in clinical trials to be an effective treatment for refractory/relapsed non-Hodgkin lymphoma(NHL).The available agents are Zevalin®and Bexxar®.They are both radiolabeled monoclonal antibodies and both target CD20 receptors present on the surface of lymphocytes.
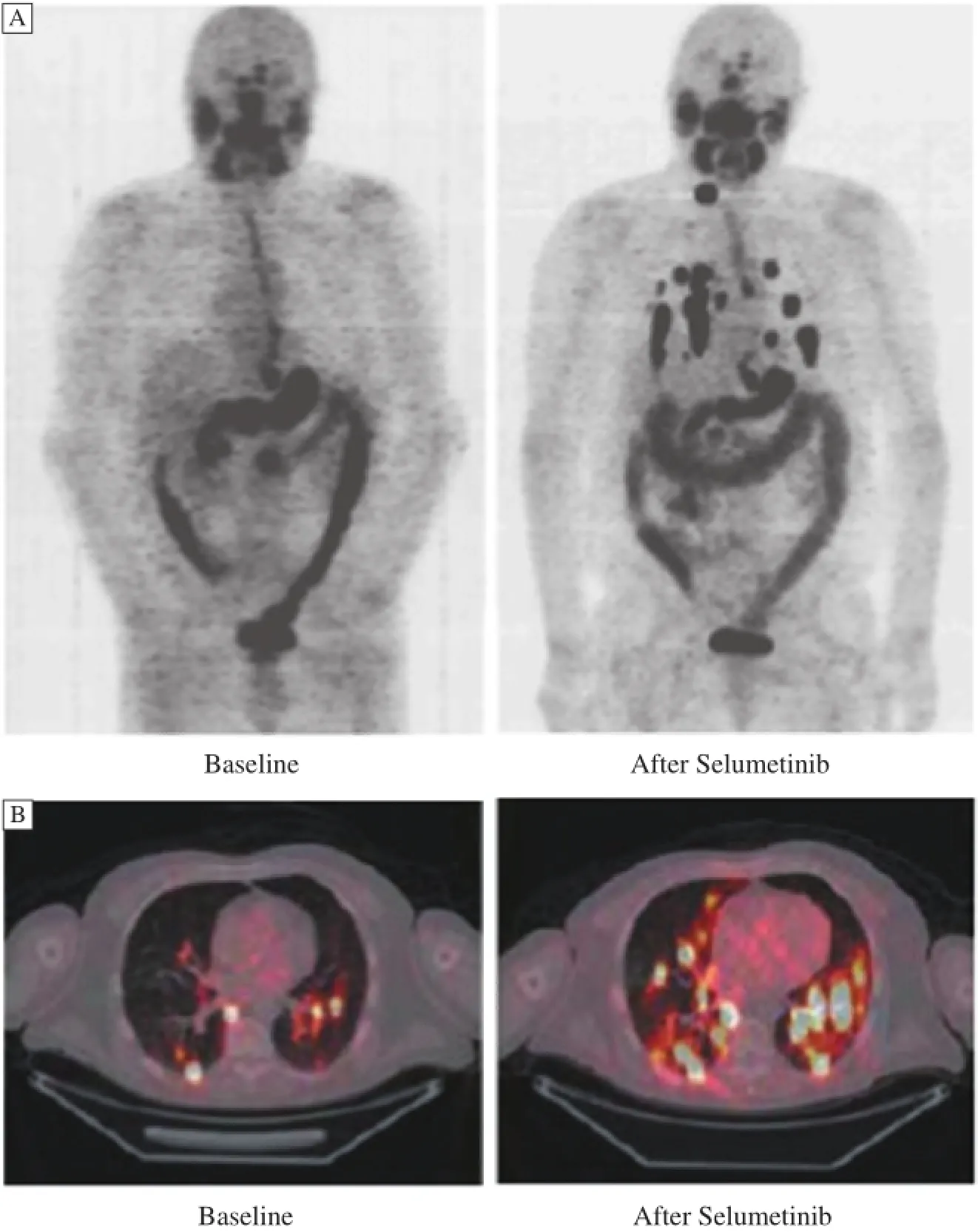
Fig.4 Whole-body maximum intensity projection images of a patient with a BRAF mutant papillary thyroid cancer.New iodine uptake is shown in nearly all previously negative lung and neck metastases.Panel A shows whole-body maximum-intensity projection images of a patient with a BRAF-mutant papillary thyroid cancer.New iodine uptake is shown in nearly all previously negative lung and neck metastases.Panel B shows fused axial PET-CT images of a patient with an NRAS-mutant,poorly differentiated thyroid cancer.Both new and significantly increased iodine uptake in lung metastases is shown.Cited from Ho AL et al.N Engl J Med,2013,368(7):623-632 with permission.
Zevalin®(ibritumomabtiuxetan,Spectrum Pharmaceuticals,Henderson,NV,USA),a yttrium-90 (90Y)radiolabeled monoclonal murine antibody,was the first radioimmunotherapy approved by the United States Food and Drug Administration(FDA)for the treatment of cancer.CD20 antigen is expressed in most cases of NHL and on normal B lymphocytes but not on stem cells,plasma cells,or non-hematopoietic tissues. In addition,the CD20 antigen does not shed from the cell surface to form free antigenthat could compete the circulating antibody[33].Because immature B lymphocytes do not have CD20 receptor,normal B lymphocytes will recover in about 9 months after treatment.After Zevalin enters the bloodstream,the monoclonal antibody ibritumomab recognizes and attaches to the CD20 antigen,allowing beta radiation emitted by the Yttrium-90 radionuclide to penetrate and damage B cells as well as neighboring cells.The Zevalin therapeutic regimen was approved by the FDA in 2002 to treat patients with relapsed or refractory,low-grade or follicular B-cell NHL.An added indication was approved by the FDA in 2009 for previously untreated follicular NHL in patients whoachieve a partial or complete response to first-line chemotherapy.The most common adverse reactions of Zevalin are cytopenias,fatigue,nasopharyngitis,nausea,abdominal pain,asthenia,cough,diarrhea,and pyrexia,while the most serious adverse reactions of Zevalin are prolonged and severe cytopenias(thrombocytopenia,anemia,lymphopenia,and neutropenia)and secondary malignancies.
A Phase III randomized controlled trial conducted in 143 patients on Zevalin therapeutic regimen with relapsed or refractory,low-grade or follicular NHL or transformed B-cell NHL revealed an overall response rate and CR rate of 80% and 30%.The rate was much higher than that in patients who received rituximab alone(56%and 16%)[34].Another multi-center Phase III trial conducted in patients with follicular NHL reported that90Y-ibritumomab tiuxetan consolidation significantly prolonged median PFS(after a median observation time of 3.5 years) of 36.5 months,compared with no further therapy(13.3 months)[35]. Though some unacceptable hematologic toxicity has been reported,the promising efficacy of Zevalin®has been demonstrated in a recent randomized study which showed that standard-dose ibritumomabtiuxetan combined with BEAM high-dose chemotherapy is safe and possibly more effective than BEAM alone(2-year survival rate,91% vs.62%)as a conditioning regimen for autologous stem cell transplantation in patients with aggressive lymphoma[36].
Bexxar,also called as Bezzar(GlaxoSmithKline),a131I radiolabeled murine monoclonal antibody(I-131 Tositumomab),was approved by the FDA for the treatment of patients with CD20 positive,follicular,NHL, with and without transformation,whose disease is refractory to Rituximab and has relapsed following chemotherapy.Tositumomab is a murine IgG2a lambda monoclonal antibody directed against the CD20 antigen.Possible mechanisms of action of the Bexxar therapeutic regimen includes induction of apoptosis,complement-dependent cytotoxicity(CDC),and antibody-dependent cellular cytotoxicity(ADCC)mediated by the antibody. Additionally,cell death is associated with ionizing radiation from the radionuclide.Adverse events of Bexxar include infection,hematologic toxicity,allergic reaction, anaphylactoid reaction,gastrointestinal symptoms,fever, nausea,sweating,hypotension,and asthenia.Fisher et al.[37]summarized the results obtained in 250 patients in 5 clinical trials that included a subset of heavily pretreated patients.In this group of patients with a considerable disease burden,the median duration of response was 12.9 months with a range from 10.9 to 17.3 months;a complete remission(CR)was seen in 30% of the patients. In the CR group,the median duration of response was almost 5 years(58.4 months),with a minimum duration of 28.3 months.A recent phase III randomized intergroup trial compared the safety and efficacy of CHOP+ Rituximab(CHOP-R)and CHOP+I-131 Tositumomab (CHOP-RIT)for 554 patients with previously untreated, advanced-stage follicular lymphomas.After a median follow-up period of 4.9 years,no evidence of significant improvement was found in 2-year PFS(80% vs.76%) and 2-year overall survival(OS)(93% vs.97%)comparing CHOP-RIT with CHOP-R[38].
A comparison between Bexxar®and Zevalin®,which was conducted in patients with low-grade refractory/ relapsed NHL,showed no statistical significance in objective responses(70.9% vs.77.8%),CR(35.5% vs.41.7%), partial remission(PR)(22.6% vs.25%)and grade 3/4 hematological toxicity(45.2% vs.61.1%)between the two agents.Because of the relatively small size of the sample,convincing evidence from further large clinical trials is needed to be reported.So far,neither Bexxar®,nor Zevalin®is approved for clinical use in China.
Radiopharmaceuticals for bone metastases
Skeletal metastases occur in many patients with different malignant tumors,especially in the advanced stages of prostate,breast,and lung cancers.Pain secondary to bone metastasis is associated with general suffering, reduced QOL,reduced self-sufficiency,increased mortality,and increased health care cost.As one of the optional treatments for palliation of painful bone metastases,internal therapy by radiopharmaceuticals is generally applied in patients with multifocal bone lesions which cannot be treated by external beam radiation therapy(EBRT). Radiopharmaceuticals are radioactive agents,administered intravenously,that enable the radiation(typically beta/electron emission)to be emitted at the sites of bone metastases.An ideal radio pharmaceutical would be a radiolabeled compound that predominantly accumu lated in bone lesions,with low toxicity to bone marrow and limited uptake by normal bone and other organs.
Strontium-89(89Sr)was the first radiopharmaceutical used in systemic radionuclide therapy for palliation of painful bone metastases.It was first reported by Pecher in 1942 for pain relief in osseous metastases[39]and was approved in the UK in 1989 and USA in 1993 as an unsealed radioisotope for internal radiation therapy.89Sr emits a pure β-particle with a maximum energy of 1.49 MeV and a physical half-life of 50.5 days. The maximum particle range in tissue is approximately 7 mm and an average soft-tissue range is 2.4 mm.89Sr is similar to calcium in chemical structure and is able tobe incorporated into the bone and reach the metastatic sites in proportion to the degree of osteoblastic activity.89Sr has a 10-fold proclivity for metastatic tumor compared to healthy bone[40].In light of the relationship between the dose of89Sr and clinical responses in terms of bone pain palliation,studies suggested a threshold dose below 0.9 MBq/kg appeared ineffective and a response barely increased above 1.5 MBq/kg with more myelosuppressive effect.A recommended dose of 148 MBq(4.0 mCi)in USA,or alternatively dose of 1.5-2.2 MBq/kg may be used[41].A randomized trial revealed less analgesic intake,pain relief,improved QOL and increased time to development of new bone metastases in patients receiving89Sr[42].This therapy attains a symptomatic benefit in 55%-80% of patients with duration of responses ranging between 2 and 17 weeks[43].Nevertheless,it did not prove to be effective in extending OS[44].As89Sr is designed for malignant diseases involving osseous metastases,combination with chemotherapy is another consideration.89Sr is currently used on a worldwide scale and is clinically available in China.
Samarium-153(153Sm)emits β-particle,with a physical half-life of 1.9 days.The β-particle has a maximum energy of 0.81 MeV,a mean energy of 0.23 MeV, and an average soft-tissue range of 0.6 mm.The β-ray is accompanied by a 103keV γ-ray,which allows simultaneous scintigraphic tracking with a gamma camera.153Sm has been chelated with bone-seeking polyphosphonates(EDTMP)and has the advantage of an optimum combination of high bone uptake,rapid blood clearance,and renal excretion when Sm is chelated with EDTMP.A dose of 1 mCi/kg for both clinical efficacy and safety was established from randomized trials for patients with painful bone metastases.There is no increase in pain relief but a greater degree of myelosuppression is present from higher administered activities. Pain relief was observed in 62%-72% of subjects with no effect on OS[45];it usually begins in 1 to 4 weeks and has lasted about 11 months[46].153Sm is also used countrywide in China.
Rhenium(Re)-1861,1-hydroxyethylidenediphosphonate(186Re-HEDP)is a bone seeking radiopharmaceutical which em its β-particle(1.07 MeV)with a maximum range of penetration of 5 mm,γ-photons (137 keV)suitable for imaging,and with a physical half-life of 89 hours.Response of pain relief can be observed in 65%-80% of patients undergoing radiotherapy with186Re-HEDP,with a mean duration of response of seven weeks[47],while there is no influence on OS.As an isotope of186Re,188Re-HEDP has also been used for bone pain palliation.It has the advantage of ready and on-demand availability of188Re from a generator and a kit is available for attaching it to HEDP.188Re-HEDP has a short physical half-life of 16.9 hrs and a higher energy of maximum beta-emission of 2.1 MeV.With similar biodistribution,dosimetry characteristics,toxicity,and benefits as186Re-HEDP in patients with osseous metastases,Chen et al. reported that188Re-HEDP significantly improved QOL in 81.5% subjects with administration of 1110 MBq(30 mCi),which enabled them to reduce their analgesic intake.
Radium-223(223Ra)is a calcium-mimetic bone-seeking radiopharmaceutical,in the form of radium dichloride.In contrast to the above mentioned β-emitters which are generally limited by adverse effect on bone marrow,223Ra predominantly emits α particles,which is characterized by highly energetic radiation and short range (<0.1 mm).It is able to induce more lethal tumoricidal effect with less surrounding tissue exposure and less adverse effects such as hematologic toxicity and myelosuppression.So far,223Ra is the only bone-seeking drug demonstrated to extend OS in metastatic castration-resistant prostate cancer(CRPC)patients,thus confirming the key role of targeting bone metastases to improve patient prognosis.A phase III trial enrolled 922 symptomatic patients with only bone metastases due to CRPC, either failing(60%)or not eligible or refused(40%)a prior docetaxel-based chemotherapy(ALSYMPCA study),who received either 6 intravenous treatments of Ra-223 at 50 kBq/kg or placebo,with an interval of 4 weeks between each treatment.Compared with placebo,223Ra significantly improved OS in patients with CRPC with bone metastases(2-sided P=0.00007;HR:0.658; 95% CI,0.581-0.832;median OS 14.9 months vs.11.3 months,respectively).Beneficial effect was also observed regarding time to first skeletal related events such as pathologic fractures and skeletal complications including nervous tissue compressions and hypercalcemia,as well as time to total serum bone-specific ALP or PSA progression.223Ra also improved QOL,especially in the pain, physical well-being,social/familial well-being,and emotional and functional well-being subscales,in terms of changes in the Functional Assessment Scale-Prostate subscale scores.The drug holds the promise of being evaluated as a viable monotherapy option in frontline or chemotherapy-progressed patients in the absence of visceral disease.223Ra was approved by the FDA in 2013 for the treatment of patients with metastatic CRPC with symptomatic bone metastases and unknown visceral metastatic disease.Its clinical use in China remains as‘need-to-be-approved’by the Chinese Food and Drug Administration.
To summarize,advances in several areas of radionuclide internal radiation therapy have been reviewed inthis article,including benign and malignant thyroid disease,RIT for NHL,and radiopharmaceuticals for palliation of painful bone metastases.These molecular mediated internal radiation therapies are gaining increasing importance by providing palliative and curative treatments for an increasing number of diseases and becoming one of the important aspects of molecular nuclear medicine that should never be neglected to keep nuclear medicine as an integrity.
Acknowledgments
The author appreciates the help from Yang Xue, Yang Ke and Zhang Yingjie.
No honoraria or other form of financial support has been received related to the development of this manuscript.
[1] Weiss SJ,Philp NJ,Am besi-Impiombato FS,et al. Thyrotropin-stimulated iodide transport mediated by adenosine 3′,5′-monophosphate and dependent on protein synthesis[J].Endocrinology,1984,114(4):1099-1107.
[2] Eng PH,Cardona GR,Fang SL,et al.Escape from the acute Wolff-Chaik off effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein[J].Endocrinology,1999,140(8):3404-3410.
[3] Ulianich L,Suzuki K,Mori A,et al.Follicular thyroglobulin(TG)suppression of thyroid-restricted genes involves the apical membrane asiologlycoprotein receptor and TG phosphorylation[J].J Biol Chem,1999,274(35): 25099-25107.
[4] Furlanetto TW,Nguyen LQ,Jameson JL.Estradio l increases proliferation and down-regulates the sodium/ iodide symporter gene in FRTL-5 cells[J].Endocrinology, 1999,140(12):5705-5711.
[5] Ajjan RA,Watson PF,Findlay C,et al.The sodium iodide symporter gene and its regulation by cytokines found in autoimmunity[J].J Endocrinol,1998,158(3):351-358.
[6] Lazar V,Bidart JM,Caillou B,et al.Expression of the Na+/I-Symporter Gene in Human Thyroid Tumors:A Comparison Study with Other Thyroid-Specific Genes[J]. J Clin Endocrinol Metab,1999,84(9):3228-3234.
[7] Hermus AR,Huysmans DAKC.Treatment of benign nodular thyroid disease[J].N Engl J Med,1998,338(20):1438-1447.
[8] Burch HB,Burman KD,Cooper DS.A 2011 Survey of Clinical Practice Patterns in the Management of Graves’Disease[J].J Clin Endocrinol Metab,2012,97(12):4549-4558
[9] Traisk F,Tallstedt L,Abraham-No rd ling M,et al. Thyroid-associated ophthalmopathy after treatment for Graves’hyperthyroidism with antithyroid d rugs or iodine-131[J].J Clin Endocrinol Metab,2009,94(10): 3700-3707.
[10]Lin Y,Jiang N.Current situation of radioiodine-131 treatment of Graves’hyperthyroidismin China-A survey from clinician and patients.EB/OL.(2013-01-15)[2013-01-23] http://csnm.medipromos.com/viewArt.asp?id=1383.
[11]Chen DY,Schneider PF,Zhang XS,et al.Striving for euthyroidism in radioiodine therapy of Graves’disease: a 12-year prospective,randomized,open-label blinded end point study[J].Thyroid,2011,21(6):647-654.
[12]Franklyn JA,Sheppard MC,Maisonneuve P.Thyroid function and mortality in patients treated for hyperthyroidism[J]. JAMA,2005,294(1):71-80.
[13]Aschebrook-Kilfoy B,Ward MH,Sabra MM,et al.Thyroid cancer incidence patterns in the United States by histologictype,1992-2006[J].Thyroid,2011,21(2):125-134.
[14]Lin Y,Li T,Liang J,et al.Predictive Value of Preablation Stimulated Thyroglobulin and Thyoglobulin/Thyoid-Stimulating Horm one Ratio in Differetiated Thyroid Cancer[J].2011,36(12):1102-1105.
[15]Li T,Lin Y,Liang J,et al.Predictive value of pre-ablation stimulated Tg in distant metastasis of papillary thyroid cancer[J].Chin J Nucl Med Mol imaging,2012,32(3): 189-191.
[16]Schlumberger M,Catargi B,Borget I,et al.Strategies of radioiodine ablation in patients with low-risk thyroid cancer[J].N Engl J Med,2012,366(18):1663-1673.
[17]Mallick U,Harmer C,Yap B,et al.Ablation with lowdose radioiodine and thyrotropin alfa in thyroid cancer[J]. N Engl J Med,2012,366(18):1674-1685.
[18]Tuttle RM,Tala H,Shah J,et al.Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation:using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system[J].Thyroid,2010,20(12):1341-1349.
[19]Ibrahimpasic T,Nixon IJ,Palmer FL,et al.Undetectable thyroglobulin after total thyroidectomy in patients with low-and intermediate-risk papillary thyroid cancer-is there a need for rad ioactive iodine therapy[J]?Surgery, 2012,152(6):1096-1105.
[20]Mazzaferri EL,Kloos RT.Current approaches to primary therapy for papillary and follicular thyroid cancer[J].J Clin Endocrinol Metab,2001,86(4):1447-1463.
[21]Durante C,Haddy N,Baudin E,et al.Long-Term Outcome of 444 Patients with Distant Metastases from Papillary and Follicular Thyroid Carcinoma:Benefits and Lim its of Radioiodine Therapy[J].J Clin Endocrinol Metab,2006,91(8):2892-2899.
[22]Carr LL,Mankoff DA,Goulart BH,et al.Phase II study of daily sunitinib in FDG-PET-positive,iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation[J].Clin Cancer Res,2010,16(21):5260-5268.
[23]Zhao D,Jin X,Li F,et al.Integrinavb3 Imaging of Radioactive Iodine-Refractory Thyroid Cancer Using99mTc-3PRGD2[J].J Nucl Med,2012,53(12):1872-1877.
[24]Oh SW,Moon SH,Park do J,et al.Combined therapy with 131I and retinoic acid in Korean patients with radioiodine-refractory papillary thyroid cancer[J].Eur J Nucl Med Mol Imaging,2011,38(10):1798-1805.
[25]Kebebew E,Lindsay S,Clark OH,et al.Results of rosiglitazone therapy in patients with thyroglobulin-positive and radioiodine-negative advanced differentiated thyroid cancer[J].Thyroid,2009,19(9):953-956.
[26]Woyach JA,Kloos RT,Ringel MD,et al.Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat inpatients with metastatic radioiodine-refractory thyroid carcinoma[J].J Clin Endocrinol Metab,2009,94(1):164-170.
[27]Provenzano MJ,Fitzgerald MP,Krager K,et al.Increased iodine uptake in thyroid carcinoma after treatment with sodium butyrate and decitabine(5-Aza-d C)[J]. Otolaryngol Head Neck Surg,2007,137(5):722-728.
[28]Ricarte-Filho JC,Ryder M,Chitale DA,et al.Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF,PIK3CA,and AKT1[J].Cancer Res,2009,69(11):4885-4893.
[29]Garcia-Rostan G,Sobrinho-Simo¨es M.Poorly differentiated thyroid carcinoma:an evolving entity[J]. Diagnostic Histopathology,2011,17(3):114-123.
[30]Chakravarty D,Santos E,Ryder M,et al.Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional B RAF activation[J].J Clin Invest,2011,121(12):4700-4711.
[31]Ho AL,Grewal RK,Leboeu f R,et al.Selumetinibenhanced radioiodine uptake in advanced thyroid cancer[J].N Engl J Med,2013,368(7):623-632.
[32]Hayes DN,Lucas AS,Tanvetyanon T.Phase II efficacy and pharmacogenomic study of Selumetinib(AZD 6244; ARRY-142886)in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements[J].Clin Cancer Res,2012,18(7):2056-2065.
[33]Einfeld DA,Brown JP,Valentine MA,et al.Molecular cloning of the human B cell CD20 receptor predicts a hydrophobic protein with multiple transmembrane domains[J].EMBO J,1988,7(3):711-717.
[34]Wiseman GA,White CA,Sparks RB,et al.Biodistribution and dosimetry results from a phase III prospectively randomized controlled trial of ZevalinTMradioimmunotherapy for low-grade,follicular,or transformed B-cell non-Hodgkin’s lymphoma[J].Crit RevOncol Hematol,2001, 39(1-2):181-194.
[35]Morschhauser F,Radford J,Van Ho of A,et al.Phase III trial of consolidation therapy with yttrium-90-ibritumomabtiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma[J].J Clin Oncol,2008,26(32):5156-5164.
[36]Shimoni A,Avivi I,Rowe JM,et al.A randomized study comparing yttrium-90 ibritumomabtiuxetan(Zevalin)and high-dose BEAM chemotherapy versus BEAM alone as the conditioning regimen before autologous stem cell transplantation in patients with aggressive lymphoma[J]. Cancer,2012,118(19):4706-4714.
[37]Fisher RI,Kaminski MS,Wahl RL,et al.Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low-grade and transformed non-Hodgkin’s lymphomas[J]. J Clin Oncol,2005,23(30):7565-7573.
[38]Press OW,Unger JM,Rimsza LM,et al.Phase III randomized intergroup trial of CHOP plus Rituximab compared with CHOP chemotherapy plus 131Iodine-Tositumomab for previously untreated follicular Non-Hodgkin lymphoma: SWOG S0016[J].J Clin Oncol,2013,31(3):314-320.
[39]Pecher P.Biological investigation with radioactive calcium and strontium.Preliminary report on the use of radioactive strontium in the treatment of metastatic bone cancer[J].U Cal Publications in Pharmacology,1942,2:117-149.
[40]Robinson RG,Blake GM,Preston DF,et al.Strontium-89:treatment results and kinetics in patients with painful metastatic prostate and breast cancer in bone[J]. Radiographics,1989,9(2):271-281.
[41]Zenda S,Nakagami Y,Toshima M,et al.Strontium-89(Sr-89)chloride in the treatment of various cancer patients with multiple bone metastases[J].Int J Clin Oncol,2014,9(4): 739-743.
[42]Nilsson S,Strang P,Ginman C,et al.Palliation of bone pain in prostate cancer using chemotherapy and strontium-89.A randomized phase II study[J].J Pain Symptom Manage,2005,29(4):352-357.
[43]Srivastava SC,Atkins HL,Krishnamurthy GT,et al. Treatment of metastatic bone pain with tin-117 m Stannic diethylenetriaminepentaacetic acid:a phase I/II clinical study[J].Clin Cancer Res,1998,4(1):61-68.
[44]McEwan AJ.Use of radionuclides for the palliation of bone metastases[J].Semin Radiat Oncol,2000,10(2):103-114.
[45]Serafini AN,Houston SJ,Resche I,et al.Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam:a double-blind placebo-controlled clinical trial[J].J Clin Oncol,1998,16(4):1574-1581.
[46]Alberts AS,Smit BJ,Louw WK,et al.Dose response relationship and multipledose efficacy and toxicity of samarium-153-EDTMP in metastatic cancer to bone[J].Radioth Oncol,1997,43(2):175-179.
[47]Maxon HR 3rd,Schroder LE,Hertzberg VS,et al.Rhenium-186(Sn)HEDP for treatment of painful osseous metastases: Results of a double-blind crossover comparison with placebo[J].J Nucl Med,1991,32(10):1877-1881.
✉Corresponding author:Yansong Lin,Department of Nuclear Medicine Peking Union Medical College Hospital,No.1 Shuaifu,Dongcheng district,Beijing 100730,China.Fax:+86-69155610100730,E-mail:linys@ pumch.cn.
Received 08 May 2014,Revised 06 June 2014,Accepted 13 October 2014, Epub 12 January 2015
R817.5,Document code:A
The author reported no conflict of interests.
©2015 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.29.20140069
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Hypercholesterolemia,low density lipoprotein receptor and proprotein convertase subtilisin/kexin-type 9
- Endothelin-1-induced mini-stroke in the dorsal hippocampus or lateral amygdala results in deficits in learning and memory
- Netrin-1 overexpression in bone marrow mesenchymal stem cells promotes functional recovery in a rat model of peripheral nerve injury
- Acute effect of aspartame-induced oxidative stress in Wistar albino rat brain
- Differential mRNA expression profiling of oral squamous cell carcinoma by high-throughput RNA sequencing
- Diacerein protects against iodoacetate-induced osteoarthritis in the femorotibial joints of rats
